Dipropyleneglycol Dimethylether, New Green Solvent for Solid-Phase Peptide Synthesis: Further Challenges to Improve Sustainability in the Development of Therapeutic Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Physical–Chemical Properties and Cost
2.2. Swelling Test
2.3. Solubility Test
2.4. Deprotection Kinetics Test
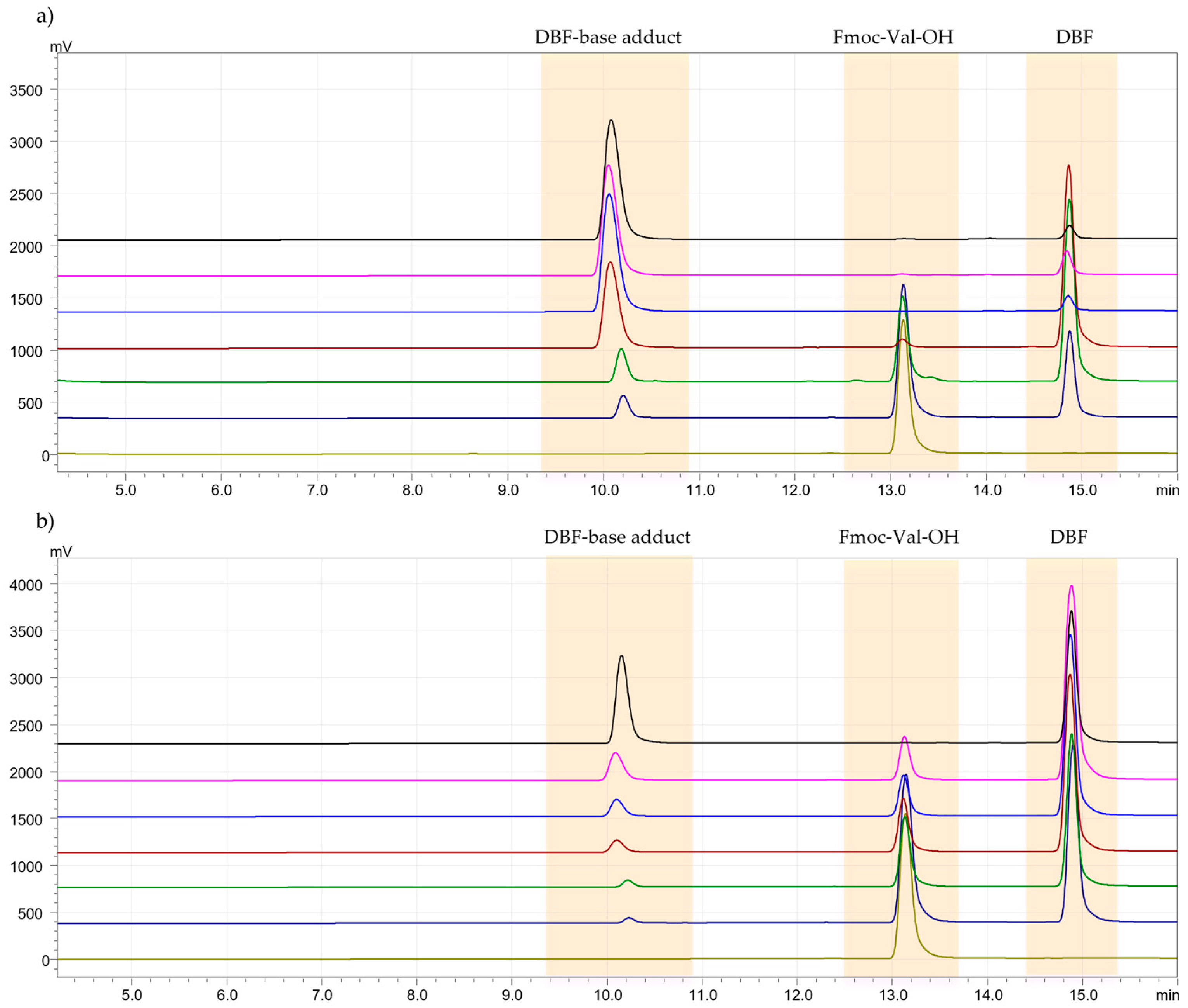
Evaluation of DBF and DBF-Base Adduct Formation
2.5. Coupling Test: Synthesis of NH2-Tyr-Ala-OH
2.6. Racemization Study
2.7. SPPS of Aib-Enkephalin and Aib-ACP
Characterization
2.8. Solvent Recycling
3. Results and Discussion
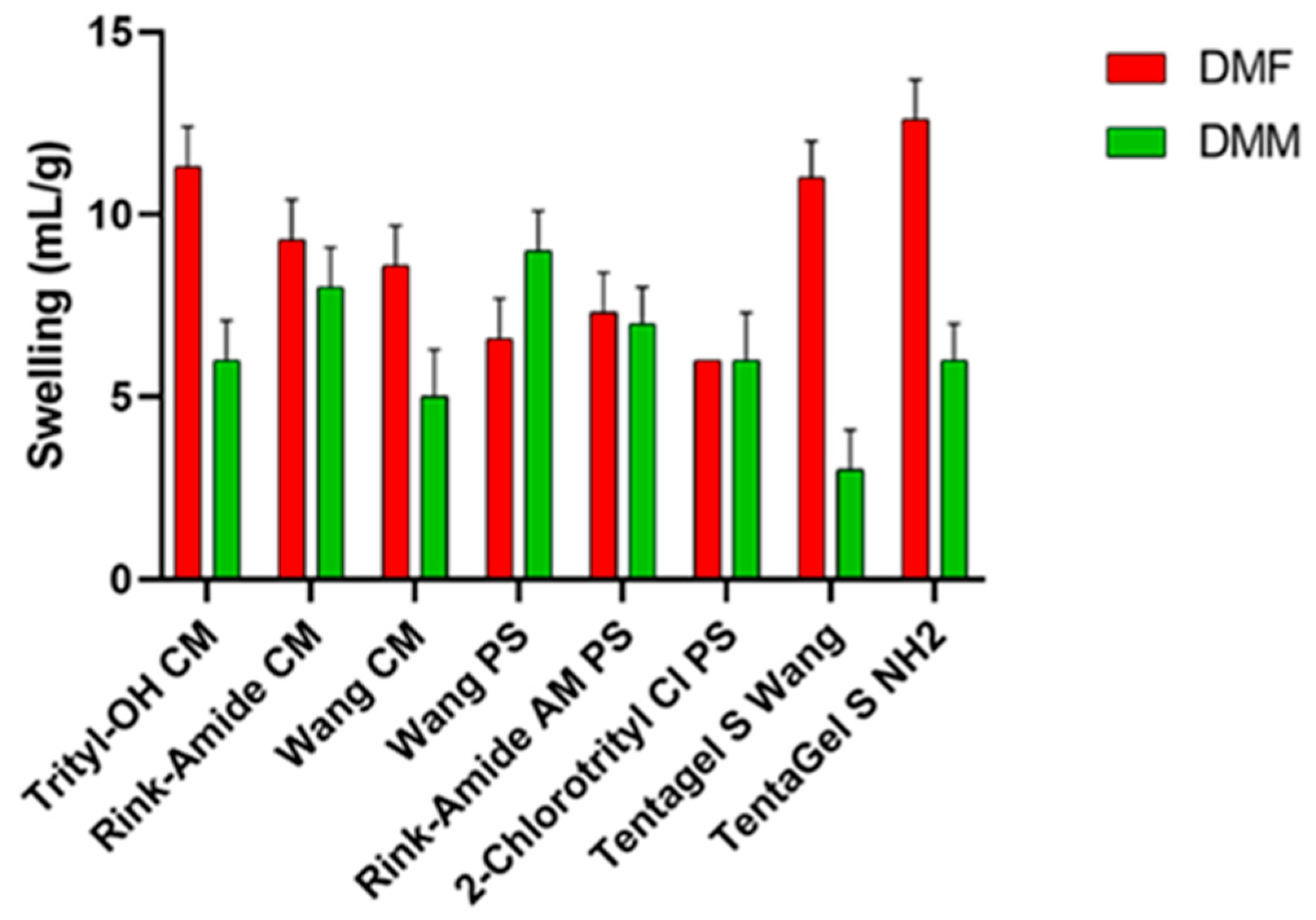
3.1. Resin Swelling Studies: Swelling Test
3.2. Solubility Test
3.3. Deprotection Kinetics Test
3.4. Coupling Test
3.5. Racemization Study
3.6. SPPS of Aib-Enkephalin and Aib-ACP
3.7. Solvent Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, V.; Egelund, P.H.G.; Johansson, H.; Le Quement, S.T.; Wojcik, F.; Sejer Pedersen, D. Greening the synthesis of peptide therapeutics: An industrial perspective. RSC Adv. 2020, 10, 42457–42492. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Jad, Y.E.; Kumar, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green Transformation of Solid-Phase Peptide Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 3671–3683. [Google Scholar] [CrossRef]
- Jad, Y.E.; Acosta, G.A.; Govender, T.; Kruger, H.G.; El Faham, A.; de la Torre, B.G.; Albericio, F. Green Solid-Phase Peptide Synthesis 2. 2-Methyltetrahydrofuran and Ethyl Acetate for Solid-Phase Peptide Synthesis under Green Conditions. ACS Sustain. Chem. Eng. 2016, 4, 6809. [Google Scholar] [CrossRef]
- Jad, Y.E.; Acosta, G.A.; Khattab, S.N.; de la Torre, B.G.; Govender, T.; Kruger, H.G.; El-Faham, A.; Albericio, F. Peptide synthesis beyond DMF: THF and ACN as excellent and friendlier alternatives. Org. Biomol. Chem. 2015, 13, 2393–2398. [Google Scholar] [CrossRef]
- Jad, Y.E.; Acosta, G.A.; Khattab, S.N.; de la Torre, B.G.; Govender, T.; Kruger, H.G.; El-Faham, A.; Albericio, F. 2-Methyltetrahydrofuran and cyclopentyl methyl ether for green solid-phase peptide synthesis. Amino Acids 2016, 48, 419–426. [Google Scholar] [CrossRef]
- Kumar, A.; Jad, Y.E.; Collins, J.M.; Albericio, F.; de la Torre, B.G. Microwave-Assisted Green Solid-Phase Peptide Synthesis Using γ-Valerolactone (GVL) as Solvent. ACS Sustain. Chem. Eng. 2018, 6, 8034. [Google Scholar] [CrossRef]
- Kumar, A.; Jad, Y.E.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green solid-phase peptide synthesis 4. γ-Valerolactone and N-formylmorpholine as green solvents for solid phase peptide synthesis. Tetrahedron Lett. 2017, 58, 2986. [Google Scholar] [CrossRef]
- Lawrenson, S.B.; Arav, R.; North, M. The greening of peptide synthesis. Green Chem. 2017, 19, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Pletscher, S.; Aemissegger, A.; Bucher, C.; Gallou, F. N-Butylpyrrolidinone as Alternative Solvent for Solid-Phase Peptide Synthesis. Org. Process Res. Dev. 2018, 22, 494. [Google Scholar] [CrossRef] [Green Version]
- Nienałtowski, T.; Krzesiński, P.; Baumert, M.E.; Skoczeń, A.; Suska-Kauf, E.; Pawłowska, J.; Kajetanowicz, A.; Grela, K. 4-Methyltetrahydropyran as a Convenient Alternative Solvent for Olefin Metathesis Reaction: Model Studies and Medicinal Chemistry Applications. ACS Sustain. Chem. Eng. 2020, 8, 18215–18223. [Google Scholar] [CrossRef] [PubMed]
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Börner, A. Organic carbonates as solvents in synthesis and catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Wang, X.; Liang, Y.; Yang, X.; Wang, Z. Facile Synthesis of N,S-Codoped Hierarchically Porous Carbon with High Volumetric Pseudocapacitance. ACS Sustain. Chem. Eng. 2017, 5, 744–751. [Google Scholar] [CrossRef]
- Pawlas, J.; Rasmussen, J.H. ReGreen SPPS: Enabling circular chemistry in environmentally sensible solid-phase peptide synthesis. Green Chem. 2019, 21, 5990–5998. [Google Scholar] [CrossRef]
- Orlandin, A.; Guryanov, I.; Ferrazzano, L.; Biondi, B.; Biscaglia, F.; Storti, C.; Rancan, M.; Formaggio, F.; Ricci, A.; Cabri, W. Carbodiimide-Mediated Beckmann Rearrangement of Oxyma-B as a Side Reaction in Peptide Synthesis. Molecules 2022, 27, 4235. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.; Bossinger, C.; Cook, P. Color test for detection of free terminal amino groups in the solid-phase synthe-sis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Sala, M.; Spensiero, A.; Esposito, F.; Scala, M.C.; Vernieri, E.; Bertamino, A.; Manfra, M.; Carotenuto, A.; Grieco, P.; Novellino, E.; et al. Development and Identification of a Novel Anti-HIV-1 Peptide Derived by Modification of the N-Terminal Domain of HIV-1 Integrase. Front. Microbiol. 2016, 7, 845. [Google Scholar] [CrossRef]
- Martelli, G.; Cantelmi, P.; Tolomelli, A.; Corbisiero, D.; Mattellone, A.; Ricci, A.; Fantoni, T.; Cabri, W.; Vacondio, F.; Ferlenghi, F.; et al. Steps towards sustainable solid phase peptide synthesis: Use and recovery of N-octyl pyrrolidone. Green Chem. 2021, 23, 4095–4106. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campins-Falcò, P.; Pèrez, B.; Sancho, R.; Lorente, M.; Sastre, G.; Gonzàlez, C. A new tool for evaluating and/or selecting analytical methods: Summarizing the information in a hexagon. Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Hudson, D. Matrix assisted synthetic transformations: A mosaic of diverse contributions. I. The pattern emerges. J. Comb. Chem. 1999, 1, 333–360. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D. Matrix assisted synthetic transformations: A mosaic of diverse contributions. II. The pattern is completed. J. Comb. Chem. 1999, 1, 403–457. [Google Scholar] [CrossRef]
- Garcia-Martin, F.; Quintanar-Audelo, M.; Garcia-Ramos, Y.; Cruz, L.J.; Gravel, C.; Furic, R.; Cruz, S.; Tulla-Puche, J.; Albericio, F. ChemMatrix, a Poly(ethylene glycol)-Based Support for the Solid-Phase Synthesis of Complex Peptides. J. Comb. Chem. 2006, 8, 213–220. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Subirós Funosas, R.; Prohens, R.; Albericio, F. COMU: A safer and more effective replacement for benzotriazole-based uronium coupling reagents. Chemistry 2009, 15, 9404–9416. [Google Scholar] [CrossRef] [PubMed]
- Luna, O.F.; Gomez, J.; Cárdenas, C.; Albericio, F.; Marshall, S.H.; Guzmán, F. Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine? Molecules 2016, 21, 1542. [Google Scholar] [CrossRef] [Green Version]
- Carpino, L.A.; Han, G.Y. The 9-fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37, 3404–3409. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Subirós-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole-Based HOBt and HOAt with a Lower Risk of Explosion. Chem. Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef]
- Liang, C.; Behnam, M.A.; Sundermann, T.R.; Klein, C.D. Phenylglycine racemization in Fmoc-based solid-phase peptide synthesis: Stereochemical stability is achieved by choice of reaction conditions. Tetrahedron Lett. 2017, 58, 2325–2329. [Google Scholar] [CrossRef]
- Fantoni, T.; Tolomelli, A.; Cabri, W. A translation of the twelve principles of green chemistry to guide the development of cross-coupling reactions. Catal. Today 2022, 397–399, 265–271. [Google Scholar] [CrossRef]
- Mattellone, A.; Corbisiero, D.; Ferrazzano, L.; Cantelmi, P.; Martelli, G.; Palladino, C.; Tolomelli, A.; Cabri, W. Speeding up sustainable solution-phase peptide synthesis using T3P® as a green coupling reagent: Methods and challenges. Green Chem. 2023, 25, 2563–2571. [Google Scholar] [CrossRef]
| Physical–Chemical Properties | Solvent | |
|---|---|---|
| DMF | DMM | |
| Boiling point | 153 °C | 175 °C |
| Density | 0.95 g/cm3 | 0.90 g/cm3 |
| Viscosity | 0.85 mm2/s | 1.12 mm2/s |
| Log P | −0.85 | 0.42 |
| Melting point | −31 °C | −80 °C |
| Flash point | 58 °C | 65 °C |
| Cost | 72.50 Eur/liter | 128.00 Eur/liter |
| Solvent | Fmoc-aa(PG)-OH | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gly | Ile | Leu | Val | Ala | Phe | Pro | Met | Tyr (tBu) | Thr (tBu) | Ser (tBu) | Asp (OtBu) | Glu (OtBu) | Cys (Trt) | Asn (Trt) | Gln (Trt) | His (Trt) | Lys (Boc) | Trp (Boc) | Arg (Pbf) | |
| DMF | ||||||||||||||||||||
| DMM | ||||||||||||||||||||
| Concentration (M) | NMP (%) | |||
|---|---|---|---|---|
| 0.1 | 10 | 20 | 30 | 40 |
| Coupling Reagents | Mixture Fmoc-Val-OH + Coupling Reagents (A–B) | Mixture Fmoc-His(Trt)-OH + Coupling Reagents (A–B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | HOBt | CDI | Oxyma Pure | HATU | COMU | DIC | HOBt/DIC (A) | OxymaPure/DIC (B) | Val + A | Val + B | His (Trt) + A | His (Trt) + B |
| DMF | ||||||||||||
| DMM | 30% NMP | 30% NMP | ||||||||||
| Entry | Solvent | Coupling Agents | Eq. | Time | T (°C) | Purity (%) |
|---|---|---|---|---|---|---|
| 1 | DMF | HOBt/DIC (1:1) | 3 | 2h | rt | 79.68 |
| 2 | DMM | HOBt/DIC (1:1) | 3 | 2h | rt | 91.14 |
| 3 | DMF | OxymaPure/DIC (1:2) | 3 | 2h | rt | 33.65 |
| 4 | DMM | OxymaPure/DIC (1:2) | 3 | 2h | rt | 83.57 |
| 5 | DMM | OxymaPure/DIC (1:2) | 3 | 10 min | 75 (MW) | 78.90 |
| Entry | Solvent | Time | T (°C) | DL b (%) |
|---|---|---|---|---|
| 6 | DMF | 2 h | rt | 48.78 |
| 7 | DMM | 2 h | rt | 65.80 |
| 8 | DMF | 10 min | 75 (MW) | 89.02 |
| 9 | DMM | 10 min | 75 (MW) | 35.29 |
| Entry | Solvent | Resin | Pentapeptide (%) | Des-Aib (%) | Other (%) |
|---|---|---|---|---|---|
| 10 | DMF | Wang PS | 89.20 | 1.39 | 9.41 |
| 11 | DMM | Wang PS | 81.76 | 8.65 | 9.59 |
| 12 | DMF | Rink amide CM | 94.88 | - | 5.12 |
| 13 | DMM | Rink amide CM | 95.60 | - | 4.40 |
| Entry | Solvent | Decapeptide (%) | Des-Aib (%) | Other (%) |
|---|---|---|---|---|
| 14 | DMF | 53.73 | - | 46.27 |
| 15 | DMM | 53.69 | 9.24 | 37.07 |
| Entry | Solvent | PMI | Recovery (Yield %) | PMI after Recovery |
|---|---|---|---|---|
| 1 | DMF | 3896.28 | - | - |
| 2 | DMM | 3750.20 | 80 | 1370.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivenzio, G.; Scala, M.C.; Marino, P.; Manfra, M.; Campiglia, P.; Sala, M. Dipropyleneglycol Dimethylether, New Green Solvent for Solid-Phase Peptide Synthesis: Further Challenges to Improve Sustainability in the Development of Therapeutic Peptides. Pharmaceutics 2023, 15, 1773. https://doi.org/10.3390/pharmaceutics15061773
Vivenzio G, Scala MC, Marino P, Manfra M, Campiglia P, Sala M. Dipropyleneglycol Dimethylether, New Green Solvent for Solid-Phase Peptide Synthesis: Further Challenges to Improve Sustainability in the Development of Therapeutic Peptides. Pharmaceutics. 2023; 15(6):1773. https://doi.org/10.3390/pharmaceutics15061773
Chicago/Turabian StyleVivenzio, Giovanni, Maria Carmina Scala, Pasquale Marino, Michele Manfra, Pietro Campiglia, and Marina Sala. 2023. "Dipropyleneglycol Dimethylether, New Green Solvent for Solid-Phase Peptide Synthesis: Further Challenges to Improve Sustainability in the Development of Therapeutic Peptides" Pharmaceutics 15, no. 6: 1773. https://doi.org/10.3390/pharmaceutics15061773