Stereoisomer-Dependent Membrane Association and Capacity for Insulin Delivery Facilitated by Penetratin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Peptide Synthesis, Purification and Labelling
2.2.2. Cell Culturing
2.2.3. Intracellular Distribution and Uptake of CF-Labelled PEN Enantiomers and TAMRA-Insulin Evaluated by Confocal Microscopy
2.2.4. Cellular Uptake of CF-Labelled PEN Enantiomers and TAMRA-Insulin Evaluated by Flow Cytometry
2.2.5. Transepithelial Permeation of Cargoes Facilitated by l-PEN and d-PEN
2.2.6. Data Analysis
3. Results
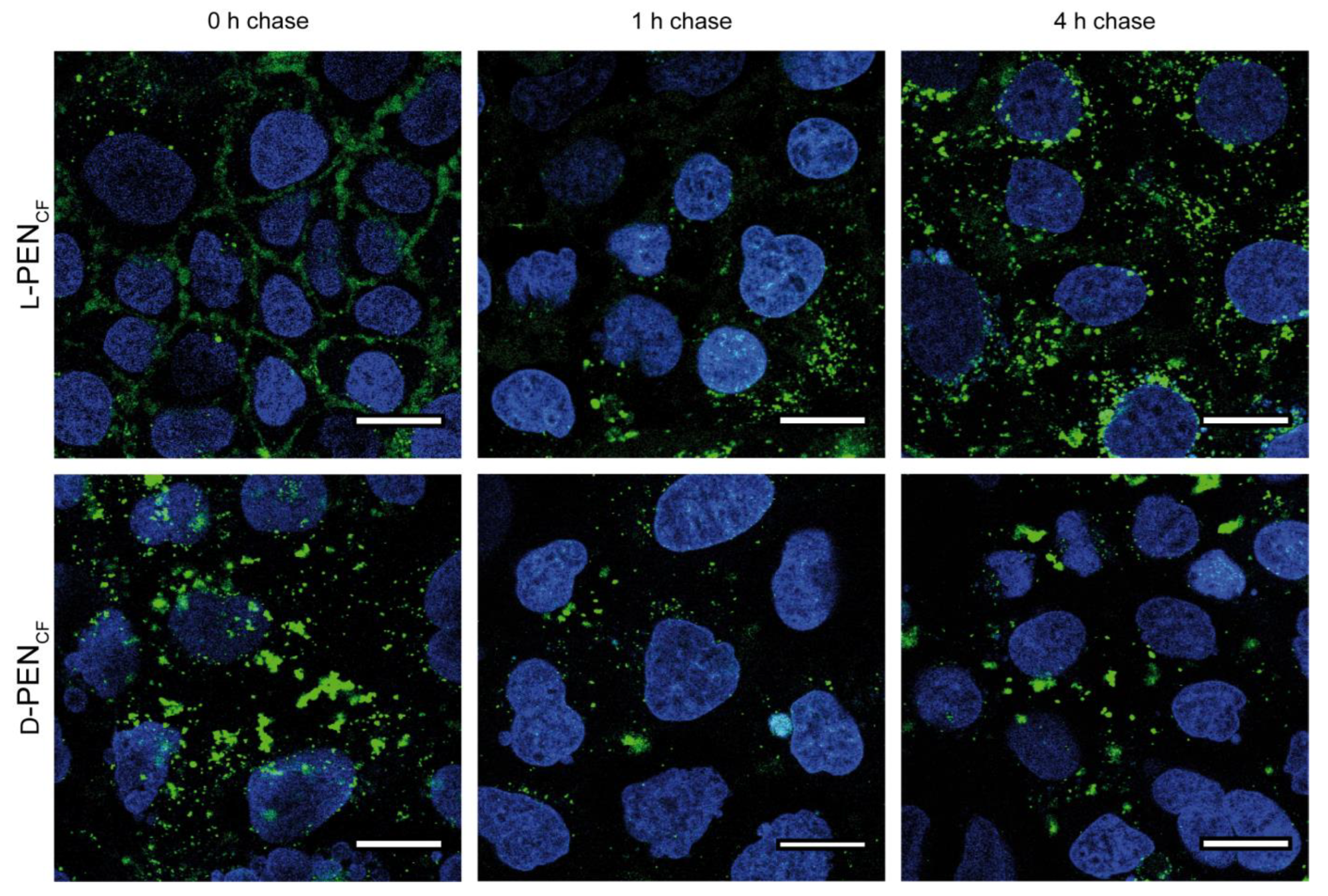
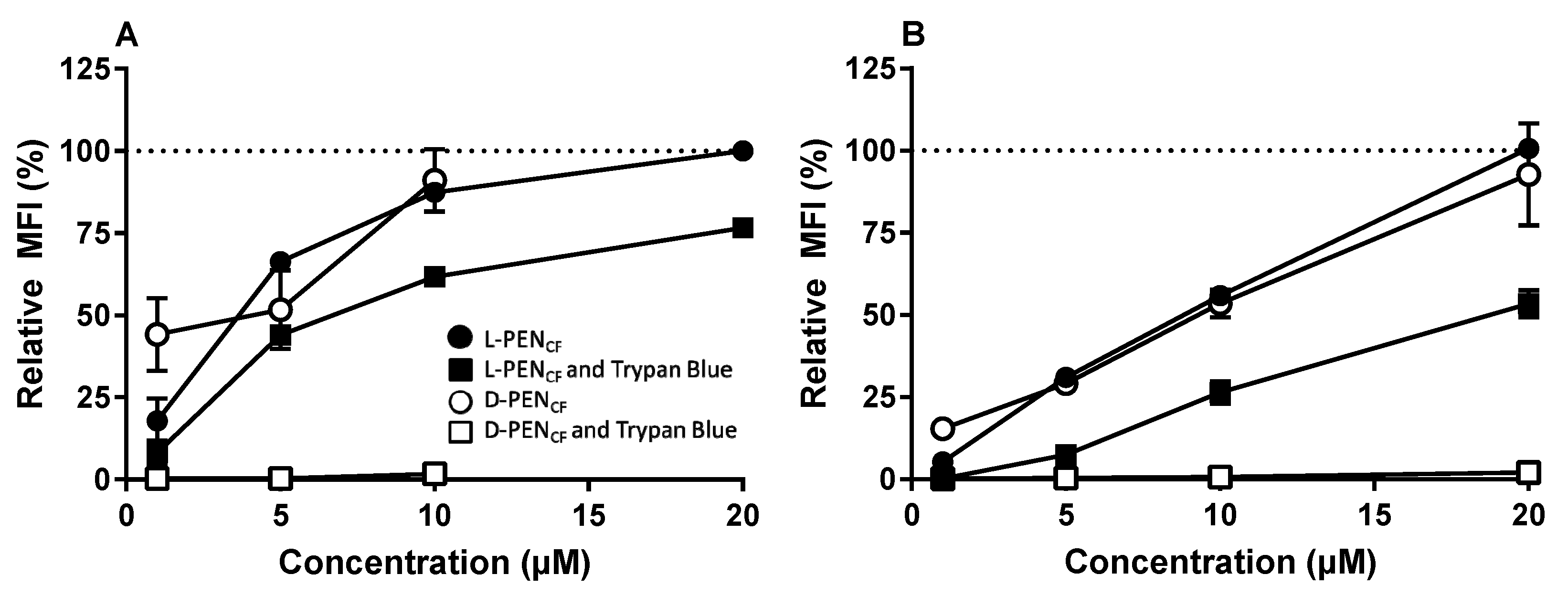
3.1. Effect of Stereoisomerism on the Uptake and Cellular Distribution of l- and d-Penetratin
3.2. l-PEN and d-PEN as Carrier Peptides for Transepithelial Peptide Delivery
3.2.1. Intracellular Delivery of TAMRA-Insulin
3.2.2. PEN-Mediated Permeation of Cargo across Caco-2 Cell Epithelium
4. Discussion
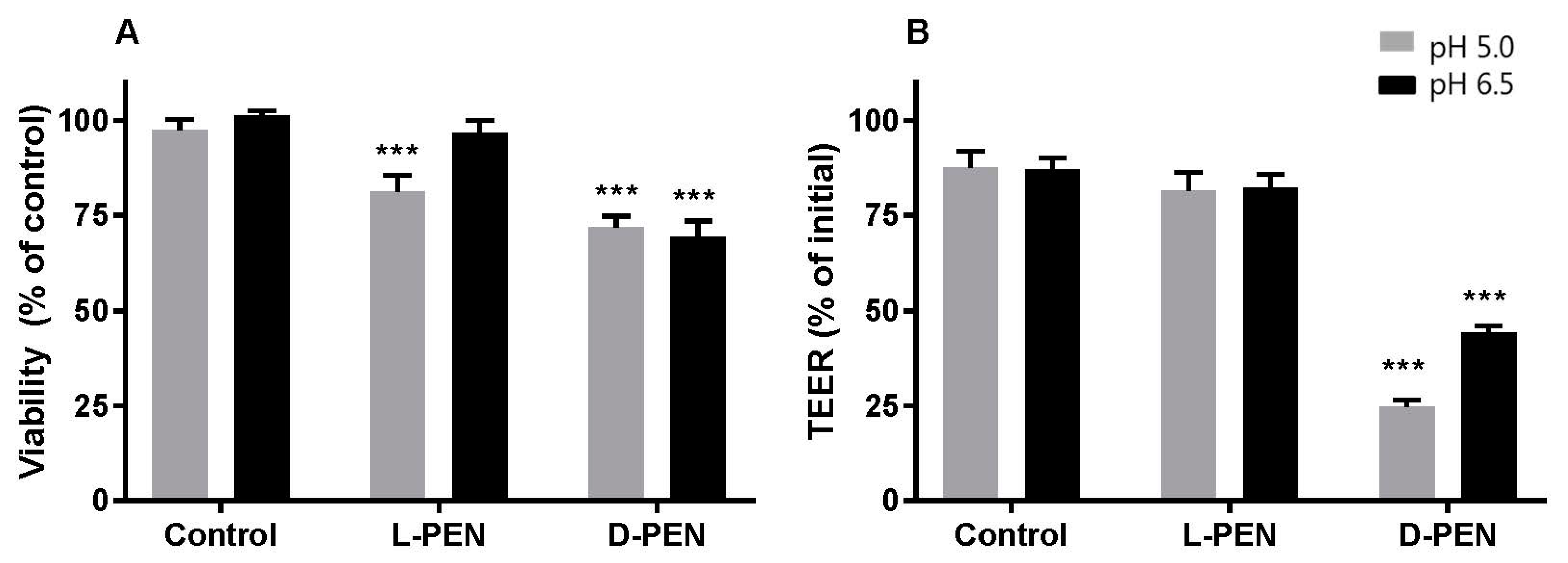
4.1. Enantiomers of PEN Interact Differently with Cell Membranes
4.2. Intracellular Delivery and Distribution of Insulin Are Mediated by Both l-PEN and d-PEN
4.3. Transepithelial Delivery of Hydrophilic Peptide Cargoes Is Mediated by Both Enantiomers, yet Preferentially by d-PEN
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristensen, M.; Birch, D.; Nielsen, H.M. Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.T.; Sayers, E.J. Cell entry of cell penetrating peptides: Tales of tails wagging dogs. J. Control. Release 2012, 161, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, R.G.; Harloff-Helleberg, S.; Werner, U.; Besenius, M.; Kristensen, M.; Nielsen, H.M. Revealing the importance of carrier-cargo association in delivery of insulin and lipidated insulin. J. Control. Release 2021, 338, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Morishita, M.; Kamei, N.; Eda, Y.; Ikeno, Y.; Takayama, K. Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int. J. Pharm. 2009, 381, 49–55. [Google Scholar] [CrossRef]
- Kristensen, M.; de Groot, A.M.; Berthelsen, J.; Franzyk, H.; Sijts, A.; Nielsen, H.M. Conjugation of cell-penetrating peptides to parathyroid hormone affects its structure, potency, and transepithelial permeation. Bioconjug. Chem. 2015, 26, 477–488. [Google Scholar] [CrossRef]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef]
- Tréhin, R.; Krauss, U.; Beck-Sickinger, A.G.; Merkle, H.P.; Nielsen, H.M. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat(47–57) through well-differentiated epithelial models. Pharm. Res. 2004, 21, 1248–1256. [Google Scholar] [CrossRef]
- Jones, A.T. Macropinocytosis: Searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 2007, 11, 670–684. [Google Scholar] [CrossRef] [Green Version]
- Padari, K.; Säälik, P.; Hansen, M.; Koppel, K.; Raid, R.; Langel, Ü.; Pooga, M. Cell transduction pathways of transportans. Bioconjug. Chem. 2005, 16, 1399–1410. [Google Scholar] [CrossRef]
- Kamei, N.; Kikuchi, S.; Takeda-Morishita, M.; Terasawa, Y.; Yasuda, A.; Yamamoto, S.; Ida, N.; Nishio, R.; Takayama, K. Determination of the optimal cell-penetrating peptide sequence for intestinal insulin delivery based on molecular orbital analysis with self-organizing maps. J. Pharm. Sci. 2013, 102, 469–479. [Google Scholar] [CrossRef]
- Zhao, M.; Weissleder, R. Intracellular cargo delivery using tat peptide and derivatives. Med. Res. Rev. 2004, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vives, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiríksdóttir, E.; Konate, K.; Langel, Ü.; Divita, G.; Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, I.D.; Goasdoué, N.; Correia, I.; Aubry, S.; Galanth, C.; Sagan, S.; Lavielle, S.; Chassaing, G. Membrane interaction and perturbation mechanisms induced by two cationic cell penetrating peptides with distinct charge distribution. Biochim. Biophys. Acta 2008, 1780, 948–959. [Google Scholar] [CrossRef]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef]
- Hallbrink, M.; Oehlke, J.; Papsdorf, G.; Bienert, M. Uptake of cell-penetrating peptides is dependent on peptide-to-cell ratio rather than on peptide concentration. Biochim. Biophys. Acta 2004, 1667, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Gräslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [Green Version]
- Lönn, P.; Kacsinta, A.D.; Cui, X.S.; Hamil, A.S.; Kaulich, M.; Gogoi, K.; Dowdy, S.F. Enhancing Endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci. Rep. 2016, 6, 32301. [Google Scholar] [CrossRef]
- El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 2009, 11, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Köhler, K.; Fotin-Mleczek, M.; Brock, R. A stepwise dissection of the intracellular fate of cationic cell-penetrating Peptides. J. Biol. Chem. 2004, 279, 12625–12635. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv. Drug Deliv. Rev. 2008, 60, 580–597. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Feighery, L.; Brayden, D.J.; McClean, S. Melittin as a permeability enhancer II: In vitro investigations in human mucus secreting intestinal monolayers and rat colonic mucosae. Pharm. Res. 2007, 24, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, R.G.; Tuelung, P.S.; Foderà, V.; Nielsen, H.M. Stereochemistry and intermolecular interactions influence carrier peptide-mediated insulin delivery. Mol. Pharm. 2023, 20, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Letoha, T.; Somlai, C.; Takács, T.; Szabolcs, A.; Jármay, K.; Rakonczay, Z., Jr.; Hegyi, P.; Varga, I.; Kaszaki, J.; Krizbai, I.; et al. A nuclear import inhibitory peptide ameliorates the severity of cholecystokinin-induced acute pancreatitis. World J. Gastroenterol. 2005, 11, 990–999. [Google Scholar] [CrossRef]
- Nakase, I.; Akita, H.; Kogure, K.; Graslund, A.; Langel, U.; Harashima, H.; Futaki, S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef]
- Verdurmen, W.P.; Bovee-Geurts, P.H.; Wadhwani, P.; Ulrich, A.S.; Hällbrink, M.; van Kuppevelt, T.H.; Brock, R. Preferential uptake of l-versus d-amino acid cell-penetrating peptides in a cell type-dependent manner. Chem. Biol. 2011, 18, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.; Franzyk, H.; Klausen, M.T.; Iversen, A.; Bahnsen, J.S.; Skyggebjerg, R.B.; Foderà, V.; Nielsen, H.M. Penetratin-mediated transepithelial insulin permeation: Importance of cationic residues and pH for complexation and permeation. AAPS J. 2015, 17, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Kamei, N.; Onuki, Y.; Takayama, K.; Takeda-Morishita, M. Mechanistic study of the uptake/permeation of cell-penetrating peptides across a caco-2 monolayer and their stimulatory effect on epithelial insulin transport. J. Pharm. Sci. 2013, 102, 3998–4008. [Google Scholar] [CrossRef]
- Birch, D.; Christensen, M.V.; Staerk, D.; Franzyk, H.; Nielsen, H.M. Stereochemistry as a determining factor for the effect of a cell-penetrating peptide on cellular viability and epithelial integrity. Biochem. J. 2018, 475, 1773–1788. [Google Scholar] [CrossRef]
- Maher, S.; Mrsny, R.J.; Brayden, D. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Del Rev. 2016, 10, 277–319. [Google Scholar] [CrossRef]
- Kristensen, M.; Nielsen, L.H.; Zor, K.; Boisen, A.; Christensen, M.V.; Berthelsen, J.; Mørck Nielsen, H. Cellular effects and delivery propensity of penetratin is influenced by conjugation to parathyroid hormone fragment 1–34 in synergy with pH. Bioconjug. Chem. 2018, 29, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafagy, E.S.; Morishita, M.; Isowa, K.; Imai, J.; Takayama, K. Effect of cell-penetrating peptides on the nasal absorption of insulin. J. Control. Release 2009, 133, 103–108. [Google Scholar] [CrossRef]
- Nielsen, E.J.; Yoshida, S.; Kamei, N.; Iwamae, R.S.; Khafagy, E.J.; Olsen, U.L.; Rahbek, B.L.; Pedersen, K.; Takayama, M. In vivo proof of concept of oral insulin delivery based on a co-administration strategy with the cell-penetrating peptide penetratin. J. Control. Release 2014, 189, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Iwase, Y.; Kamei, N.; Khafagy, E.S.; Miyamoto, M.; Takeda-Morishita, M. Use of a non-covalent cell-penetrating peptide strategy to enhance the nasal delivery of interferon beta and its PEGylated form. Int. J. Pharm. 2016, 510, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illien, F.; Rodriguez, N.; Amoura, M.; Joliot, A.; Pallerla, M.; Cribier, S.; Burlina, F.; Sagan, S. Quantitative fluorescence spectroscopy and flow cytometry analyses of cell-penetrating peptides internalization pathways: Optimization, pitfalls, comparison with mass spectrometry quantification. Sci. Rep. 2016, 6, 36938. [Google Scholar] [CrossRef] [Green Version]
- Birch, D.; Christensen, M.V.; Staerk, D.; Franzyk, H.; Nielsen, H.M. Fluorophore labelling of a cell-penetrating peptide induces differential effects on its cellular distribution and affects cell viability. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 2483–2494. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth 2012, 9, 676–682. Available online: http://www.nature.com/nmeth/journal/v9/n7/abs/nmeth.2019.html#supplementary-information (accessed on 26 January 2022). [CrossRef] [Green Version]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Sayers, E.J.; Cleal, K.; Eissa, N.G.; Watson, P.; Jones, A.T. Distal phenylalanine modification for enhancing cellular delivery of fluorophores, proteins and quantum dots by cell penetrating peptides. J. Control. Release 2014, 195, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, C.L.; Brennan, P.; Fegan, C.; Takayama, K.; Nakase, I.; Futaki, S.; Jones, A.T. Cellular uptake, distribution and cytotoxicity of the hydrophobic cell penetrating peptide sequence PFVYLI linked to the proapoptotic domain peptide PAD. J. Control. Release 2009, 140, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudi, E.; Boscolo, B.; Inserra, G.; Laurenti, E.; Traversa, S.; Barbero, L.; Ferrari, R.P. The interaction of the cell-penetrating peptide penetratin with heparin, heparansulfates and phospholipid vesicles investigated by ESR spectroscopy. J. Pept. Sci. 2005, 11, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Seelig, J. Contributions of glycosaminoglycan binding and clustering to the biological uptake of the nonamphipathic cell-penetrating peptide WR9. Biochemistry 2011, 50, 4650–4664. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Aoyama, Y.; Khafagy, E.S.; Henmi, M.; Takeda-Morishita, M. Effect of different intestinal conditions on the intermolecular interaction between insulin and cell-penetrating peptide penetratin and on its contribution to stimulation of permeation through intestinal epithelium. Eur. J. Pharm. Biopharm. 2015, 94, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kremsmayr, T.; Aljnabi, A.; Blanco-Canosa, J.B.; Tran, H.N.T.; Emidio, N.B.; Muttenthaler, M. On the utility of chemical strategies to improve peptide gut stability. J. Med. Chem. 2022, 65, 6191–6206. [Google Scholar] [CrossRef]




| Compound | Mass (Da) | Log P | Note |
|---|---|---|---|
| Insulin | 5808 | −13.1 | Hydrophilic two-chain peptide |
| Mannitol | 182 | −3.7 | Sugar-derived polyol (paracellular marker) |
| Vancomycin | 1449 | −4.4 | Hydrophilic tricyclic glycopeptide |
| Cyclosporin | 1202 | 3.6 | Hydrophobic monocyclic peptide |
| pH 6.5 | pH 7.4 | |||
|---|---|---|---|---|
| Relative Uptake (%) | MFI (A.N.) | Relative Uptake (%) | MFI (A.N.) | |
| TAMRA-Insulin | ||||
| Without Trypan Blue | 100.0 ± 1.6 | 48.0 ± 1.2 | 100.0 ± 2.2 | 41.2 ± 2.9 |
| With Trypan Blue | 103.4 ± 5.6 | 49.6 ± 1.9 | 100.8 ± 4.3 | 40.8 ± 2.6 |
| TAMRA-Insulin + l-PEN | ||||
| Without Trypan Blue | 126.5 ± 7.7 | 60.4 ± 2.6 | 152.6 ± 6.9 | 61.9 ± 1.5 *** |
| With Trypan Blue | 124.7 ± 7.3 | 62.3 ± 5.1 | 151.6 ± 2.8 | 62.1 ± 4.5 *** |
| TAMRA-Insulin + d-PEN | ||||
| Without Trypan Blue | 148.2 ± 2.0 | 71.1 ± 1.4 | 181.1 ± 15.3 | 72.6 ± 1.6 |
| With Trypan Blue | 120.0 ± 7.1 | 59.1 ± 2.0 * | 164.6 ± 10.5 | 65.9 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birch, D.; Sayers, E.J.; Christensen, M.V.; Jones, A.T.; Franzyk, H.; Nielsen, H.M. Stereoisomer-Dependent Membrane Association and Capacity for Insulin Delivery Facilitated by Penetratin. Pharmaceutics 2023, 15, 1672. https://doi.org/10.3390/pharmaceutics15061672
Birch D, Sayers EJ, Christensen MV, Jones AT, Franzyk H, Nielsen HM. Stereoisomer-Dependent Membrane Association and Capacity for Insulin Delivery Facilitated by Penetratin. Pharmaceutics. 2023; 15(6):1672. https://doi.org/10.3390/pharmaceutics15061672
Chicago/Turabian StyleBirch, Ditlev, Edward J. Sayers, Malene V. Christensen, Arwyn T. Jones, Henrik Franzyk, and Hanne M. Nielsen. 2023. "Stereoisomer-Dependent Membrane Association and Capacity for Insulin Delivery Facilitated by Penetratin" Pharmaceutics 15, no. 6: 1672. https://doi.org/10.3390/pharmaceutics15061672





