Application of Amniotic Membrane in Skin Regeneration
Abstract
:1. Introduction
2. Method
3. Structure and Composites of Amniotic Membrane
4. Preparation Method of Amniotic Membrane
5. Applications of Amniotic Membrane on The Skin Regeneration
5.1. Wound Healing
5.1.1. Wound Dressing
5.1.2. Stem Cell
5.1.3. Polymer Combination
5.1.4. Skin Graft
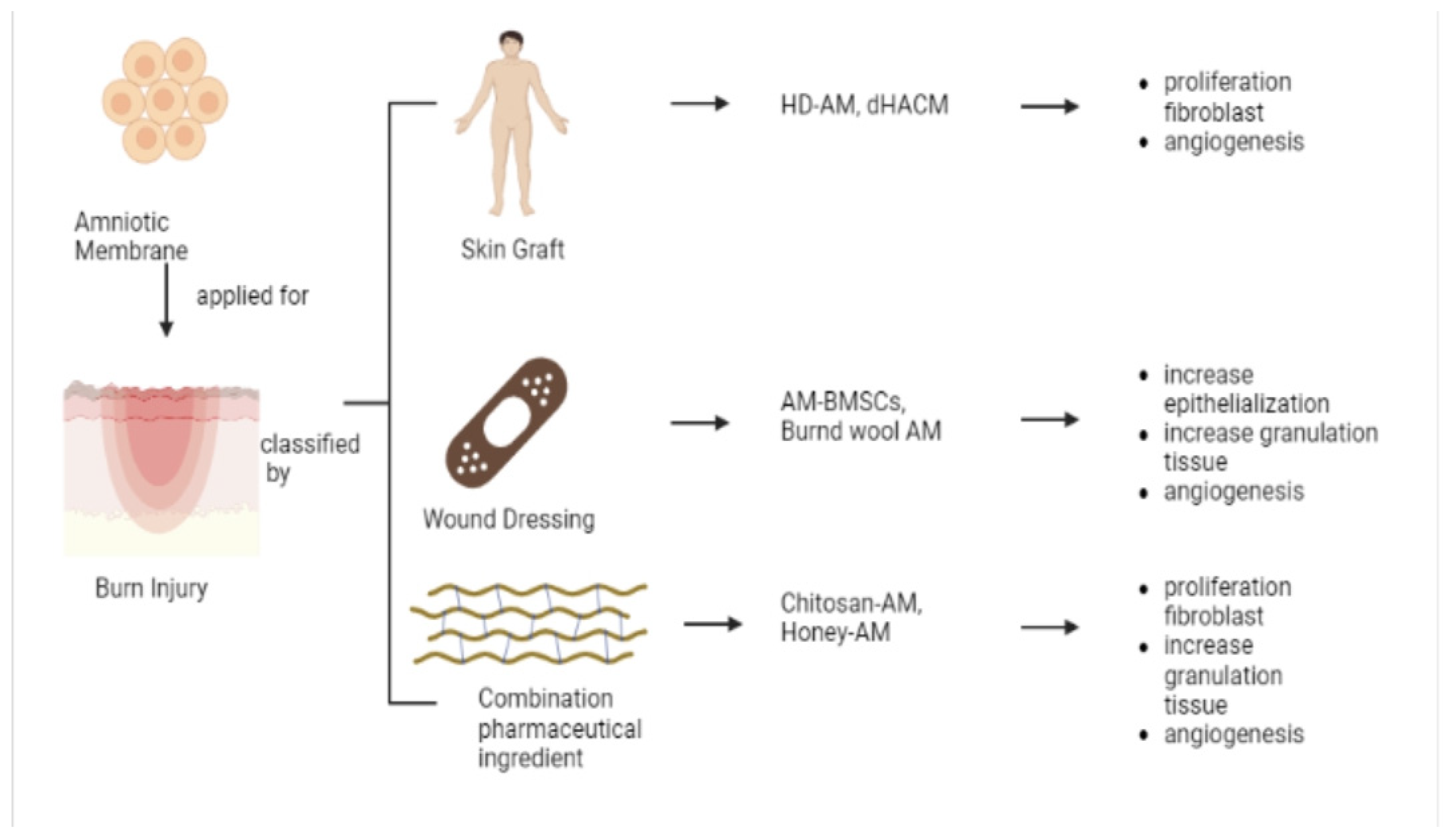
5.2. Burn Injury
5.2.1. Skin Graft
5.2.2. Wound Dressing
5.2.3. Pharmaceutical Ingredient Combination
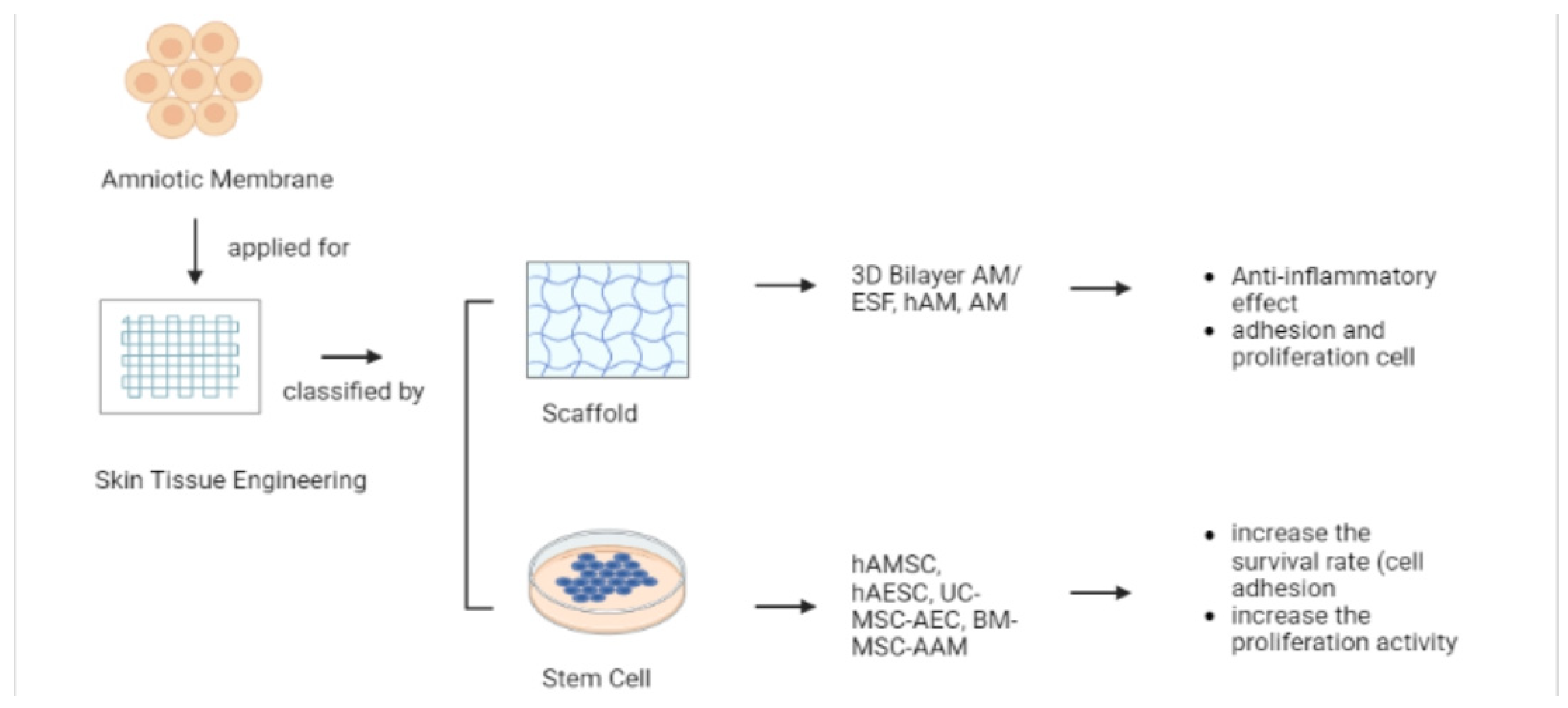
5.3. Skin Tissue Engineering (TE)
5.3.1. Scaffold
5.3.2. Stem Cell
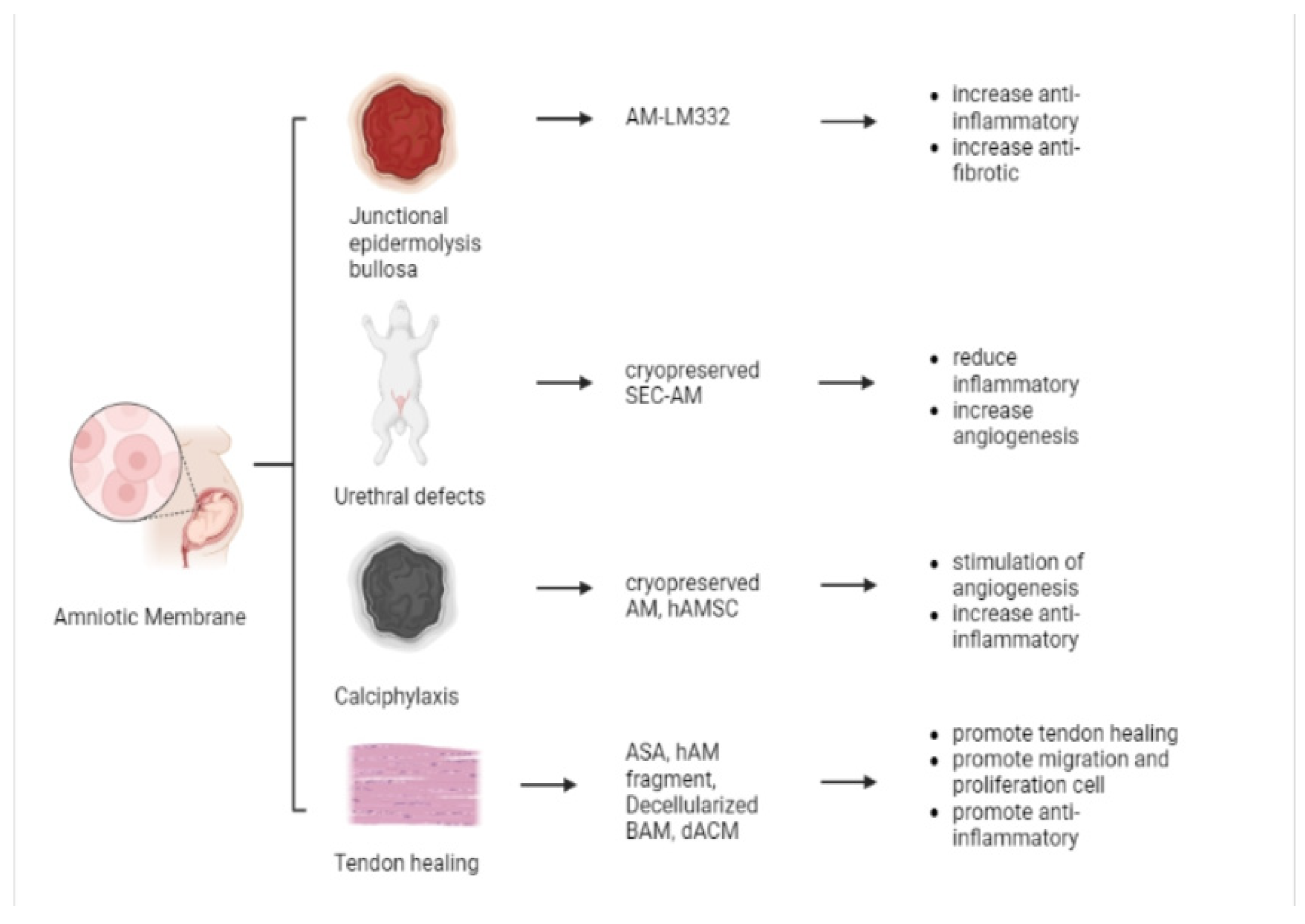
5.4. Other Applications
5.4.1. Junctional Epidermolysis Bullosa (JEB)
5.4.2. Urethral Defects
5.4.3. Calciphylaxis
5.4.4. Tendon Healing
6. Author’s Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Amniotic membrane |
| ECM | Extracellular matrix |
| b-FGF | Basic fibroblast growth factor |
| EGF | Epidermal growth factor |
| KGF | Keratocyte growth factor |
| HGF | Hepatocyte growth factor |
| HIV | Human immunodeficiency virus |
| HCV | Hepatitis C Virus |
| HBs | hepatitis b virus |
| PBS | phosphate buffer saline |
| RPMI | Roswell Park Memorial Institute |
| EDTA | ethylenediaminetetraacetic acid |
| NaCl | natrium chloride |
| AEC | amnion epithelial cells |
| AMSC | amniotic membrane mesenchymal stem cell |
| HAMSCs | human amniotic mesenchymal stem cells |
| DMEM-F12 | Dulbecco’s modified eagle medium/nutrient mixture F-12 |
| hAECs | human amniotic epithelial stem cells |
| HAM | human amniotic membrane |
| hAAM | human acellular amniotic membrane |
| CM | conditioned media |
| dHACM | dehydrated human amnion/chorion membrane |
| dHACA | dehydrated human amnion and chorion allograft |
| dACM | dehydrated human amnion/chorion membranes |
| VEGF | vascular endothelial growth factor |
| α-SMA | smooth muscle alpha-actin |
| TGF-β1 | transforming growth factor-beta-1 |
| TGF-β1 | transforming growth factor-beta-3 |
| HLA | human leucocyte antigen |
| BCN | bicomponent-network |
| dHAM | decellularized human amniotic membrane |
| dHAMMA | decellularized human amniotic membrane methacrylate |
| MA | methacrylic anhydride |
| GelMAdHAMMA | methacrylated gelatin decellularized Human Amniotic Membrane methacrylate |
| GelMA | methacrylated gelatin |
| DE | dermal equivalent |
| BM-SCs | bone marrow stromal cells |
| hDAM | human dehydrated amniotic membrane |
| MSS | Manchester Scar Scale |
| BM-SCs-hDAM | bone marrow stromal cells human dehydrated amniotic membrane |
| AV | aloe vera |
| AME | amniotic membrane extract |
| HAAM | human acellular amniotic membrane |
| dHACA | human amnion and chorion allograft |
| TESS | skin tissue engineering substitutes |
| DFU | diabetic foot ulcer |
| hAMSCs-CM | media conditioned by human amniotic mesenchymal stem cells |
| hAECs-CM | media conditioned by human amniotic epithelial stem cells |
| LOXL2 | lysyl-oxidase-2 |
| JNK | c-jun N-terminal kinase |
| WJ-MSC | Wharton’s jelly mesenchymal stem cells |
| DF | dermal fibroblast |
| MSCs | mesenchymal Stem Cell |
| MenSCs | menstrual blood-derived stem cell |
| DAM | decellularized human amniotic membrane |
| MenSCs-DAM | menstrual blood-derived stem cell decellularized human amniotic membrane |
| hAAM | human acellular amniotic membrane |
| rHFSC | rat hair follicle stem cell |
| EdU | 5-ethynyl-2-deoxyuridine |
| H&E | hematoxylin and eosin |
| rHFSChAAM | rat hair follicle stem cells human acellular amniotic membrane |
| iPSC | induced pluripotent stem cells |
| EpSCs | epithelial stem cells |
| iPSC-EpSC | induced pluripotent stem cells- epithelial stem cells |
| ADMSC | adipose-derived mesenchymal stem cells |
| AAM | acellular amniotic membrane |
| STSG | split-thickness skin grafts |
| HD-AM | hyperdry amniotic membrane |
| BMSC | bone marrow stem cells |
| BAWD | bioactive wound dressing |
| AME | amniotic membrane extract |
| PMN | polymorphonuclear |
| TE | tissue engineering |
| PMNL | human polymorphonuclear cells |
| TNFα | tumor necrosis factor-alpha |
| COLI | collagen I |
| MMP3 | matrix metalloproteinase-3 |
| ESF | electrospun nanofiber silk fibroin |
| AT-MSCs | adipose tissue-derived mesenchymal stem cells |
| VEGFa | vascular endothelial growth factor |
| bFGF | basic fibroblast growth factor |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| UC-MSC | umbilical cord mesenchymal stem cells |
| FGF-7 | fibroblast growth factor 7 |
| RSF | random skin flaps |
| BM-MSCs | bone marrow mesenchymal stem cells |
| JEB | junctional epidermolysis bullosa |
| EB | epidermolysis bullosa |
| SEC | skin epithelial cell sheet |
| cryo-SEC-AM | cryopreserved skin epithelial cell sheet amniotic membrane |
| VAS | visual analog scale |
| ASA | amniotic suspension allograft |
References
- Datta, S.; Rameshbabu, A.P.; Bankoti, K.; Maity, P.P.; Das, D.; Pal, S.; Roy, S.; Sen, R.; Dhara, S. Oleoyl-Chitosan-Based Nanofiber Mats Impregnated with Amniotic Membrane Derived Stem Cells for Accelerated Full-Thickness Excisional Wound Healing. ACS Biomater. Sci. Eng. 2017, 3, 1738–1749. [Google Scholar] [CrossRef]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human Amniotic Mesenchymal Stem Cells and Their Paracrine Factors Promote Wound Healing by Inhibiting Heat Stress-Induced Skin Cell Apoptosis and Enhancing Their Proliferation through Activating PI3K/AKT Signaling Pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Huang, S.; Wen, J.; Jiao, Y.; Su, X.; Shi, G.; Huang, J. PF-127 Hydrogel plus Sodium Ascorbyl Phosphate Improves Wharton’s Jelly Mesenchymal Stem Cell-Mediated Skin Wound Healing in Mice. Stem Cell Res. Ther. 2020, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Cañada, C.; Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic Wound Healing by Amniotic Membrane: TGF-β and EGF Signaling Modulation in Re-Epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Hoffman, T.; Sathyamoorthy, M.; Lerch, A.; Jacob, V.; Moorman, M.; Kuang, J.Q.; Danilkovitch, A. A Viable Lyopreserved Amniotic Membrane Modulates Diabetic Wound Microenvironment and Accelerates Wound Closure. Adv. Wound Care 2019, 8, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Duarte, I.G.L.; Duval-Araujo, I. Amniotic Membrane as a Biological Dressing in Infected Wound Healing in Rabbits. Acta Circ. Bras. 2014, 29, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic Membrane and Its Epithelial and Mesenchymal Stem Cells as an Appropriate Source for Skin Tissue Engineering and Regenerative Medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Lu, M.; Wang, T.; Dian, D.; Zhong, Y.; Aleahmad, M. Human Amniotic Membrane as a Delivery Vehicle for Stem Cell-Based Therapies. Life Sci. 2021, 272, 119157. [Google Scholar] [CrossRef]
- Schulze, U.; Hampel, U.; Sel, S.; Goecke, T.W.; Thäle, V.; Garreis, F.; Paulsen, F. Fresh and Cryopreserved Amniotic Membrane Secrete the Trefoil Factor Family Peptide 3 That Is Well Known to Promote Wound Healing. Histochem. Cell Biol. 2012, 138, 243–250. [Google Scholar] [CrossRef]
- Hossain, M.L.; Rahman, M.A.; Siddika, A.; Adnan, M.H.; Rahman, H.; Diba, F.; Hasan, M.Z.; Asaduzzaman, S.M. Burn and Wound Healing Using Radiation Sterilized Human Amniotic Membrane and Centella Asiatica Derived Gel: A Review. Regen. Eng. Transl. Med. 2020, 6, 347–357. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human Amniotic Membrane Transplantation: Different Modalities of Its Use in Ophthalmology. World J. Transpl. 2014, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Shaifur Ra, M.; Asaduzzama, S.M.; Shahedur, R.M. Properties and Therapeutic Potential of Human Amniotic Membrane. Asian J. Dermatol. 2014, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, R.; Krishnan, L.K.; Nair, R.P.; Kalliyana Krishnan, V. Reinforcement of Amniotic Membrane with Fibrin Coated Poly-[Lactide-Co-Glycolide-Co-Caprolactone] Terpolymer Containing Silver Nanoparticles for Potential Wound Healing Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 810–819. [Google Scholar] [CrossRef]
- Bemenderfer, T.B.; Anderson, R.B.; Odum, S.M.; Davis, W.H. Effects of Cryopreserved Amniotic Membrane-Umbilical Cord Allograft on Total Ankle Arthroplasty Wound Healing. J. Foot Ankle Surg. 2019, 58, 97–102. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, Y.; Du, J.; Wang, H.; Li, W.; Liu, Z. Effects of Amniotic Extraction on Epithelial Wound Healing and Stromal Remodelling after Excimer Laser Keratectomy in Rabbit Cornea. Chin. J. Ophthalmol. 2014, 50, 42–50. [Google Scholar] [CrossRef]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic Membrane: From Structure and Functions to Clinical Applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Chehelcheraghi, F.; Eimani, H.; Homayoonsadraie, S.; Torkaman, G.; Amini, A.; Majd, H.A.; Shemshadi, H. Effects of Acellular Amniotic Membrane Matrix and Bone Marrow-Derived Mesenchymal Stem Cells in Improving Random Skin Flap Survival in Rats. Iran. Red Crescent Med. J. 2016, 18, e25588. [Google Scholar] [CrossRef] [Green Version]
- Nouri, M.; Ebrahimi, M.; Bagheri, T.; Fatemi, M.J.; Najafbeygi, A.; Araghi, S.; Molaee, M. Healing Effects of Dried and Acellular Human Amniotic Membrane and Mepitelas for Coverage of Skin Graft Donor Areas; A Randomized Clinical Trial. Bull Emerg. Trauma 2018, 6, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Ozkaya, O.; Egemen, O.; Yesilada, A.; Sakiz, D.; Ugurlu, K. The Effect of Nonpreserved Human Amniotic Membrane on the Survival of Ischaemic Skin Flaps in Rats. J. Plast Reconstr. Aesthet. Surg. 2012, 65, 1700–1705. [Google Scholar] [CrossRef]
- Moreno, S.E.; Massee, M.; Koob, T.J. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFβ-SMAD Pathway In Vitro. JID Innov. 2021, 1, 100020. [Google Scholar] [CrossRef]
- Kim, S.S.; Song, C.K.; Shon, S.K.; Lee, K.Y.; Kim, C.H.; Lee, M.J.; Wang, L. Effects of Human Amniotic Membrane Grafts Combined with Marrow Mesenchymal Stem Cells on Healing of Full-Thickness Skin Defects in Rabbits. Cell Tissue Res. 2009, 336, 59–66. [Google Scholar] [CrossRef]
- Campelo, M.B.D.; Santos, J.d.A.F.; Maia Filho, A.L.M.; Ferreira, D.C.L.; Sant’Anna, L.B.; De Oliveira, R.A.; Maia, L.F.; Arisawa, E.Â.L. Effects of the Application of the Amniotic Membrane in the Healing Process of Skin Wounds in Rats. Acta Circ. Bras. 2018, 33, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Hatzfeld, A.S.; Pasquesoone, L.; Germain, N.; Danzé, P.M.; Drucbert, A.S.; Tardivel, M.; Bongiovanni, A.; Duquennoy-Martinot, V.; Guerreschi, P.; Marchetti, P. Benefits of Cryopreserved Human Amniotic Membranes in Association with Conventional Treatments in the Management of Full-Thickness Burns. Int. Wound J. 2019, 16, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Tan, E.K.; Mandrycky, C.; He, H.; O’Connell, J.; Tseng, S.C.G. Comparison of Cryopreserved Amniotic Membrane and Umbilical Cord Tissue with Dehydrated Amniotic Membrane/Chorion Tissue. J. Wound Care 2014, 23, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakabadze, Z.; Chakhunashvili, D.; Gogilashvili, K.; Ediberidze, K.; Chakhunashvili, K.; Kalandarishvili, K.; Karalashvili, L. Bone Marrow Stem Cell and Decellularized Human Amniotic Membrane for the Treatment of Nonhealing Wound after Radiation Therapy. Exp. Clin. Transplant. 2019, 17, 92–98. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Kesting, M.R.; Paulitschke, P.; Stöckelhuber, M.; von Bomhard, A. Development of a Tissue-Engineered Skin Substitute on a Base of Human Amniotic Membrane. J. Tissue Eng. 2019, 10, 2041731418825378. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The Healing Effect of Wharton’s Jelly Stem Cells Seeded on Biological Scaffold in Chronic Skin Ulcers: A Randomized Clinical Trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The Repairing of Full-Thickness Skin de Fi Ciency and Its Biological Mechanism Using Decellularized Human Amniotic Membrane as the Wound Dressing. Mater. Sci. Eng. C 2017, 77, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Sous Naasani, L.I.; Damo Souza, A.F.; Rodrigues, C.; Vedovatto, S.; Azevedo, J.G.; Santin Bertoni, A.P.; Da Cruz Fernandes, M.; Buchner, S.; Wink, M.R. Decellularized Human Amniotic Membrane Associated with Adipose Derived Mesenchymal Stromal Cells as a Bioscaffold: Physical, Histological and Molecular Analysis. Biochem. Eng. J. 2019, 152, 107366. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Bandehpour, M.; Mozafari, M.; Hashemi, A.; Ghanbarian, H.; Sameni, M.; Salimi, M.; Gholami, M.; Samadikuchaksaraei, A. Decellularized Human Amniotic Membrane: More Is Needed for an Efficient Dressing for Protection of Burns against Antibiotic-Resistant Bacteria Isolated from Burn Patients. Burns 2015, 41, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; El-Derby, A.; Abd Elkodous, M.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the Amniotic Membrane in Tissue Engineering and Regeneration: The Hundred-Year Challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Nelson, R.A.; Sunnon, K.; Reid, T.; Clouse, C.; Kock, N.D.; Jackson, J.; Soker, S.; Atala, A. Amnion Membrane Hydrogel and Amnion Membrane Powder Accelerate Wound Healing in a Full Thickness Porcine Skin Wound Model. Stem Cells Transl. Med. 2020, 9, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Son, D.; Choi, T.H.; Jung, S.; Kwon, S.; Kim, J.; Han, K. Evaluation of an Amniotic Membrane-Collagen Dermal Substitute in the Management of Full-Thickness Skin Defects in a Pig. Arch. Plast. Surg. 2013, 40, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; Zhao, F.; Jiang, H.; Kang, Y.; Song, Y.; Lin, X.; Shi, P.; Zhang, T.; Pang, X. LOXL2 from Human Amniotic Mesenchymal Stem Cells Accelerates Wound Epithelialization by Promoting Differentiation and Migration of Keratinocytes. Aging 2020, 12, 12960–12986. [Google Scholar] [CrossRef]
- Yu, S.C.; Xu, Y.Y.; Li, Y.; Xu, B.; Sun, Q.; Li, F.; Zhang, X.G. Construction of Tissue Engineered Skin with Human Amniotic Mesenchymal Stem Cells and Human Amniotic Epithelial Cells. Eur. Rev. Med. Pharm. Sci. 2015, 19, 4627–4635. [Google Scholar]
- Tousi, S.M.T.R.; Amirizadeh, N.; Nasirinezhad, F.; Nikougoftar, M.; Ganjibakhsh, M.; Aboutaleb, N. A Rapid and Cost-Effective Protocol for Isolating Mesenchymal Stem Cells from the Human Amniotic Membrane. Galen Med. J. 2017, 6, 9. [Google Scholar] [CrossRef]
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of Amniotic Stem Cells. Cell Reprogram. 2014, 16, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; De Toro, F.J.; Blanco, F.J. Isolation and Characterization of Mesenchymal Stem Cells from Human Amniotic Membrane. Tissue Eng. Part C Methods 2010, 17, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Tauzin, H.; Rolin, G.; Viennet, C.; Saas, P.; Humbert, P.; Muret, P. A Skin Substitute Based on Human Amniotic Membrane. Cell Tissue Bank 2014, 15, 257–265. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Baumann, C.; Stoeckelhuber, M.; Hasler, R.; Mücke, T.; Steinsträßer, L.; Drecoll, E.; Wolff, K.D.; Kesting, M.R. Amniotic Membrane as Part of a Skin Substitute for Full-Thickness Wounds: An Experimental Evaluation in a Porcine Model. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1245–1256. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of Burn Wound Healing Gel Prepared from Human Amniotic Membrane and Aloe Vera Extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, E.S.; Sheikh, E.S.; Fetterolf, D.E. Use of Dehydrated Human Amniotic Membrane Allografts to Promote Healing in Patients with Refractory Non Healing Wounds. Int. Wound J. 2013, 11, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Zhang, C.; Hu, J.; Chen, J.; Du, W.; Liu, F.; Ren, W.; Wang, J.; Quan, R. Feasibility of Repairing Full-Thickness Skin Defects by IPSC-Derived Epithelial Stem Cells Seeded on a Human Acellular Amniotic Membrane. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Xiong, J.; Shao, S.; Xu, S.; Ni, H.; Wang, Y.; Ji, K. Hair Follicle Morphogenesis in the Treatment of Mouse Full-Thickness Skin Defects Using Composite Human Acellular Amniotic Membrane and Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 8281235. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhou, H.; Du, W.; Huang, X.; Zheng, X.; Zhang, C.; Hu, H.; Wang, J.; Quan, R. Hair Follicle Stem Cells Combined with Human Allogeneic Acellular Amniotic Membrane for Repair of Full Thickness Skin Defects in Nude Mice. J. Tissue Eng. Regen. Med. 2020, 14, 723–735. [Google Scholar] [CrossRef]
- Xue, S.L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.C. Human Acellular Amniotic Membrane Implantation for Lower Third Nasal Reconstruction: A Promising Therapy to Promote Wound Healing. Burns Trauma 2018, 6, 24. [Google Scholar] [CrossRef]
- Farzamfar, S.; Salehi, M.; Ehterami, A.; Naseri-Nosar, M.; Vaez, A.; Zarnani, A.H.; Sahrapeyma, H.; Shokri, M.R.; Aleahmad, M. Promotion of Excisional Wound Repair by a Menstrual Blood-Derived Stem Cell-Seeded Decellularized Human Amniotic Membrane. Biomed. Eng. Lett. 2018, 8, 393–398. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, C.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Cui, W.; Ge, Z. Photo-Crosslinkable Amniotic Membrane Hydrogel for Skin Defect Healing. Acta Biomater. 2021, 125, 197–207. [Google Scholar] [CrossRef]
- Samadikuchaksaraei, A.; Mehdipour, A.; Habibi Roudkenar, M.; Verdi, J.; Joghataei, M.T.; As’adi, K.; Amiri, F.; Dehghan Harati, M.; Gholipourmalekabadi, M.; Karkuki Osguei, N. A Dermal Equivalent Engineered with TGF-Β3 Expressing Bone Marrow Stromal Cells and Amniotic Membrane: Cosmetic Healing of Full-Thickness Skin Wounds in Rats. Artif. Organs 2016, 40, E266–E279. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yan, Y.; Dai, Y.; Jin, S.; Liu, Y.; Chen, J.; Teng, J. A Sponge-like Double-Layerwound Dressing with Chitosan and Decellularized Bovine Amniotic Membrane for Promoting Diabeticwound Healing. Polymers 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.S.; Mohammadi, A.A.; Moshirabadi, K.; Zardosht, M. Effect of Dermal Fibroblasts and Mesenchymal Stem Cells Seeded on an Amniotic Membrane Scaffold in Skin Regeneration: A Case Series. J. Cosmet. Dermatol. 2021, 20, 4040–4047. [Google Scholar] [CrossRef]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.J.; Keller, J.; Li, W.W. A Prospective, Randomised, Controlled, Multi-Centre Comparative Effectiveness Study of Healing Using Dehydrated Human Amnion/Chorion Membrane Allograft, Bioengineered Skin Substitute or Standard of Care for Treatment of Chronic Lower Extremity Diabetic Ul. Int. Wound J 2015, 12, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Glat, P.; Orgill, D.P.; Galiano, R.; Armstrong, D.; Serena, T.; Didomenico, L.A.; Kaufman, J.; Carter, M.J.; Jacobs, A.M.; Zelen, C.M. Placental Membrane Provides Improved Healing Efficacy and Lower Cost Versus a Tissue-Engineered Human Skin in the Treatment of Diabetic Foot Ulcerations. Plast Reconstr. Surg. Glob. Open 2019, 7, e2371. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Choi, S.; Cho Lee, A.R. Effect of Freeze Dried Bovine Amniotic Membrane Extract on Full Thickness Wound Healing. Arch. Pharm. Res. 2013, 36, 472–478. [Google Scholar] [CrossRef]
- Ahuja, N.; Jin, R.; Powers, C.; Billi, A.; Bass, K. Dehydrated Human Amnion Chorion Membrane as Treatment for Pediatric Burns. Adv. Wound Care 2020, 9, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Vaheb, M.; Kohestani, B.M.; Karrabi, M.; Khosrojerdi, M.; Khajeh, M.; Shahrestanaki, E.; Sahebkar, M. Evaluation of Dried Amniotic Membrane on Wound Healing at Split-Thickness Skin Graft Donor Sites: A Randomized, Placebo-Controlled, Double-Blind Trial. Adv. Ski. Wound Care 2020, 33, 636–641. [Google Scholar] [CrossRef]
- Tanideh, N.; Keshavarzi, F.; Hemat Zadeh, A.; Daneshi, S.; Koohi-Hosseinabadi, O.; Mokhtari, M.; Sedighi, A.; Asadi-Yousefabad, S.-L. Healing Effects of Human Amniotic Membrane and Burned Wool on the Second-Degree Burn in Rats. Galen Med. J. 2020, 9, e1759. [Google Scholar] [CrossRef]
- Oba, J.; Okabe, M.; Yoshida, T.; Soko, C.; Fathy, M.; Amano, K.; Kobashi, D.; Wakasugi, M.; Okudera, H. Hyperdry Human Amniotic Membrane Application as a Wound Dressing for a Full-Thickness Skin Excision after a Third-Degree Burn Injury. Burns Trauma 2020, 8, tkaa014. [Google Scholar] [CrossRef]
- Salehi, S.H.; As’adi, K.; Mousavi, S.J.; Shoar, S. Evaluation of Amniotic Membrane Effectiveness in Skin Graft Donor Site Dressing in Burn Patients. Indian J. Surg. 2015, 77, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Momeni, M.; Zarehaghighi, M.; Hajimiri, M.; Khorasani, G.; Dinarvand, R.; Nekookar, A.; Sodeifi, N.; Khosravani, P.; Shayanasl, N.; Ebrahimi, M. In Vitro and in Vivo Investigation of a Novel Amniotic-Based Chitosan Dressing for Wound Healing. Wound Repair Regen. 2018, 26, 87–101. [Google Scholar] [CrossRef] [PubMed]
- rad, F.S.; Beheshti, A.; Zangivand, A.A.; Shafigh, Y. The Effect of Honey-Impregnated Human Placenta Membrane on Burn Wound Healing in Rat. Comp. Clin. Path 2015, 24, 263–268. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Samadikuchaksaraei, A.; Seifalian, A.M.; Urbanska, A.M.; Ghanbarian, H.; Hardy, J.G.; Omrani, M.D.; Mozafari, M.; Reis, R.L.; KunduSilk, S.C. Fibroin/Amniotic Membrane 3D Bi-Layered Artificial Skin. Biomed. Mater. 2018, 13, 35003. [Google Scholar] [CrossRef] [Green Version]
- Chehelcheraghi, F.; Abbaszadeh, A.; Tavafi, M. Skin Mast Cell Promotion in Random Skin Flaps in Rats Using Bone Marrow Mesenchymal Stem Cells and Amniotic Membrane. Iran Biomed. J. 2018, 22, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, R.; Choudhery, M.S.; Mehmood, A.; Khan, S.N.; Riazuddin, S. In Vitro Differentiation Potential of Human Placenta Derived Cells into Skin Cells. Stem Cells Int. 2015, 2015, 841062. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, A.; Singh, D.; Malviya, A. Use of Gamma-Irradiated Amniotic Membrane for the Healing of Split Skin Graft Donor Site. Tissue Eng. Regen. Med. 2013, 10, 110–114. [Google Scholar] [CrossRef]
- Castiglia, D.; Fortugno, P.; Condorelli, A.G.; Barresi, S.; De Luca, N.; Pizzi, S.; Neri, I.; Graziano, C.; Trojan, D.; Ponzin, D.; et al. A Novel Phenotype of Junctional Epidermolysis Bullosa with Transient Skin Fragility and Predominant Ocular Involvement Responsive to Human Amniotic Membrane Eyedrops. Genes 2021, 12, 716. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, J.; Gu, Y.; Shao, J.; Zhou, S.; Zhuang, J.; Song, L.; Wang, X. Cryopreserved Skin Epithelial Cell Sheet Combined with Acellular Amniotic Membrane as an Off-the-Shelf Scaffold for Urethral Regeneration. Mater. Sci. Eng. C 2021, 122, 111926. [Google Scholar] [CrossRef]
- Antonio, J.; Martínez, L.; Valiente, M.R.; Ana, C.F.; Sergio, M.G.; Sanchís, C.; José, C.; Pascual, F. Use of Cryopreserved Human Amniotic Membrane in the Treatment of Skin Ulcers Secondary to Calciphylaxis. Dermathol. Ther. 2021, 34, 1–4. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, J.; Xiao, Y.; Liu, K.; Cui, Y.; Xu, F.; Ren, W.; Yuan, Y.; Jiang, C.; Ning, S.; et al. A Novel Long-Term Intravenous Combined with Local Treatment with Human Amnion-Derived Mesenchymal Stem Cells for a Multidisciplinary Rescued Uremic Calciphylaxis Patient and the Underlying Mechanism. J. Mol. Cell Biol. 2022, 14, mjac010. [Google Scholar] [CrossRef]
- De Girolamo, L.; Ambra, L.F.M.; Orfei, C.P.; McQuilling, J.P.; Kimmerling, K.A.; Mowry, K.C.; Johnson, K.A.; Phan, A.T.; Whited, J.L.; Gomoll, A.H. Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy. Cells 2019, 8, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicodemo, M.d.C.; das Neves, L.R.; Aguiar, J.C.; Brito, F.d.S.; Ferreira, I.; Sant’Anna, L.B.; Raniero, L.J.; Martins, R.Á.L.; Barja, P.R.; Arisawa, E.A.L.S. Amniotic Membrane as an Option for Treatment of Acute Achilles Tendon Injury in Rats. Acta Circ. Bras. 2017, 32, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Mcquilling, J.P.; Sanders, M.; Poland, L.; Sanders, M.; Basadonna, G.; Waldrop, N.E.; Mowry, K.C. A Diabetic Animal Model. Lab. Anim. Res. 2021, 31, 19–25. [Google Scholar]
- Rahavian, A.H.; Hazrati, E.; Azar, D.A.; Allameh, F.; Hojjati, S.A.; Javanmard, B.; Hamidi, R. Using Dry Human Amniotic Membrane in Secondary Intention Wound Healing after Urological Cancer Surgery: The First Randomized Clinical Trial in Iran. Int. J. Cancer Manag. 2021, 14, e111421. [Google Scholar] [CrossRef]
- Hortensius, R.A.; Ebens, J.H.; Dewey, M.J.; Harley, B.A.C. Incorporation of the Amniotic Membrane as an Immunomodulatory Design Element in Collagen Scaffolds for Tendon Repair. ACS Biomater. Sci. Eng. 2018, 4, 4367–4377. [Google Scholar] [CrossRef]
- Yang, J.J.; Jang, E.C.; Song, K.S.; Lee, J.S.; Kim, M.K.; Chan, S.H. The Effect of Amniotic Membrane Transplantation on Tendon-Healing in a Rabbit Achilles Tendon Model. Tissue Eng. Regen. Med. 2010, 7, 323–329. [Google Scholar]
- Hortensius, R.A.; Ebens, J.H.; Harley, B.A.C. Immunomodulatory Effects of Amniotic Membrane Matrix Incorporated into Collagen Scaffolds. J. Biomed. Mater. Res. A 2016, 104, 1332–1342. [Google Scholar] [CrossRef] [Green Version]
- Sari, R.; Larasati, G.S.; Kuncorowati, N.G.; Syaify, A. Platelet-Rich Fibrin (PRF) Membranes Accelerate Open Wound Healing Better than Amniotic Membranes: A Histological Study on the Proliferation Phase. Wound Med. 2020, 31, 100190. [Google Scholar] [CrossRef]
- Islam, M.M.; Hossain, M.L.; Diba, F.; Hasan, M.Z.; Juliana, F.M.; Asaduzzaman, S.M. The Combined Effect of Amniotic Membrane and Moringa Oleifera Leaves Derived Gel for Wound and Burn Healing in Rat Model. Regen. Eng. Transl. Med. 2018, 4, 177–186. [Google Scholar] [CrossRef]
- Rana, M.M.; Rahman, M.S.; Ullah, M.A.; Siddika, A.; Hossain, M.L.; Akhter, M.S.; Hasan, M.Z.; Asaduzzaman, S.M. Amnion and Collagen-Based Blended Hydrogel Improves Burn Healing Efficacy on a Rat Skin Wound Model in the Presence of Wound Dressing Biomembrane. Biomed. Mater. Eng. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A Review on Tissue Engineering, Application, and Storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Kesting, M.R.; Stoeckelhuber, M.; Von Bomhard, A. Evaluation of Tissue-Engineered Skin on Base of Human Amniotic Membrane for Wound Healing. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2320. [Google Scholar] [CrossRef]
- Riha, S.M.; Maarof, M.; Fauzi, M.B. Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers 2021, 13, 1546. [Google Scholar] [CrossRef]
- Tian, B.; Song, L.; Liang, T.; Li, Z.; Ye, X.; Fu, Q.; Li, Y. Repair of Urethral Defects by an Adipose Mesenchymal Stem Cell-Porous Silk Fibroin Material. Mol. Med. Rep. 2018, 18, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Aygün, H.; Çakar, A.; Atilla, H.A. Tendon Healing and Repair: A Review of Current Approaches. Kafkas Univ. Vet. Fak. Derg. 2013, 19, A243–A248. [Google Scholar] [CrossRef]
- Prakash, S.; Kalra, P.; Dhal, A. Flexor Tendon Repair with Amniotic Membrane. Int. Orthop. 2020, 44, 2037–2045. [Google Scholar] [CrossRef]

| No | Application | Preparation Method | Experimental Settings (Target Tissue/Cells) | Ref. |
|---|---|---|---|---|
| 1 | Wound Healing | HAM de-epithelialization | In vitro (human epidermal keratinocytes) | [40] |
| Lyophilized amnion membrane powder, Amnion hydrogel | In vivo (porcine skin) | [33] | ||
| Fresh HAM | In vivo (porcine skin) | [41] | ||
| Dried amniotic membranes | In vitro (human keratinocytes (HaCaT), human fibroblasts (HFF1 cell line); in vivo (Wistar rats)) | [42] | ||
| Dried AM | In vivo (porcine skin) | [34] | ||
| Dehydrated human amniotic membrane allografts | Clinical exam (4 patients) | [43] | ||
| HAM de-epithelialization | In vivo (rabbit) | [22] | ||
| Dried human acellular amniotic membrane (hAAM) | In Vitro (NS) | [44] | ||
| Dried amniotic membrane | Clinical exams (20 patients) | [19] | ||
| Decellularized HAM | In vivo (mice) | [45] | ||
| Dried amniotic membrane | In vivo (mice) | [46] | ||
| Freeze Dried HAM | Clinical exams | [47] | ||
| Conditioned media (CM) of hAMSC | In vitro (human mesenchymal stem cell adipogenic differentiation medium); in vivo (mice) | [2] | ||
| Decellularized human amniotic membrane | In vivo (Wistar rats) | [48] | ||
| Decellularization of amniotic membrane, AM Hydrogel | In vitro (human fibroblasts); In vivo (rabbits) | [49] | ||
| Human dehydrated amniotic membrane | In vitro (TGF-β3); in vivo (Wistar rats) | [50] | ||
| Conditioned Media of hAMSC and hAEC | In vitro (lysyl oxidase-like 2 (LOXL2); in vivo (mice)) | [35] | ||
| Decellularized Bovine Amniotic membrane | In vitro (human foreskin fibroblasts; in vivo (mice)) | [51] | ||
| Decellularized Amniotic membrane | Clinical exams (5 patients) | [52] | ||
| Dehydrated human amnion/chorion membrane (dHACM) | Clinical exams (65 patients) | [53] | ||
| Dehydrated human amnion and chorion allograft (dHACA) | Clinical exams (72 patients) | [54] | ||
| Decellularized human amniotic membrane | In vivo (VEGF, α-SMA, TGF-β1; rats) | [29] | ||
| Lyophilized Bovine AM | In vitro (human foreskin fibroblasts); in vivo (rabbits) | [55] | ||
| 2 | Burn Injury | Cryopreserved HAM | In vivo (porcine skin; clinical exam) | [24] |
| Decellularized and lyophilized human amniotic membrane | In vivo (rats) | [26] | ||
| Dehydrated human amnion chorion membranes (dHACM) | Clinical exam (30 patients) | [56] | ||
| Dried AM | Clinical exam (35 patients) | [57] | ||
| Fresh HAM | In vivo (male rats) | [58] | ||
| Hyperdry human amniotic membrane | In vivo (mice) | [59] | ||
| Dried amniotic membrane | Clinical exams (42 patients) | [60] | ||
| Cryopreserved HAM | In vivo (rats) | [61] | ||
| Fresh AM | In vivo (rats) | [62] | ||
| 3 | Tissue engineering (TE) skin | Decellularization of amniotic membrane | In vitro (NS) | [63] |
| Fresh AM | In vivo (rats) | [64] | ||
| Amniotic epithelial cells (AECs) | In vitro | [65] | ||
| Fresh HAM | In vivo (Wistar rats) | [23] | ||
| Fresh HAM | Clinical exams (30 patients) | [66] | ||
| De-epithelialization of the hAM | In vitro (keratinocytes and fibroblasts) | [27] | ||
| Fresh AM | In vivo (Wistar rats) | [18] | ||
| Fresh AM | In vivo (Wistar rats) | [20] | ||
| Isolation of human amniotic mesenchymal stem cells and human amniotic epithelial cells | In vitro (phycoerythrinor fluorescein isothiocyanate-conjugated monoclonal antibodies against human CD34, CD73, CD90, CD105, and HLA-DR) | [36] | ||
| 4 | Junctional Epidermolysis Bullosa | AM eye drop | Clinical exams (NS) | [67] |
| 5 | Urethral Defects | Decellularized Amniotic Membrane | In vitro (NS); in vivo (rabbits) | [68] |
| 6 | Calciphylaxis | Cryopreserved human amniotic membrane | Clinical exams (1 patient) | [69] |
| Isolation of human amnion-derived mesenchymal stem cells (hAMSC) | Preclinical exams (mice and rats) | [70] | ||
| 7 | Tendon healing | Amniotic suspension allograft | Preclinical exams (rats) | [71] |
| Fresh AM | In vivo (Wistar rats) | [72] | ||
| Dehydrated human amnion/chorion membranes (dACMs) | In vivo (rats) | [73] | ||
| Fresh AM | Clinical exams (17 patients) | [74] | ||
| Lyophilized AM | In vitro (interleukin 6 (IL-6) and interleukin 8 (IL-8)) | [75] | ||
| Decellularized bovine amniotic membrane | In vivo (rabbit) | [76] | ||
| Decellularized AM | In vitro (TGF-β1) | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriani, N.; Wilar, G.; Narsa, A.C.; Mohammed, A.F.A.; Wathoni, N. Application of Amniotic Membrane in Skin Regeneration. Pharmaceutics 2023, 15, 748. https://doi.org/10.3390/pharmaceutics15030748
Fitriani N, Wilar G, Narsa AC, Mohammed AFA, Wathoni N. Application of Amniotic Membrane in Skin Regeneration. Pharmaceutics. 2023; 15(3):748. https://doi.org/10.3390/pharmaceutics15030748
Chicago/Turabian StyleFitriani, Nurul, Gofarana Wilar, Angga Cipta Narsa, Ahmed F. A. Mohammed, and Nasrul Wathoni. 2023. "Application of Amniotic Membrane in Skin Regeneration" Pharmaceutics 15, no. 3: 748. https://doi.org/10.3390/pharmaceutics15030748








