CD73-Positive Cell Spheroid Transplantation Attenuates Colonic Atrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. DSS-Induced Colitis Model
2.3. Isolation and Intravenous Administration of CD73-Positive Cells
2.4. Spheroid Formation and Trans-anal Transplantation
2.5. Flow Cytometry
2.6. Collection of Conditioned Media (CM)
2.7. Cytometric Bead Array (CBA)
2.8. Cell Proliferation and Contraction Assays
2.9. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.10. Acquisition of Clinical Samples
2.11. mRNA Sequencing (mRNA-Seq)
2.12. Histological Analyses
2.13. Statistical Analysis
3. Results
3.1. Intravenous Injection of CD73+ Cells Attenuates Tissue Destruction in DSS-Induced Colitis
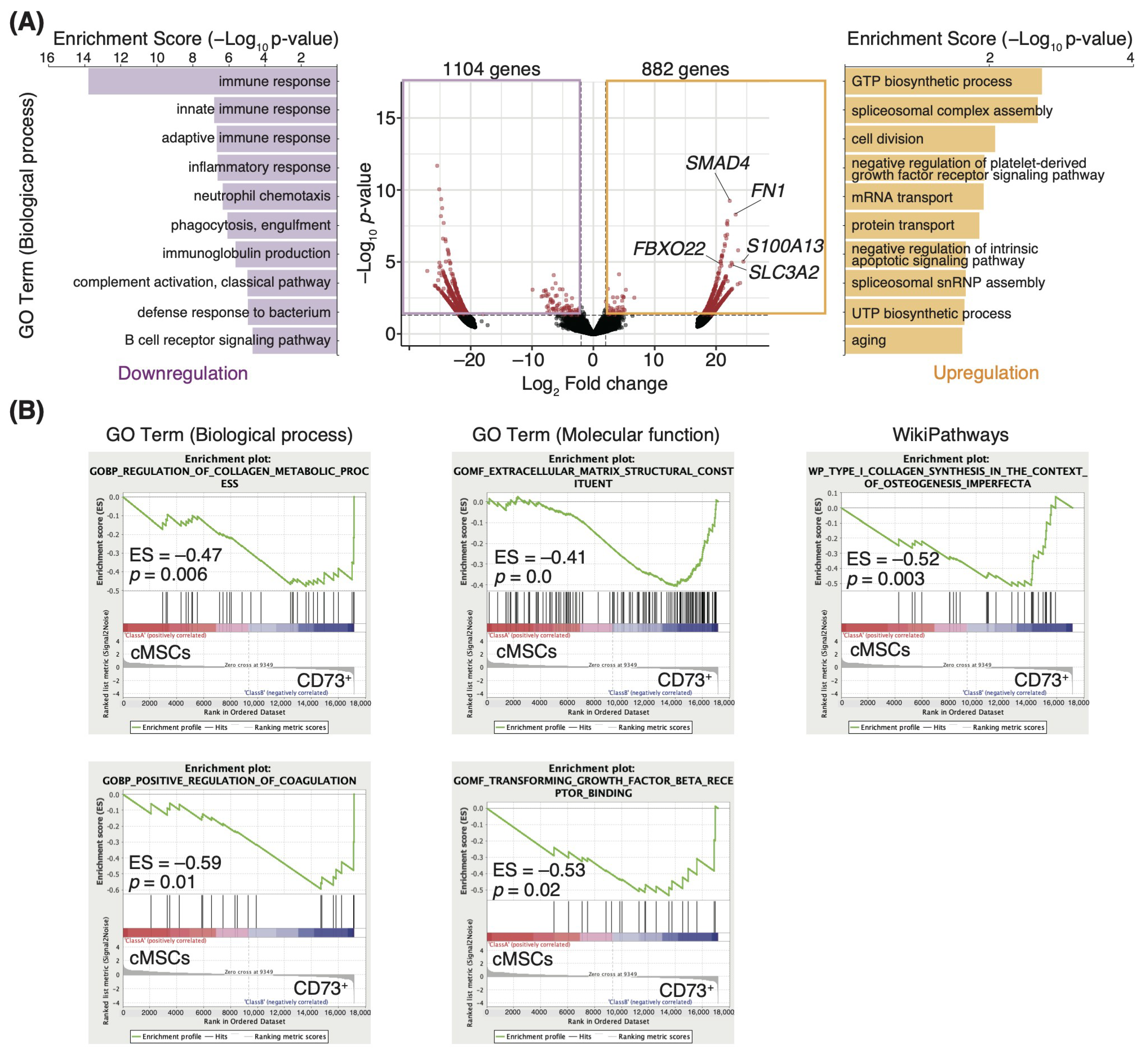
3.2. ECM-Related Genes Are Enriched in the CD73+ Cell Population
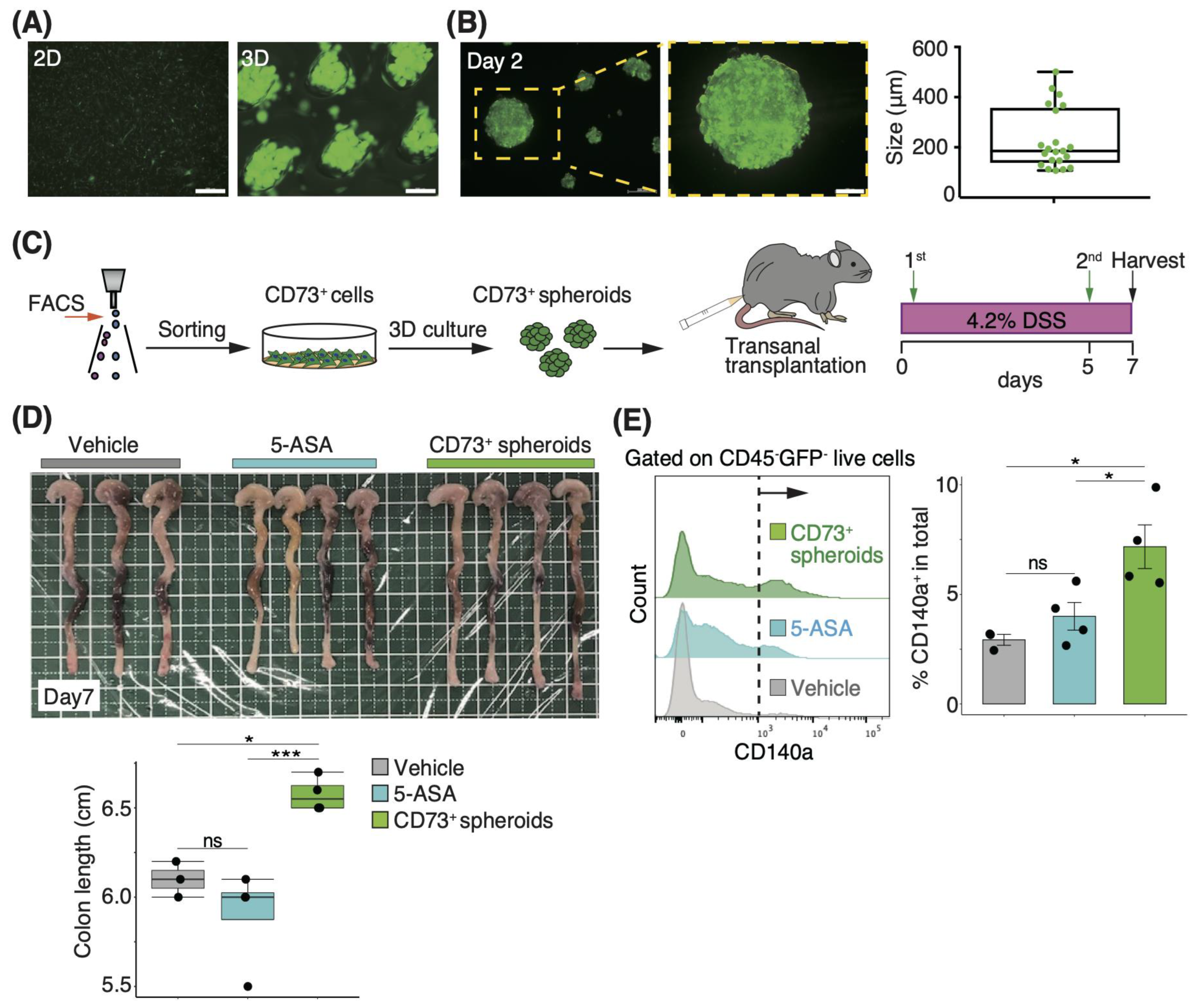
3.3. Three-Dimensional Spheroids of CD73+ Cells Have More Engraftment Potential than Two-Dimensional Cells in Trans-Anal Transplantation
3.4. Transplantation of CD73+ Cell Spheroids onto the Colonic Lumen Prevents Mucosal Atrophy
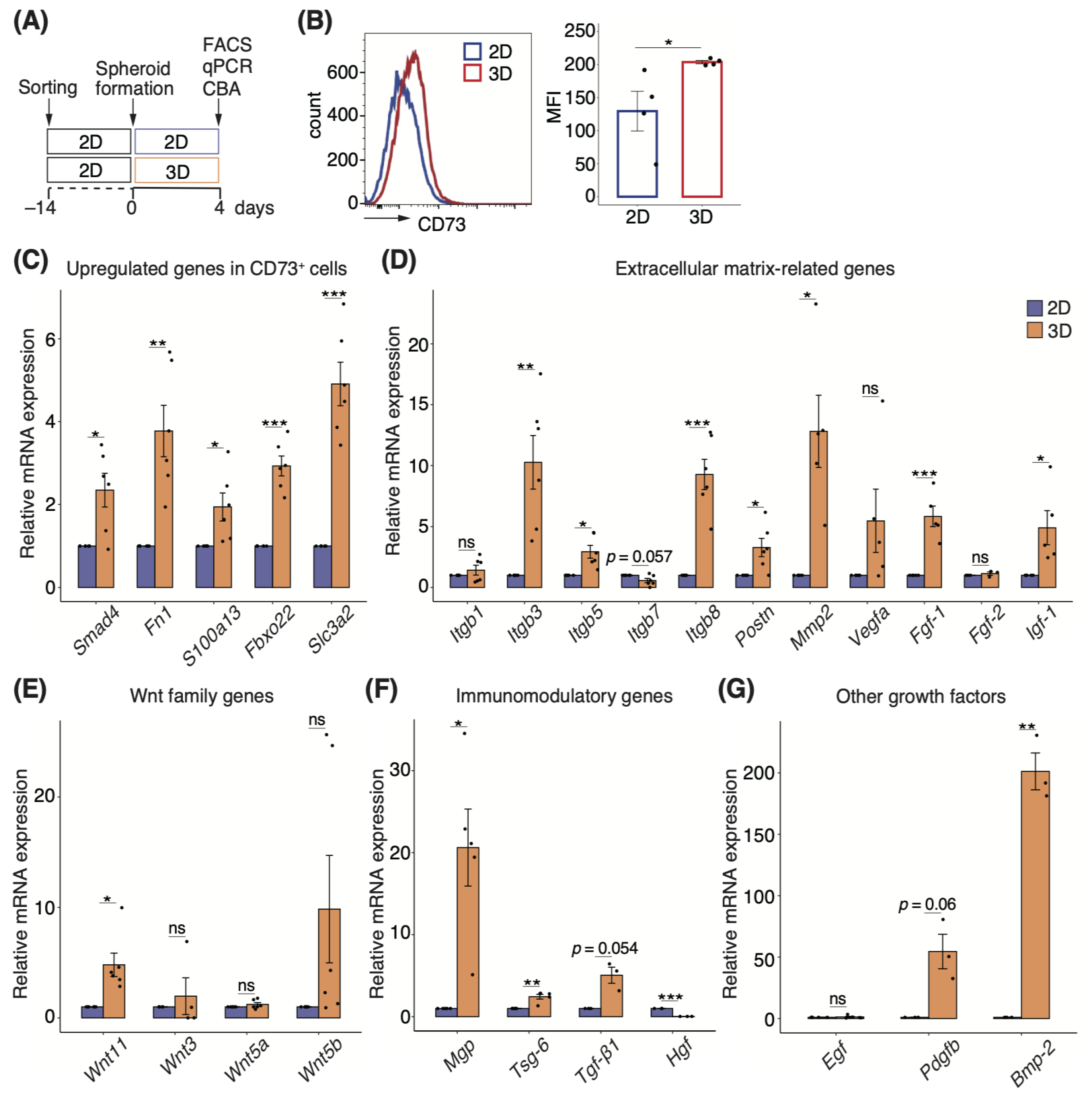
3.5. Three-Dimensional Spheroids Enhance the Properties of CD73+ Cells
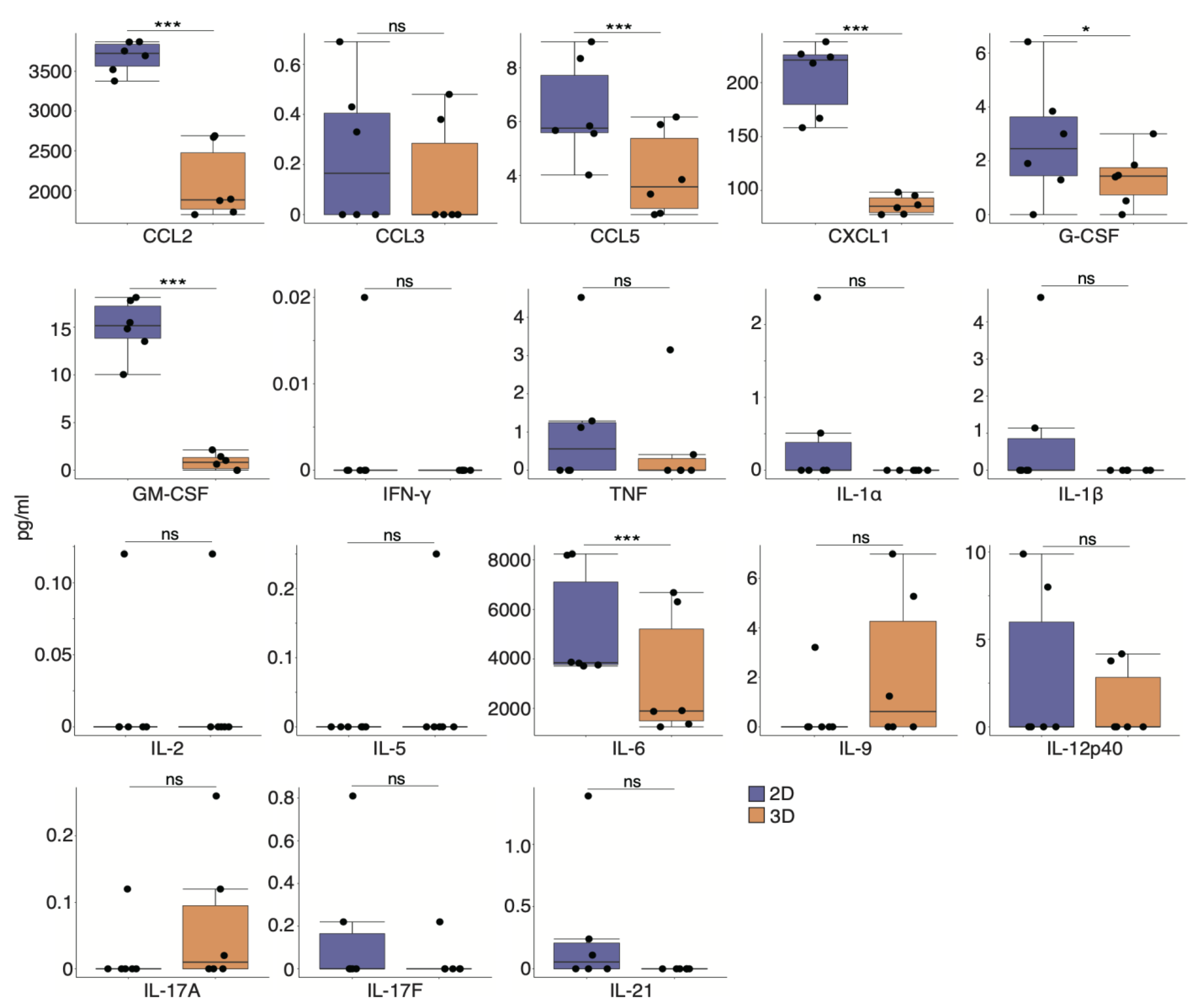
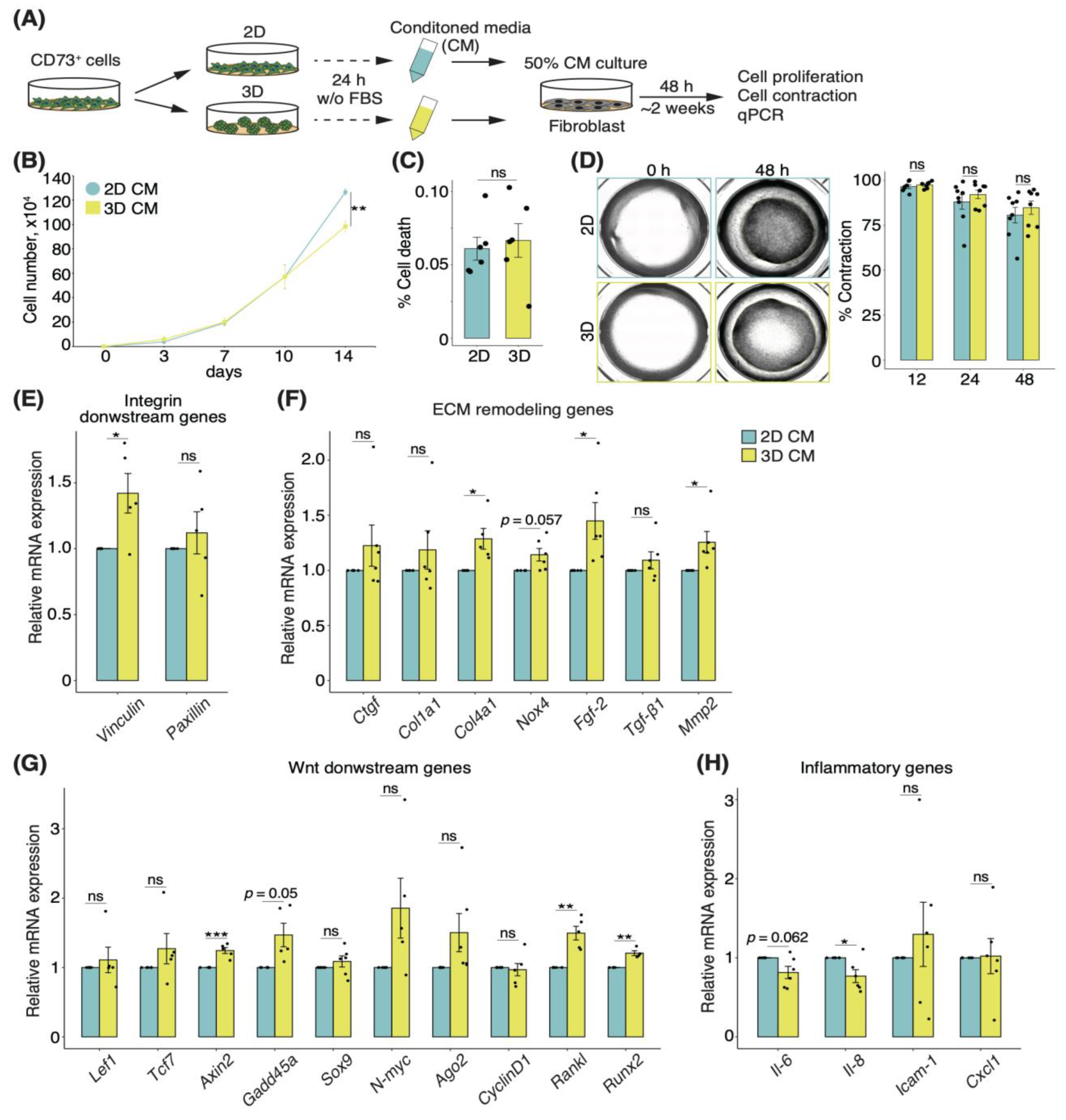
3.6. Secretory Factors of CD73+ Cell Spheroids Induce Alterations of ECM Remodeling in Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, F.E.; Seidelin, J.B.; Yin, X.; Mead, B.E.; Tong, Z.; Li, Y.; Karp, J.M.; Nielsen, O.H. Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Mol. Med. 2017, 9, 558–570. [Google Scholar] [CrossRef]
- Okabayashi, S.; Kobayashi, T.; Hibi, T. Inflammatory Bowel Disease in Japan-Is It Similar to or Different from Westerns? J. Anus Rectum Colon 2020, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rey, E.; Anderson, P.; Gonzalez, M.A.; Rico, L.; Buscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef]
- Wang, M.; Liang, C.; Hu, H.; Zhou, L.; Xu, B.; Wang, X.; Han, Y.; Nie, Y.; Jia, S.; Liang, J.; et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci. Rep. 2016, 6, 30696. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Fda, C.; Schneider, N.; Pinto, F.O.; Meyer, F.S.; Visioli, F.; Pfaffenseller, B.; Lopez, P.L.; Passos, E.P.; Cirne-Lima, E.O.; Meurer, L.; et al. Intravenous vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World J. Gastroenterol. 2014, 20, 18228–18239. [Google Scholar] [CrossRef]
- Grace Suto, E.; Mabuchi, Y.; Suzuki, N.; Koyanagi, A.; Kawabata, Y.; Ogata, Y.; Ozeki, N.; Nakagawa, Y.; Muneta, T.; Sekiya, I.; et al. High capacity of purified mesenchymal stem cells for cartilage regeneration. Inflamm. Regen. 2015, 35, 078–085. [Google Scholar] [CrossRef] [Green Version]
- Rostovskaya, M.; Anastassiadis, K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS ONE 2012, 7, e51221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chai, C.; Wang, R.; Feng, Y.; Huang, L.; Zhang, Y.; Xiao, X.; Yang, S.; Zhang, Y.; Zhang, X. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin. Transl. Med. 2021, 11, e650. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Kim, B.S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [Google Scholar] [CrossRef]
- Niibe, K.; Ohori-Morita, Y.; Zhang, M.; Mabuchi, Y.; Matsuzaki, Y.; Egusa, H. A Shaking-Culture Method for Generating Bone Marrow Derived Mesenchymal Stromal/Stem Cell-Spheroids With Enhanced Multipotency in vitro. Front. Bioeng. Biotechnol. 2020, 8, 590332. [Google Scholar] [CrossRef]
- Suto, E.G.; Mabuchi, Y.; Suzuki, N.; Suzuki, K.; Ogata, Y.; Taguchi, M.; Muneta, T.; Sekiya, I.; Akazawa, C. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73(+) population and exhibit efficacy after transplantation. Sci. Rep. 2017, 7, 4838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suto, E.G.; Mabuchi, Y.; Toyota, S.; Taguchi, M.; Naraoka, Y.; Itakura, N.; Matsuoka, Y.; Fujii, Y.; Miyasaka, N.; Akazawa, C. Advantage of fat-derived CD73 positive cells from multiple human tissues, prospective isolated mesenchymal stromal cells. Sci. Rep. 2020, 10, 15073. [Google Scholar] [CrossRef]
- McCarthy, N.; Kraiczy, J.; Shivdasani, R.A. Cellular and molecular architecture of the intestinal stem cell niche. Nat. Cell Biol. 2020, 22, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Pasztoi, M.; Ohnmacht, C. Tissue Niches Formed by Intestinal Mesenchymal Stromal Cells in Mucosal Homeostasis and Immunity. Int. J. Mol. Sci. 2022, 23, 5181. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Degirmenci, B.; Moor, A.E.; Herr, P.; Zimmerli, D.; Moor, M.B.; Hausmann, G.; Cantu, C.; Aguet, M.; Basler, K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016, 15, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386 e317. [Google Scholar] [CrossRef] [Green Version]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019, 178, 714–730 e722. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Mizutani, T.; Mochizuki, W.; Matsumoto, T.; Nozaki, K.; Sakamaki, Y.; Ichinose, S.; Okada, Y.; Tanaka, T.; Watanabe, M.; et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014, 28, 1752–1757. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Hoshino, J.; Yamazaki, C.; Sekiguchi, T.; Miyauchi, S.; Horie, K. Effects of Chondroitin Sulfate on Colitis Induced by Dextran Sulfate Sodium in Rats. Jpn. J. Pharmacol. 2001, 85, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Ernst, M.; Preaudet, A.; Putoczki, T. Non-invasive assessment of the efficacy of new therapeutics for intestinal pathologies using serial endoscopic imaging of live mice. J. Vis. Exp. 2015, 97, e52383. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Ditzel, N.; Isern, J.; Ekblom, M.; Mendez-Ferrer, S.; Kassem, M.; Scheding, S. Low/negative expression of PDGFR-alpha identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Rep. 2014, 3, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Severe, N.; Karabacak, N.M.; Gustafsson, K.; Baryawno, N.; Courties, G.; Kfoury, Y.; Kokkaliaris, K.D.; Rhee, C.; Lee, D.; Scadden, E.W.; et al. Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping. Cell Stem Cell 2019, 25, 570–583 e577. [Google Scholar] [CrossRef]
- Schiller, M.; Javelaud, D.; Mauviel, A. TGF-beta-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wang, C.; Zhou, J.; Liang, Q.; He, F.; Li, F.; Li, Y.; Chen, J.; Zhang, F.; Han, C.; et al. Fibronectin 1 activates WNT/beta-catenin signaling to induce osteogenic differentiation via integrin beta1 interaction. Lab. Invest. 2020, 100, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Hayrabedyan, S.; Kyurkchiev, S.; Kehayov, I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J. Reprod. Immunol. 2005, 67, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, H.; Zhao, Y.; Zhang, X.; Liu, J.; Pan, Y.; Bai, J.; Zhang, H. Knockdown of FBXO22 inhibits melanoma cell migration, invasion and angiogenesis via the HIF-1alpha/VEGF pathway. Invest. N. Drugs 2020, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Feral, C.C.; Zijlstra, A.; Tkachenko, E.; Prager, G.; Gardel, M.L.; Slepak, M.; Ginsberg, M.H. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J. Cell Biol. 2007, 178, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Hisamatsu, D.; Ohno-Oishi, M.; Nakamura, S.; Mabuchi, Y.; Naka-Kaneda, H. Growth differentiation factor 6 derived from mesenchymal stem/stromal cells reduces age-related functional deterioration in multiple tissues. Aging 2016, 8, 1259. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef]
- Chapman, T.P.; Frias Gomes, C.; Louis, E.; Colombel, J.F.; Satsangi, J. Review article: Withdrawal of 5-aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 73–84. [Google Scholar] [CrossRef]
- Puxeddu, I. Eotaxin/CCL11, RANTES/CCL5 and MCP-1/CCL2 modulate airway fibroblast properties in vitro*1. J. Allergy Clin. Immunol. 2004, 113, S186. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohns Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef] [Green Version]
- Kolachala, V.L.; Bajaj, R.; Wang, L.; Yan, Y.; Ritzenthaler, J.D.; Gewirtz, A.T.; Roman, J.; Merlin, D.; Sitaraman, S.V. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J. Biol. Chem. 2007, 282, 32965–32973. [Google Scholar] [CrossRef] [Green Version]
- Feral, C.C.; Nishiya, N.; Fenczik, C.A.; Stuhlmann, H.; Slepak, M.; Ginsberg, M.H. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Dalmasso, G.; Torkvist, L.; Halfvarson, J.; Yan, Y.; Laroui, H.; Shmerling, D.; Tallone, T.; D’Amato, M.; Sitaraman, S.V.; et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J. Clin. Invest. 2011, 121, 1733–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambartsumian, N.; KlingelhoÈfer, J.; Grigorian, M.; Christensen, C.; Kriajevska, M.; Tulchinsky, E.; Georgiev, G.; Berezin, V.; Bock, E.; Rygaard, J. The metastasis-associated Mts1 (S100A4) protein could act as an angiogenic factor. Oncogene 2001, 20, 4685–4695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupack, D.G.; Cheresh, D.A. ECM remodeling regulates angiogenesis: Endothelial integrins look for new ligands. Sci. STKE 2002, 2002, pe7. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Soldi, R.; Bagala, C.; Micucci, I.; Bellum, S.; Tarantini, F.; Prudovsky, I.; Maciag, T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J. Biol. Chem. 2001, 276, 22544–22552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Dai, C. Key Fibrogenic Signaling. Curr. Pathobiol. Rep. 2015, 3, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, S.E. Mechanisms of tissue repair: From wound healing to fibrosis. Int. Ernational. J. Biochem. Cell Biol. 1997, 29, 5–17. [Google Scholar] [CrossRef]
- Nishina, T.; Deguchi, Y.; Ohshima, D.; Takeda, W.; Ohtsuka, M.; Shichino, S.; Ueha, S.; Yamazaki, S.; Kawauchi, M.; Nakamura, E.; et al. Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer. Nat. Commun. 2021, 12, 2281. [Google Scholar] [CrossRef]
- Abdu-Allah, H.H.; El-Shorbagi, A.; Abdel-Moty, S.G.; El-Awady, R.; Abdel-Alim, A. 5-Aminosalyclic acid (5-ASA): A unique anti-inflammatory salicylate. Med. Chem. 2016, 6, 306–315. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-kappaB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, K.S. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res. Ther 2020, 11, 397. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther 2020, 11, 345. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Liao, Y.; Huang, W.; Lai, X.; Luo, J.; Du, C.; Lin, J.; Zhang, Z.; Qiu, D.; Liu, Q.; et al. Mesenchymal stromal cells-derived matrix Gla protein contribute to the alleviation of experimental colitis. Cell Death Dis. 2018, 9, 691. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; McLaughlin, N.; Sharkey, J.; Pommey, S.; Denoyer, D.; Dwyer, K.M.; Smyth, M.J. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 1547–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune SuppressionTumor CD73 Impairs Antitumor T-Cell Responses. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, M.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Zhang, B. Targeting CD73 to augment cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 66–76. [Google Scholar] [CrossRef]
- Liu, X.H.; Wu, X.R.; Lan, N.; Zheng, X.B.; Zhou, C.; Hu, T.; Chen, Y.F.; Cai, Z.R.; Chen, Z.X.; Lan, P.; et al. CD73 promotes colitis-associated tumorigenesis in mice. Oncol. Lett. 2020, 20, 1221–1230. [Google Scholar] [CrossRef]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepp, O.; Bezu, L.; Yamazaki, T.; Di Virgilio, F.; Smyth, M.J.; Kroemer, G.; Galluzzi, L. ATP and cancer immunosurveillance. EMBO J. 2021, 40, e108130. [Google Scholar] [CrossRef]
- Bahreyni, A.; Samani, S.S.; Khazaei, M.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Therapeutic potentials of adenosine receptors agonists and antagonists in colitis; Current status and perspectives. J. Cell Physiol. 2018, 233, 2733–2740. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisamatsu, D.; Itakura, N.; Mabuchi, Y.; Ozaki, R.; Suto, E.G.; Naraoka, Y.; Ikeda, A.; Ito, L.; Akazawa, C. CD73-Positive Cell Spheroid Transplantation Attenuates Colonic Atrophy. Pharmaceutics 2023, 15, 845. https://doi.org/10.3390/pharmaceutics15030845
Hisamatsu D, Itakura N, Mabuchi Y, Ozaki R, Suto EG, Naraoka Y, Ikeda A, Ito L, Akazawa C. CD73-Positive Cell Spheroid Transplantation Attenuates Colonic Atrophy. Pharmaceutics. 2023; 15(3):845. https://doi.org/10.3390/pharmaceutics15030845
Chicago/Turabian StyleHisamatsu, Daisuke, Natsumi Itakura, Yo Mabuchi, Rion Ozaki, Eriko Grace Suto, Yuna Naraoka, Akari Ikeda, Lisa Ito, and Chihiro Akazawa. 2023. "CD73-Positive Cell Spheroid Transplantation Attenuates Colonic Atrophy" Pharmaceutics 15, no. 3: 845. https://doi.org/10.3390/pharmaceutics15030845





