Colloidal Silver Nanoparticles Obtained via Radiolysis: Synthesis Optimization and Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
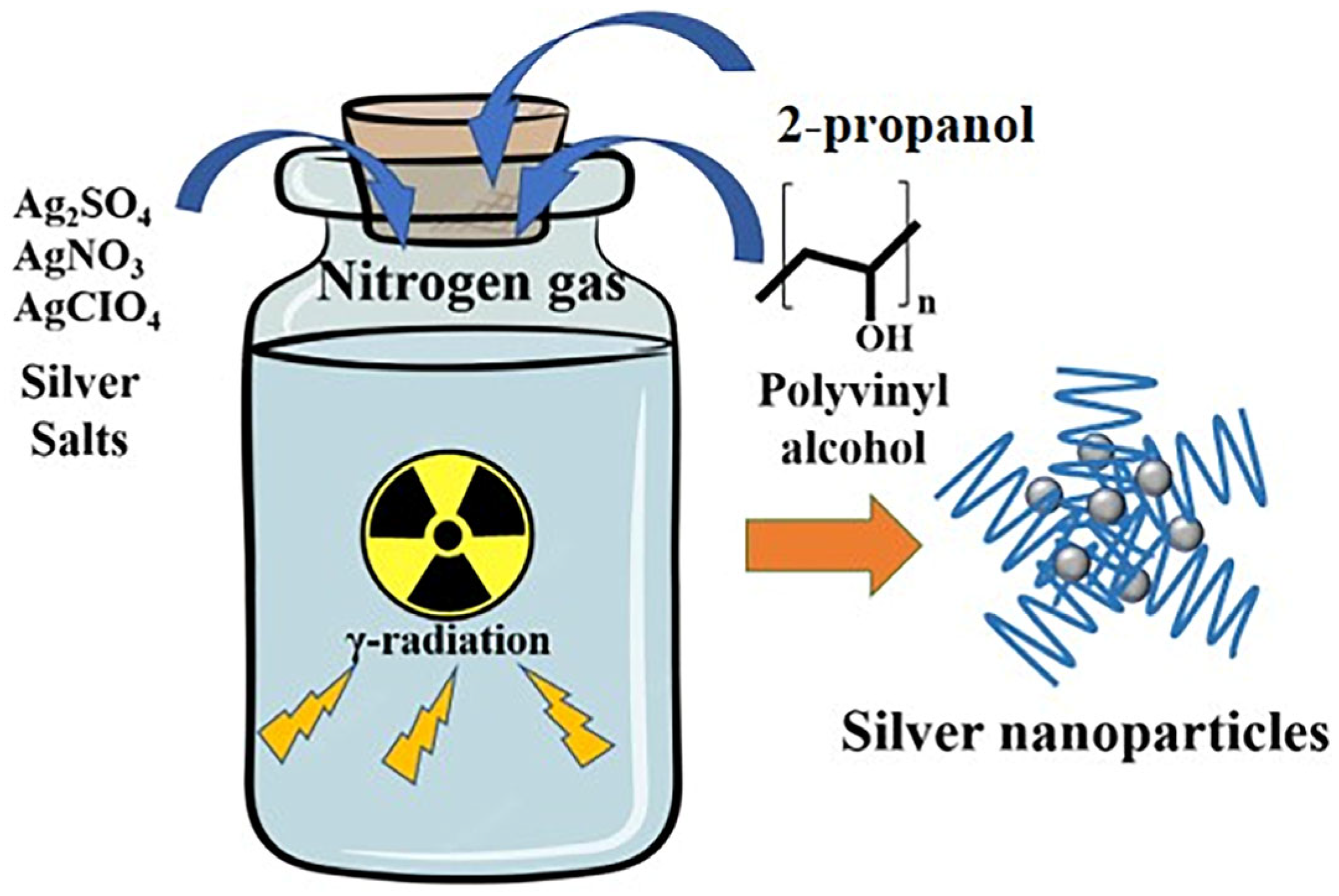
2.2. Preparation of AgNPs
2.3. Characterization
2.4. In Vitro Evaluation of Antibacterial Ability
3. Results and Discussion
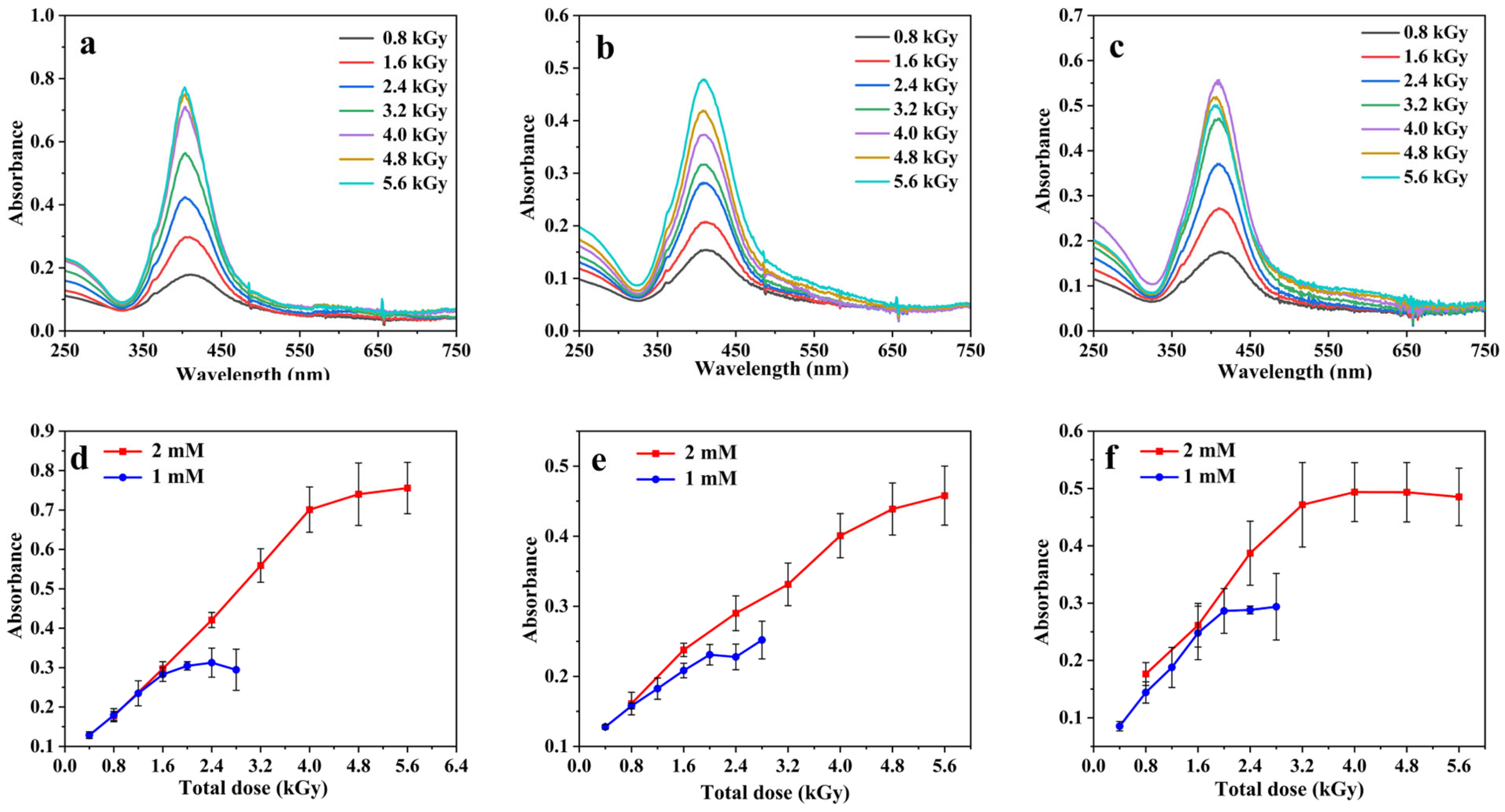
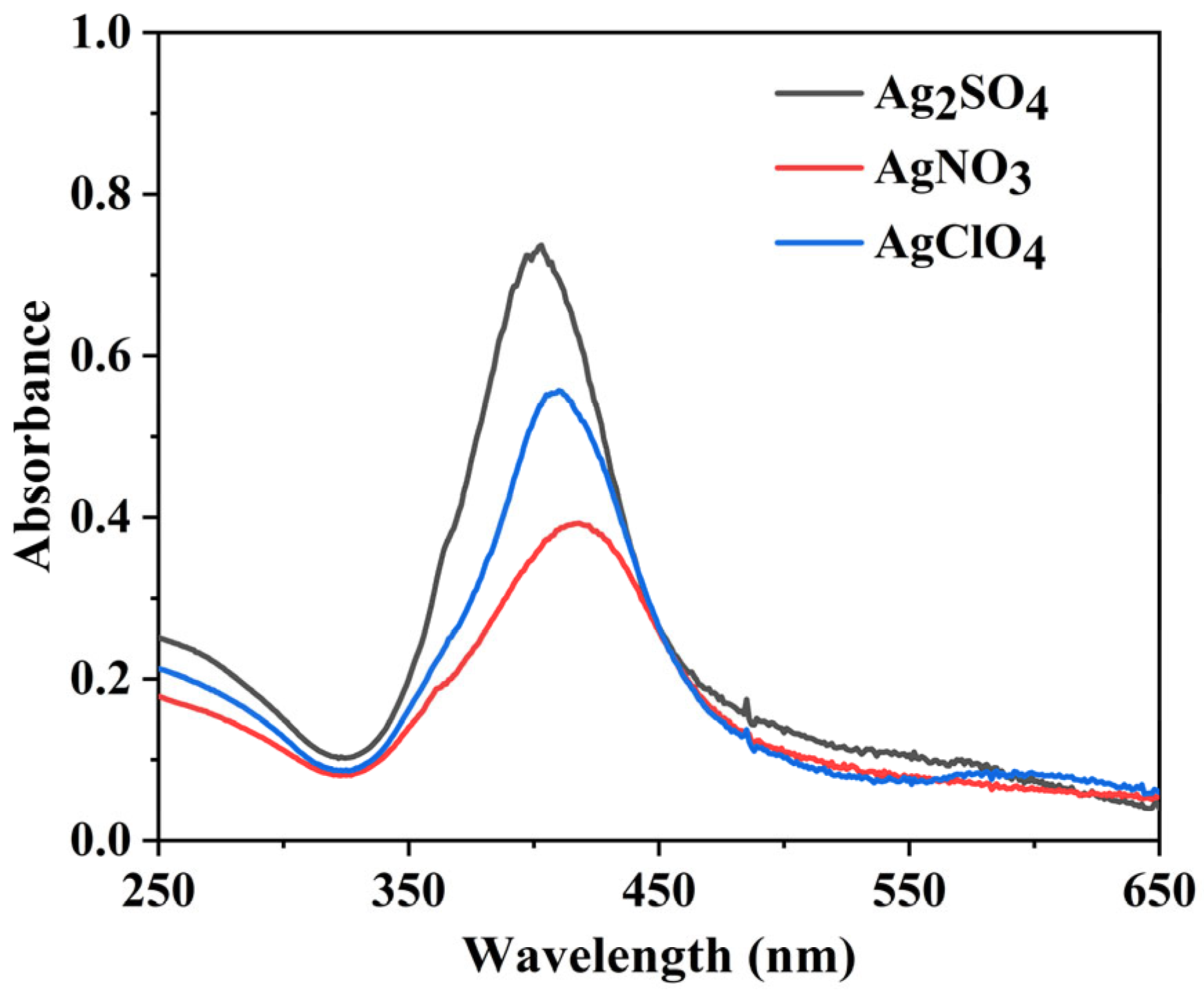
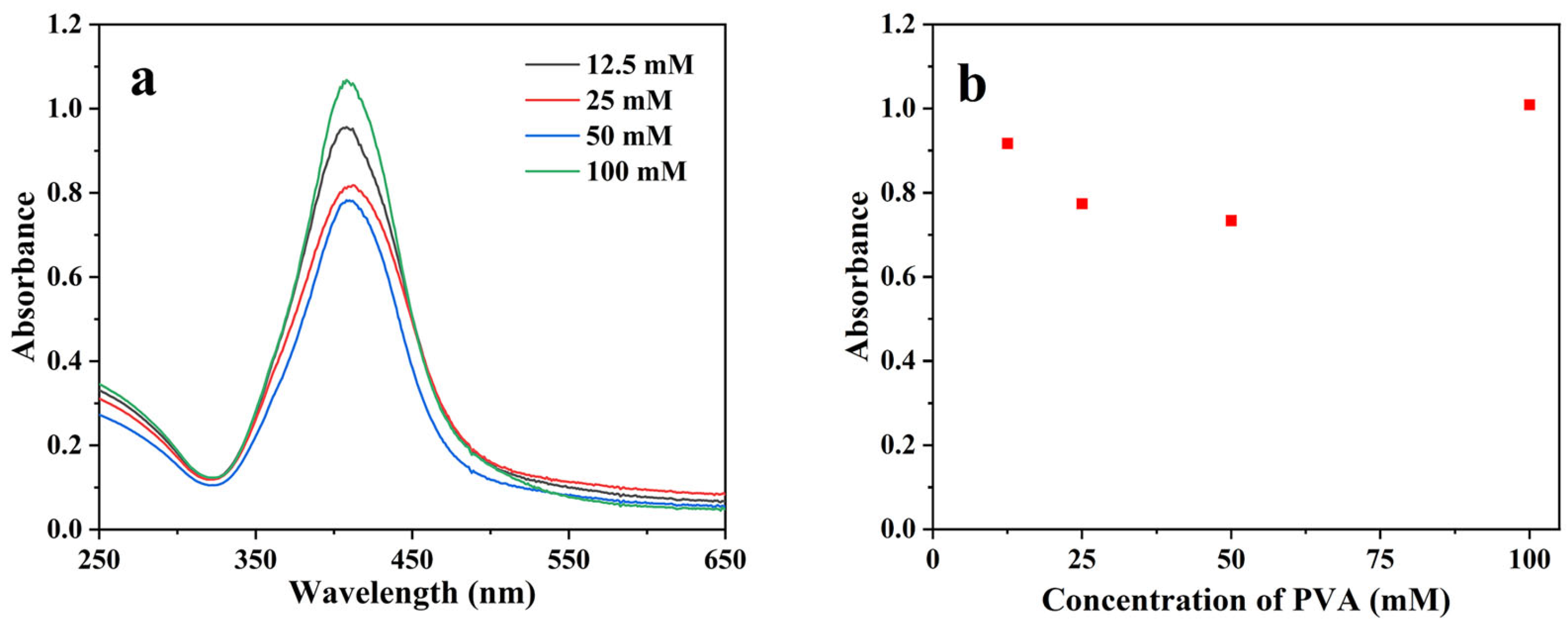
3.1. AgNP Synthesis and Optimization
 e−aq, H•, OH•, H3O+, H2O2, H2
e−aq, H•, OH•, H3O+, H2O2, H2
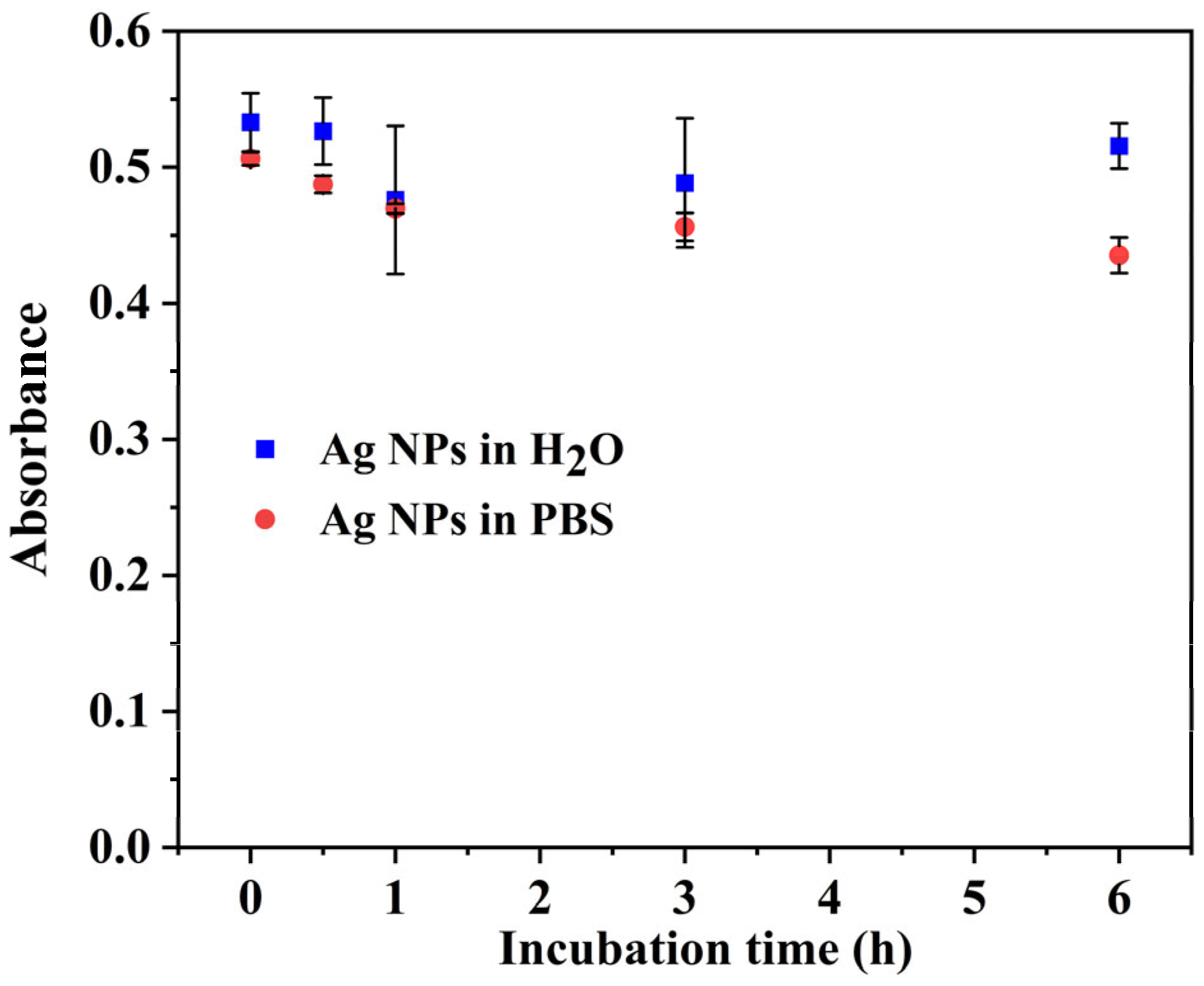
3.2. AgNPs Stability Studies
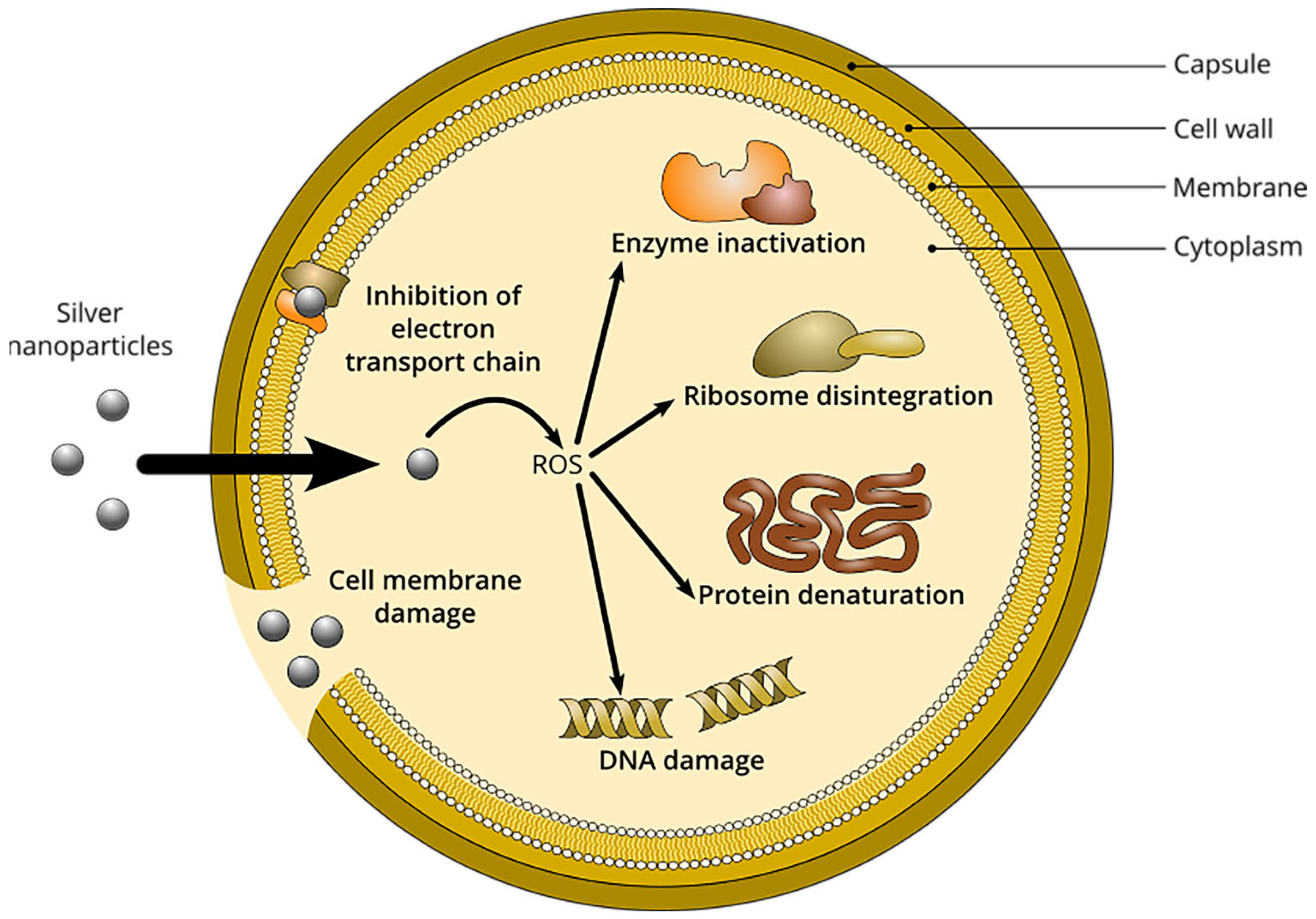
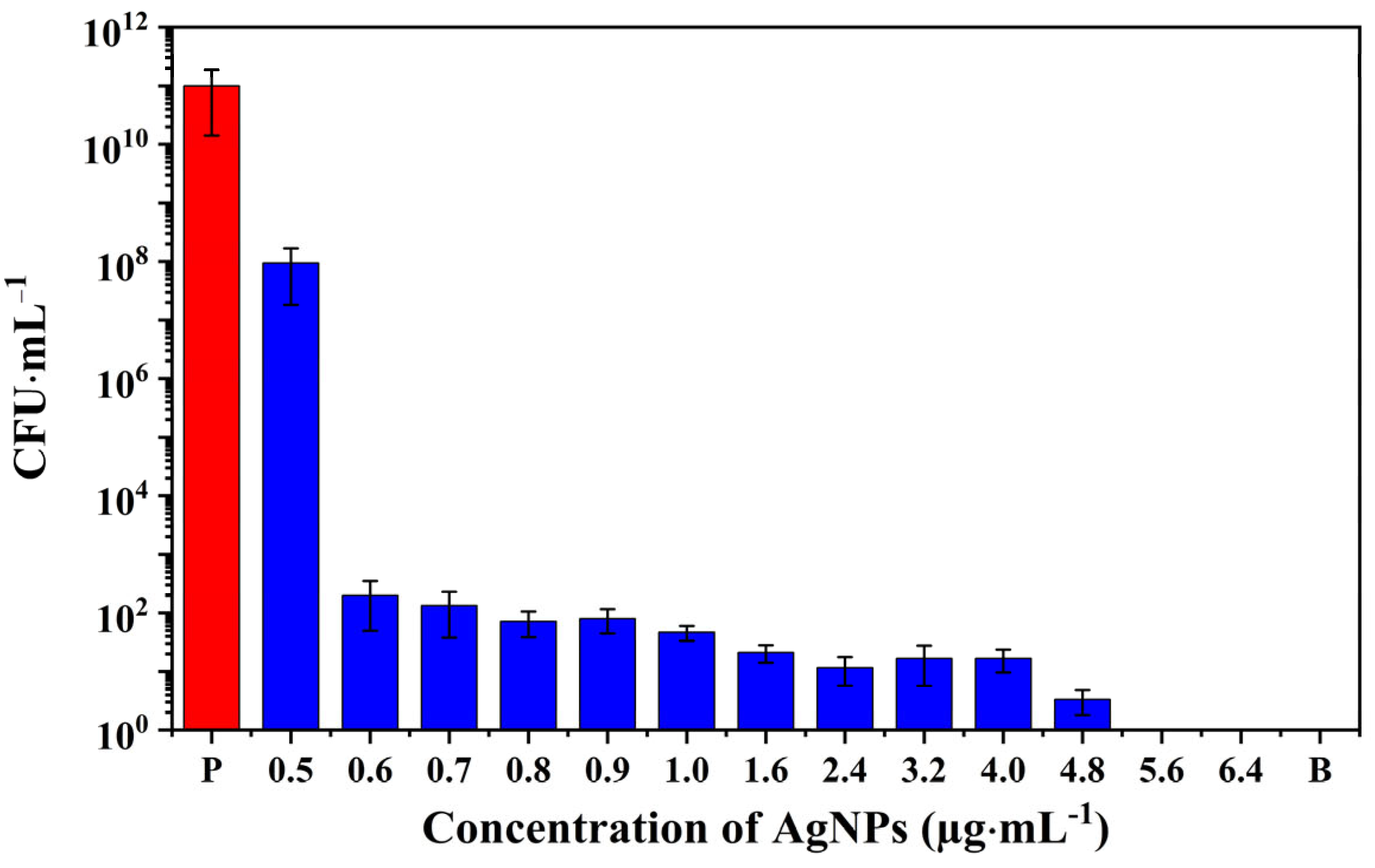
3.3. Antibacterial Activity of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaming, D.; Remita, H. Nanotechnology: From the Ancient Time to Nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Shahidi, S. Magnetic Nanoparticles Application in the Textile Industry—A Review. J. Ind. Text. 2021, 50, 970–989. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver Nanoparticle Applications and Human Health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. The Role of Antibiotics and Antibiotic Resistance in Nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Hanif, Z.; Khan, Z.A.; Choi, D.; La, M.; Park, S.J. One-Pot Synthesis of Silver Nanoparticle Deposited Cellulose Nanocrystals with High Colloidal Stability for Bacterial Contaminated Water Purification. J. Environ. Chem. Eng. 2021, 9, 105535. [Google Scholar] [CrossRef]
- Williams, D. The Relationship between Biomaterials and Nanotechnology. Biomaterials 2008, 29, 1737–1738. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupova, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and Characterization of Size- and Shape-Controlled Silver Nanoparticles. Phys. Sci. Rev. 2018, 4, 20170082. [Google Scholar]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Tăbăran, A.-F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver Nanoparticles for the Therapy of Tuberculosis. Int. J. Nanomed. 2020, 15, 2231–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J.C.; Yip, H.Y.; Li, Q.; Kwong, K.W.; Xu, A.-W.; Wong, P.K. Ambient Light Reduction Strategy to Synthesize Silver Nanoparticles and Silver-Coated TiO2 with Enhanced Photocatalytic and Bactericidal Activities. Langmuir 2003, 19, 10372–10380. [Google Scholar] [CrossRef]
- Pascu, B.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Bumm, L.A.; Grad (mBuriac), O.; Nemeş, N.S.; Mihalcea, C.; Duda-Seiman, D.M. Silver Nanoparticle Synthesis via Photochemical Reduction with Sodium Citrate. Int. J. Mol. Sci. 2023, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Abidi, W.; Remita, H. Gold Based Nanoparticles Generated by Radiolytic and Photolytic Methods. Recent Pat. Eng. 2010, 4, 170–188. [Google Scholar] [CrossRef]
- Xin, X.; Qi, C.; Xu, L.; Gao, Q.; Liu, X. Green Synthesis of Silver Nanoparticles and Their Antibacterial Effects. Front. Chem. Eng. 2022, 4, 941240. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon Orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.-L.; Delcourt, A.M.-O. Radiation-Induced Synthesis of Mono- and Multi-Metallic Clusters and Nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Remita, H.; Lampre, I.; Mostafavi, M.; Balanzat, E.; Bouffard, S. Comparative Study of Metal Clusters Induced in Aqueous Solutions by γ-Rays, Electron or C6+ Ion Beam Irradiation. Radiat. Phys. Chem. 2005, 72, 575–586. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Endo-Kimura, M.; Wang, K.; Ohtani, B.; Bahena Uribe, D.; Rodríguez-López, J.L.; Remita, H. Inhibition of Fungal Growth Using Modified TiO2 with Core@ Shell Structure of Ag@CuO Clusters. ACS Appl. Bio. Mater. 2019, 2, 5626–5633. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Mostafavi, M.; Dey, G.R.; Francois, L.; Belloni, J. Transient and Stable Silver Clusters Induced by Radiolysis in Methanol. J. Phys. Chem. A 2002, 106, 10184–10194. [Google Scholar] [CrossRef] [Green Version]
- Henglein, A. Electronics of Colloidal Nanometer Particles. Ber. Bunsenges. Phys. Chem. 1995, 99, 903–913. [Google Scholar] [CrossRef]
- Mostafavi, M.; Marignier, J.L.; Amblard, J.; Belloni, J. Nucleation Dynamics of Silver Aggregates Simulation of Photographic Development Processes. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1989, 34, 605–617. [Google Scholar] [CrossRef]
- Ray, P.; Clément, M.; Martini, C.; Abdellah, I.; Beaunier, P.; Rodriguez-Lopez, J.-L.; Huc, V.; Remita, H.; Lampre, I. Stabilisation of Small Mono- and Bimetallic Gold–Silver Nanoparticles Using Calix [8] Arene Derivatives. New J. Chem. 2018, 42, 14128–14137. [Google Scholar] [CrossRef]
- Sheikh, N.; Akhavan, A.; Kassaee, M.Z. Synthesis of Antibacterial Silver Nanoparticles by γ-Irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2009, 42, 132–135. [Google Scholar] [CrossRef]
- Du, B.D.; Phu, D.V.; Duy, N.N.; Lan, N.T.K.; Lang, V.T.K.; Thanh, N.V.K.; Phong, N.T.P.; Hien, N.Q. Preparation of Colloidal Silver Nanoparticles in Poly( N -Vinylpyrrolidone) by γ-Irradiation. J. Exp. Nanosci. 2008, 3, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Hassabo, A.G.; Nada, A.A.; Ibrahim, H.M.; Abou-Zeid, N.Y. Impregnation of Silver Nanoparticles into Polysaccharide Substrates and Their Properties. Carbohydr. Polym. 2015, 122, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Uttayarat, P.; Eamsiri, J.; Tangthong, T.; Suwanmala, P. Radiolytic Synthesis of Colloidal Silver Nanoparticles for Antibacterial Wound Dressings. Adv. Mater. Sci. Eng. 2015, 2015, 376082. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Bahry, T.; Marignier, J.-L.; Guigner, J.-M.; Gervais, M.; Baiz, S.; Goubard, F.; Remita, S. A Novel Radiation Chemistry-Based Methodology for the Synthesis of PEDOT/Ag Nanocomposites. Mater. Chem. Front. 2017, 1, 879–892. [Google Scholar] [CrossRef]
- Pascu, B.; Negrea, A.; Ciopec, M.; Davidescu, C.M.; Negrea, P.; Gherman, V.; Duteanu, N. New Generation of Antibacterial Products Based on Colloidal Silver. Materials 2020, 13, 1578. [Google Scholar] [CrossRef] [Green Version]
- Khozemy, E.E.; Nasef, S.M.; Mahmoud, G.A. Synthesis and Characterization of Antimicrobial Nanocomposite Hydrogel Based on Wheat Flour and Poly (Vinyl Alcohol) Using γ-Irradiation. Adv. Polym. Technol. 2018, 37, 3252–3261. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Ma, H.; Cui, M.; Yang, B.; Wang, R.; Zhu, Y.; Gu, X.; Sha, Y.; Zhang, X. Radiation Syntheses of Modified Poly (Lactic Acid) Fabrics with Hydrophilic and Antibacterial Properties. Prog. Org. Coat. 2023, 176, 107393. [Google Scholar] [CrossRef]
- Nakamura, S.; Ando, N.; Sato, M.; Ishihara, M. Ultraviolet Irradiation Enhances the Microbicidal Activity of Silver Nanoparticles by Hydroxyl Radicals. Int. J. Mol. Sci. 2020, 21, 3204. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Yang, D.; Qiu, X.; Xie, Y.; Zhang, X. Microwave-Mediated Fabrication of Silver Nanoparticles Incorporated Lignin-Based Composites with Enhanced Antibacterial Activity via Electrostatic Capture Effect. J. Colloid Interface Sci. 2021, 583, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and Characterization of Active Films Based on Starch-PVA, Containing Silver Nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Gachard, E.; Remita, H.; Khatouri, J.; Keita, B.; Nadjo, L.; Belloni, J. Radiation-Induced and Chemical Formation of Gold Clusters. New J. Chem. 1998, 22, 1257–1265. [Google Scholar] [CrossRef]
- Hart, E.J. Research Potentials of the Hydrated Electron. Acc. Chem. Res. 1969, 2, 161–167. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, Growth and Properties of Nanoclusters Studied by Radiation Chemistry: Application to Catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Schwarz, H.A.; Dodson, R.W. Reduction Potentials of CO2− and the Alcohol Radicals. J. Phys. Chem. 1989, 93, 409–414. [Google Scholar] [CrossRef]
- Henglein, A. The Reactivity of Silver Atoms in Aqueous Solutions (A γ-Radiolysis Study). Ber. Bunsenges. Phys. Chem. 1977, 81, 556–561. [Google Scholar] [CrossRef]
- Belloni, J. Metal Nanocolloids. Curr. Opin. Colloid Interface Sci. 1996, 1, 184–196. [Google Scholar] [CrossRef]
- Mulvaney, P.; Henglein, A. Formation of Unstabilized Oligomeric Silver Clusters during the Reduction of Ag+ Ions in Aqueous Solution. Chem. Phys. Lett. 1990, 168, 391–394. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Self-Assembly of Silver Nanoparticles in a Polymer Solvent: Formation of a Nanochain through Nanoscale Soldering. Mater. Chem. Phys. 2005, 90, 221–224. [Google Scholar] [CrossRef]
- Ulanski, P.; Bothe, E.; Rosiak, J.M.; von Sonntag, C. OH-Radical-Induced Crosslinking and Strand Breakage of Poly (Vinyl Alcohol) in Aqueous Solution in the Absence and Presence of Oxygen. A Pulse Radiolysis and Product Study. Macromol. Chem. Phys. 1994, 195, 1443–1461. [Google Scholar] [CrossRef]
- Treguer, M.; de Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.; de Keyzer, R. Dose Rate Effects on Radiolytic Synthesis of Gold−Silver Bimetallic Clusters in Solution. J. Phys. Chem. B 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Becaro, A.A.; Jonsson, C.M.; Puti, F.C.; Siqueira, M.C.; Mattoso, L.H.C.; Correa, D.S.; Ferreira, M.D. Toxicity of PVA-Stabilized Silver Nanoparticles to Algae and Microcrustaceans. Environ. Nanotechnol. Monit. Manag. 2015, 3, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammawithan, S.; Siritongsuk, P.; Nasompag, S.; Daduang, S.; Klaynongsruang, S.; Prapasarakul, N.; Patramanon, R. A Biological Study of Anisotropic Silver Nanoparticles and Their Antimicrobial Application for Topical Use. Vet. Sci. 2021, 8, 177. [Google Scholar] [CrossRef]
- Li, W.-R.; Sun, T.-L.; Zhou, S.-L.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Huang, X.-M. A Comparative Analysis of Antibacterial Activity, Dynamics, and Effects of Silver Ions and Silver Nanoparticles against Four Bacterial Strains. Int. Biodeterior. Biodegrad. 2017, 123, 304–310. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Verma, R.; Prasad, P.; Verma, R.; Das, S.N.; Singh, J.; Singh, R.P. Preparation, Antibacterial Activity, and Electrocatalytic Detection of Hydrazine Based on Biogenic CuFeO2/PANI Nanocomposites Synthesized Using Aloe Barbadensis Miller. New J. Chem. 2022, 46, 8805–8816. [Google Scholar] [CrossRef]
- Shantkriti, S.; Pradeep, M.; Unish, K.K.; Das, V.; Nidhin, S.; Gugan, K.; Murugan, A. Bioynthesis of Silver Nanoparticles Using Dunaliella Salina and Its Antibacterial Applications. Appl. Surf. Sci. Adv. 2023, 13, 100377. [Google Scholar]
- Singh, P.; Singh, K.R.; Singh, J.; Das, S.N.; Singh, R.P. Tunable Electrochemistry and Efficient Antibacterial Activity of Plant-Mediated Copper Oxide Nanoparticles Synthesized by Annona Squamosa Seed Extract for Agricultural Utility. RSC Adv. 2021, 11, 18050–18060. [Google Scholar] [CrossRef] [PubMed]
- Vertelov, G.K.; Krutyakov, Y.A.; Efremenkova, O.V.; Olenin, A.Y.; Lisichkin, G.V. A Versatile Synthesis of Highly Bactericidal Myramistin® Stabilized Silver Nanoparticles. Nanotechnology 2008, 19, 355707. [Google Scholar] [CrossRef]
- Bartel, M.; Markowska, K.; Strawski, M.; Wolska, K.; Mazur, M. Silver-Decorated Gel-Shell Nanobeads: Physicochemical Characterization and Evaluation of Antibacterial Properties. Beilstein J. Nanotechnol. 2020, 11, 620–630. [Google Scholar] [CrossRef]
- Ambi, A.; Parikh, N.; Vera, C.; Burns, K.; Montano, N.; Sciorra, L.; Epstein, J.; Zeng, D.; Traba, C. Anti-Infection Silver Nanoparticle Immobilized Biomaterials Facilitated by Argon Plasma Grafting Technology. Biofouling 2018, 34, 273–286. [Google Scholar] [CrossRef]
- Cowan, M.M.; Abshire, K.Z.; Houk, S.L.; Evans, S.M. Antimicrobial Efficacy of a Silver-Zeolite Matrix Coating on Stainless Steel. J. Ind. Microbiol. Biotechnol. 2003, 30, 102–106. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S. Green Synthesis of Silver Nanoparticles Using the Flower Extract of Abelmoschus Esculentus for Cytotoxicity and Antimicrobial Studies. Int. J. Nanomed. 2021, 16, 3343. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Bernardo, W.L.; Boriollo, M.F.G.; Tonon, C.C.; da Silva, J.J.; Cruz, F.M.; Martins, A.L.; Höfling, J.F.; Spolidorio, D.M.P. Antimicrobial Effects of Silver Nanoparticles and Extracts of Syzygium Cumini Flowers and Seeds: Periodontal, Cariogenic and Opportunistic Pathogens. Arch. Oral Biol. 2021, 125, 105101. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N. Characterization, Antibacterial, Total Antioxidant, Scavenging, Reducing Power and Ion Chelating Activities of Green Synthesized Silver, Copper and Titanium Dioxide Nanoparticles Using Artemisia Haussknechtii Leaf Extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2066–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valodkar, M.; Bhadoria, A.; Pohnerkar, J.; Mohan, M.; Thakore, S. Morphology and Antibacterial Activity of Carbohydrate-Stabilized Silver Nanoparticles. Carbohydr. Res. 2010, 345, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.T.; Anh, L.T.; Phuc, L.H.; Vinh, L.K.; Tung, N.T.; Phuong, P.H. Green Preparation of Carbon Quantum Dots and Its Silver Nanoparticles Composite against Carbapenem-Resistant Acinetobacter Baumannii. Appl. Nanosci. 2023, 13, 4109–4118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez Miranda, M.; Liu, W.; Godinez-Leon, J.A.; Amanova, A.; Houel-Renault, L.; Lampre, I.; Remita, H.; Gref, R. Colloidal Silver Nanoparticles Obtained via Radiolysis: Synthesis Optimization and Antibacterial Properties. Pharmaceutics 2023, 15, 1787. https://doi.org/10.3390/pharmaceutics15071787
Menéndez Miranda M, Liu W, Godinez-Leon JA, Amanova A, Houel-Renault L, Lampre I, Remita H, Gref R. Colloidal Silver Nanoparticles Obtained via Radiolysis: Synthesis Optimization and Antibacterial Properties. Pharmaceutics. 2023; 15(7):1787. https://doi.org/10.3390/pharmaceutics15071787
Chicago/Turabian StyleMenéndez Miranda, Mario, Wenbo Liu, Jesus Alfredo Godinez-Leon, Aisara Amanova, Ludivine Houel-Renault, Isabelle Lampre, Hynd Remita, and Ruxandra Gref. 2023. "Colloidal Silver Nanoparticles Obtained via Radiolysis: Synthesis Optimization and Antibacterial Properties" Pharmaceutics 15, no. 7: 1787. https://doi.org/10.3390/pharmaceutics15071787




