Spanlastics as a Potential Approach for Enhancing the Nose-To-Brain Delivery of Piperine: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Management of Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation of Piperine Loaded Spanlastics (PIP-SPL)
2.2. Optimization of Spanlastics Using Box–Behnken Design (BBD)
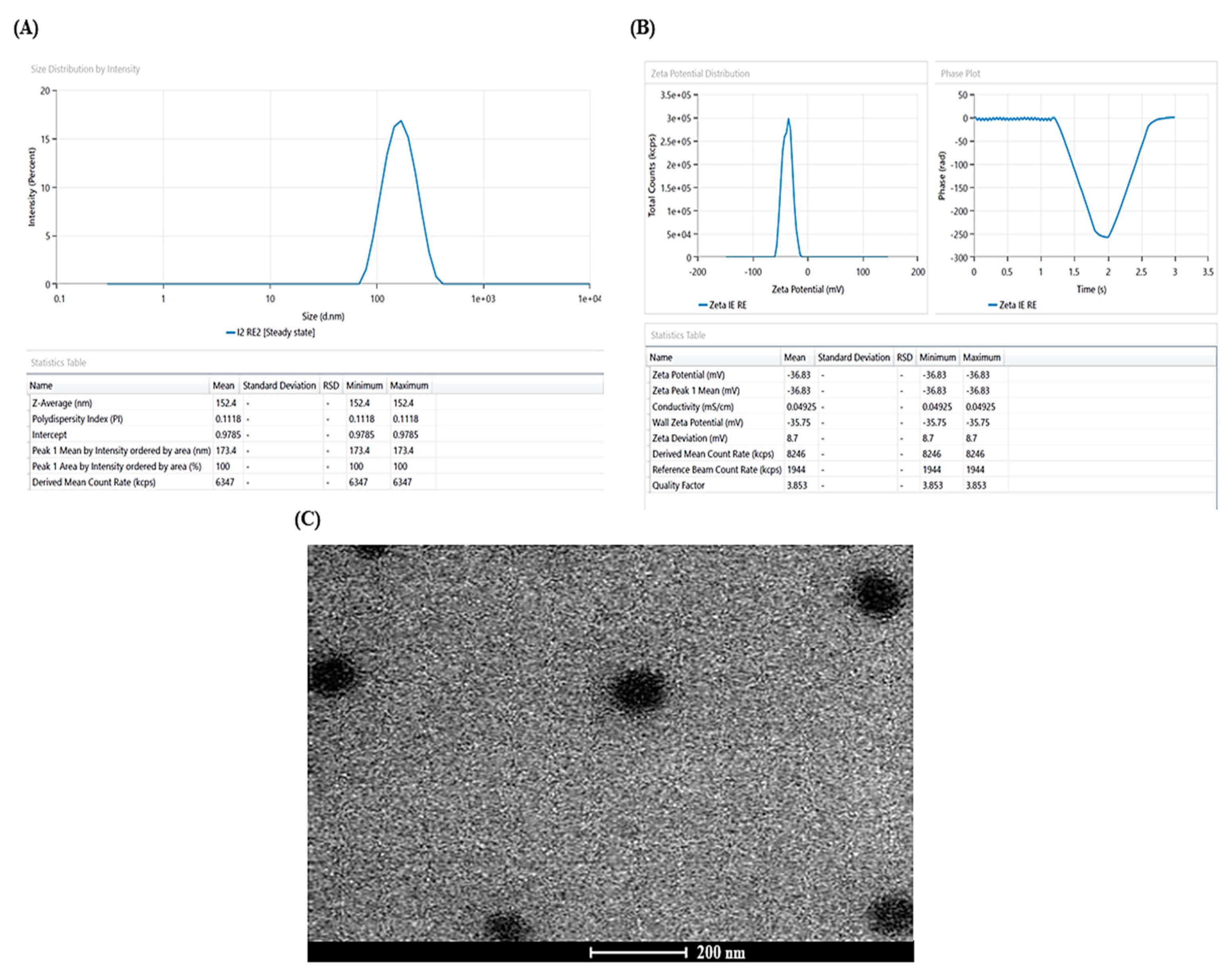
2.3. Characterization of PIP-SPLopt
2.3.1. Vesicle Size, Zeta Potential, and Polydispersity Index (PDI)
2.3.2. Entrapment Efficiency
2.4. Spanlastics Morphology
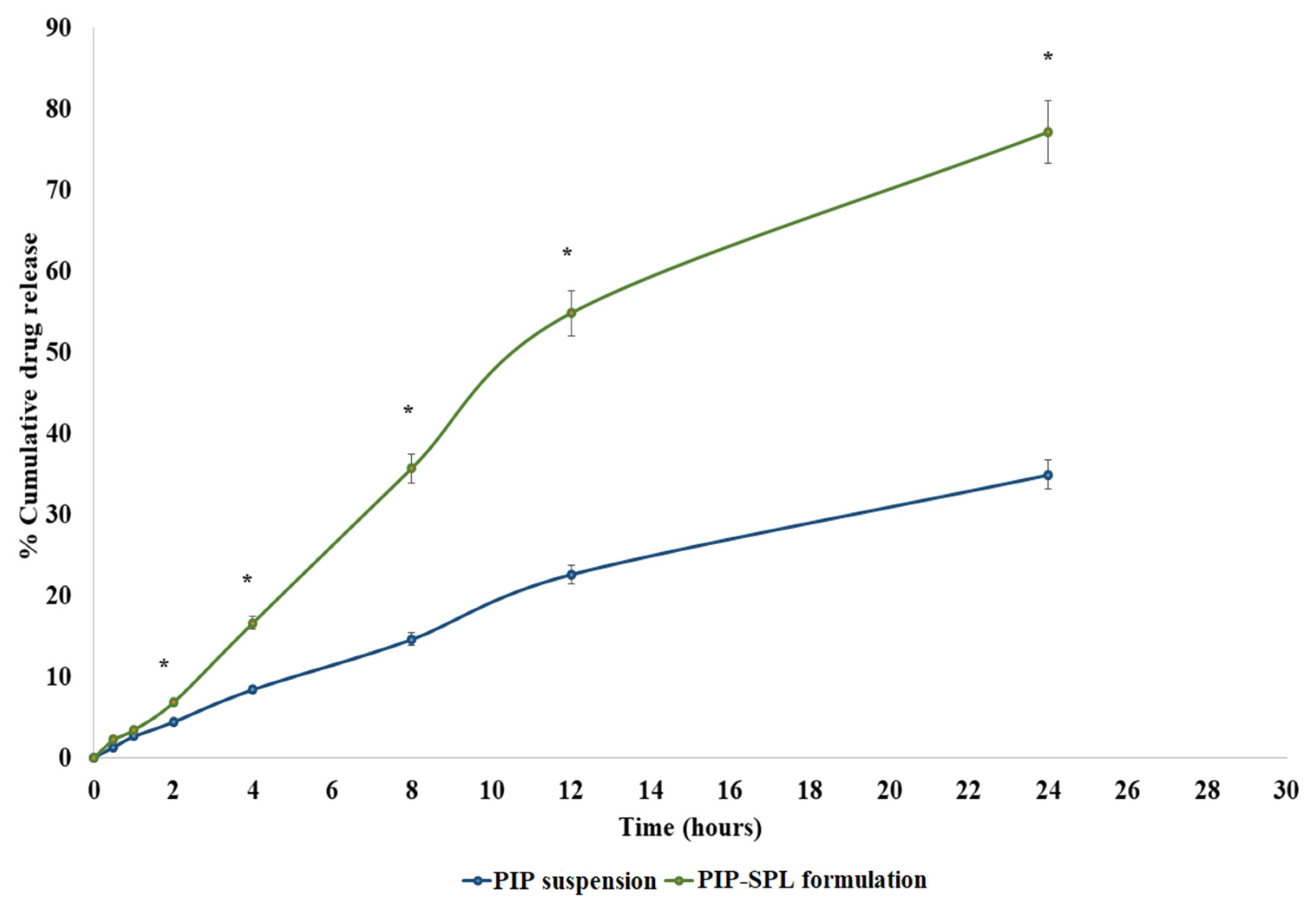
2.5. In Vitro PIP Release Study
2.6. Nasal Permeation Study
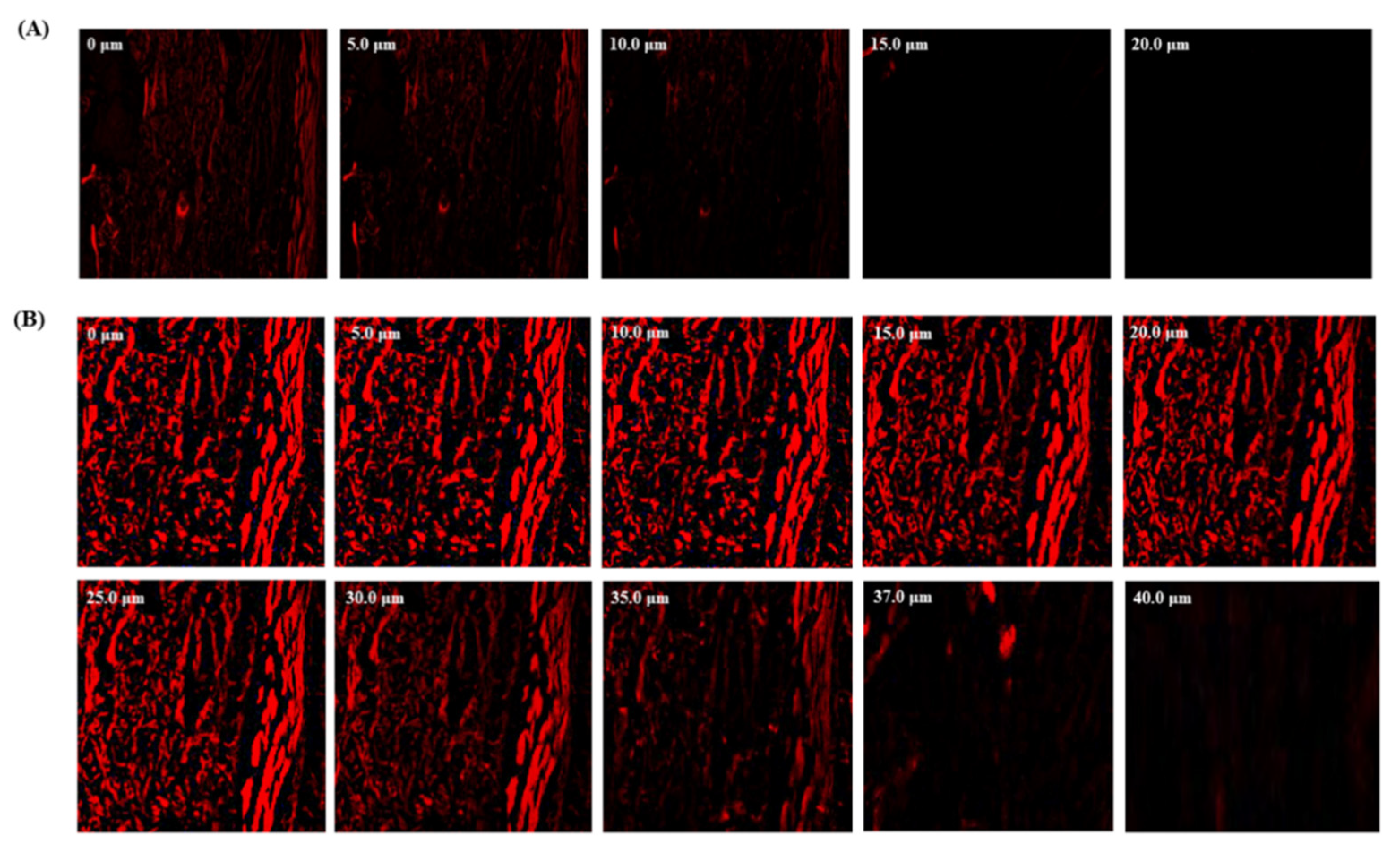
2.7. Confocal Laser Scanning Microscopy (CLSM)
2.8. Nasal Toxicity Study
2.9. Blood-Brain Distribution Study
2.10. Anticonvulsant Activity
2.10.1. Pentylenetetrazole (PTZ)-Induced Seizure
2.10.2. Effect on Neuromuscular Coordination
2.10.3. Biochemical Estimation
2.10.4. Histopathological Study
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of PIP-SPL by BBD
3.1.1. Response (1): Effect of Independent Variables on PDI
3.1.2. Response (2): Effect of Independent Variables on Vesicle Size
3.1.3. Response (3): Effect of Independent Variables on Entrapment Efficiency
3.2. Characterization
3.3. Spanlastic Morphology
3.4. In Vitro Drug Release Study
3.5. Nasal Permeation Study
3.6. Confocal Laser Scanning Microscopy
3.7. Nasal Toxicity Study
3.8. Blood–Brain Distribution Study
3.9. Anticonvulsant Activity
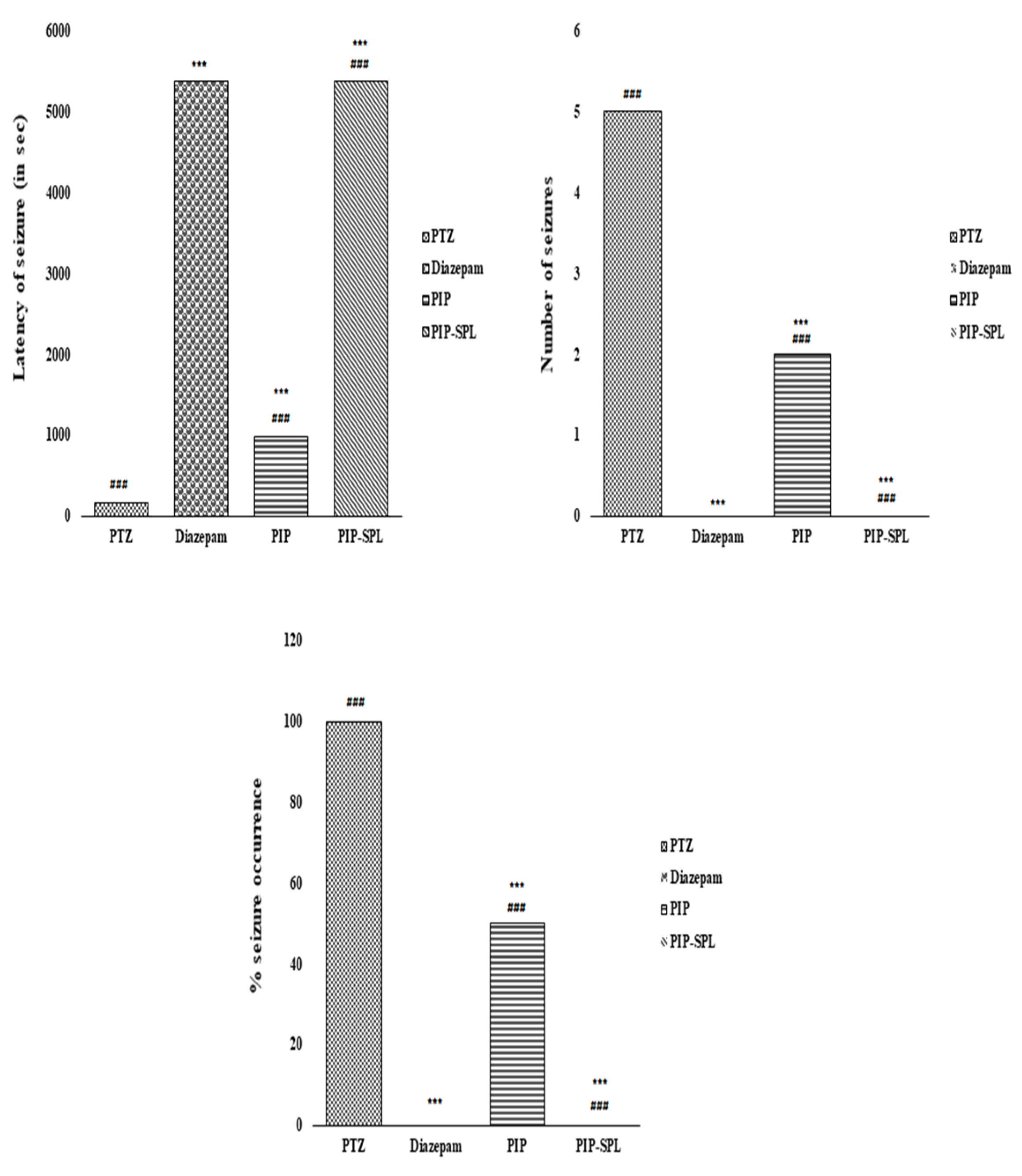
3.9.1. Effect of PIP-SPL Formulation on PTZ-Induced Seizures in Rats
3.9.2. Effect of PIP-SPL Formulation on Neuromuscular Coordination in Rats
3.9.3. Effect of PIP-SPL on PTZ Kindling-Induced Oxidative Biomarkers on the Brain
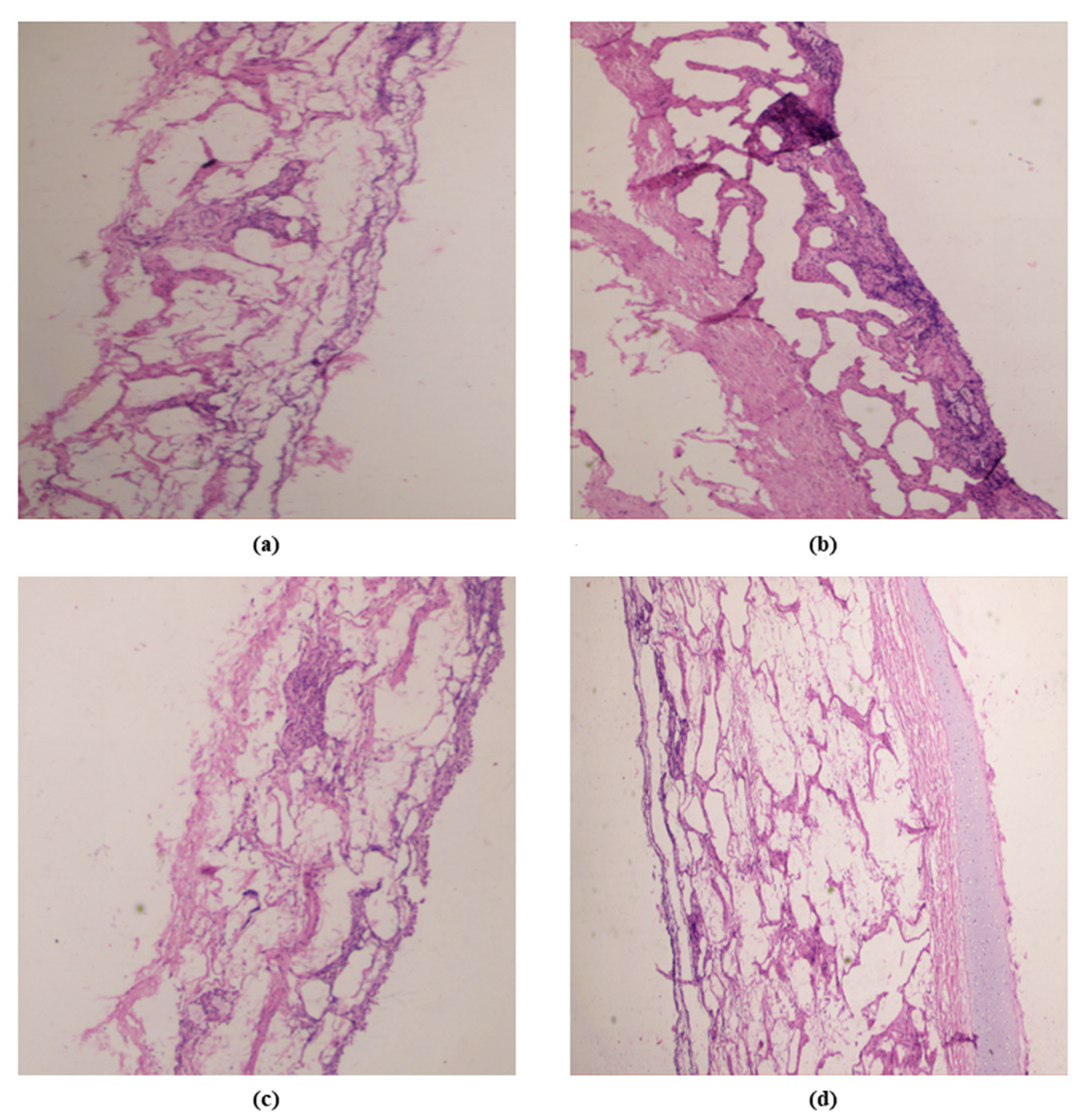
3.9.4. Histological Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scharfman, H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- Hesdorffer, D.C.; Beck, V.; Begley, C.E.; Bishop, M.L.; Cushner-Weinstein, S.; Holmes, G.L.; Shafer, P.O.; Sirven, J.I.; Austin, J.K. Research implications of the Institute of medicine report, epilepsy across the Spectrum: Promoting health and understanding. Epilepsia 2013, 54, 207–216. [Google Scholar] [CrossRef]
- Uruaka, C.I.; Georgwill, O. Evaluation of the anticonvulsant, hypnotic and anxiolytic-like effects of methanol seed extract of Dennettia tripetala in mice. J. Afr. Ass. Physiol. Sci. 2020, 8, 41–49. [Google Scholar]
- Ettinger, A.B.; Argoff, C.E. Use of antiepileptic drugs for nonepileptic conditions: Psychiatric disorders and chronic pain. Neurotherapeutics 2007, 4, 75–83. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Katz, A.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017, 76, 24–31. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Ali, A.; Aqil, M.; Mujeeb, M.; Sultana, Y. Investigation on utility of some novel terpenes on transungual delivery of fluconazole for the management of onychomycosis. J. Cosmet. Dermatol. 2022, 21, 5103–5110. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Aqil, M.; Mujeeb, M.; Sultana, Y. Investigation on Utility of Some Novel Terpenes on Transungual Delivery of Terbinafine for the Management of Onychomycosis. Dermatol. Pract. Concept. 2022, e2022202. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Aqil, M.; Mujeeb, M. Application of QbD based approach in development and validation of RP-HPLC method for simultaneous estimation of Methotrexate and Baicalin in Dual-Drug Loaded Liposomes. Biomed. Chromatogr. 2023, e5581. [Google Scholar] [CrossRef]
- Karri, V. Piperine: A valuable alkaloid from piper species. Int. J. Pharm. Sci. 2014, 6, 34–38. [Google Scholar]
- Adin, S.N.; Gupta, I.; Ahad, A.; Aqil, M.; Mujeeb, M. A developed high-performance thin-layer chromatography method for the determination of baicalin in Oroxylum indicum L. and its antioxidant activity. JPC-J Planar. Chromat. 2022, 35, 383–393. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Aqil, M.; Mujeeb, M. Computer-aided Box-Behnken outlook towards optimization of extraction of baicalin from Oroxylum indicum L. stem barks. Pharmacogn. Mag. 2022, 18, 808–814. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Aqil, M.; Mujeeb, M.; Ahad, A. BBD Driven Optimization of Extraction of Therapeutically Active Xanthanoid Mangiferin from Mangifera indica L. Leaves and its Antioxidant Activity. Pharmacogn. Res. 2023, 15, 84–93. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Aqil, M. High-performance thin-layer chromatography method development and validation for quantification of naringin in different extracts of Citrus sinensis L. and its antioxidant activity. JPC-J Planar. Chromat. 2022, 35, 463–471. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Aqil, M.; Mujeeb, M.; Sultana, Y. Quality by design-based development and validation of an HPLC method for simultaneous estimation of pregabalin and piperine in dual drug-loaded liposomes. Biomed Chromatogr. 2022, 37, e5510. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Adin, S.N.; Aqil, M.; Mujeeb, M. QbD-based extraction of Naringin from Citrus sinensis L. peel and its antioxidant activity. Pharmacogn. Res. 2023, 15, 145–154. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Aqil, M.; Mujeeb, M.; Ahad, A. COMPUTER-AIDED BOX–BEHNKEN OUTLOOK TOWARDS OPTIMIZATION OF EXTRACTION OF PIPERINE FROM PIPER LONGUM L. FRUITS. World J. Pharm. Res. 2022, 11, 1439–1455. [Google Scholar] [CrossRef]
- Khandhar, A.; Patel, S.; Patel, A.; Zaveri, M.; Lecturer, S. Chemistry and pharmacology of Piper Longum L. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 67–76. [Google Scholar]
- Kaur, J.; Famta, P.; Famta, M.; Mehta, M.; Satija, S.; Sharma, N.; Vyas, M.; Khatik, G.L.; Chellappan, D.K.; Dua, K.; et al. Potential Anti-epileptic Phytoconstituents: An Updated Review. J. Ethnopharmacol. 2021, 268, 113565. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, R.; Jafarzadeh, S.; Kashi, T.; Dinarvand, R.; Tahriri, M.; Rakhshan, V.; Esfandyari-Manesh, M. Preparation, Characterization and Evaluation of Drug Release Properties of Simvastatin-loaded PLGA Microspheres. Iran. J. Pharm. Res. 2016, 15, 205–211. [Google Scholar] [PubMed]
- Praveen, A.; Aqil, M.; Imam, S.; Ahad, A.; Moolakkadath, T.; Ahmad, F. Lamotrigine encapsulated intra-nasal nanoliposome formulation for epilepsy treatment: Formulation design, characterization and nasal toxicity study. Colloids Surf. B Biointerfaces 2018, 174, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [PubMed]
- Beyrami, M.; Karimi, E.; Oskoueian, E. Synthesized chrysin-loaded nanoliposomes improves cadmium-induced toxicity in mice. Environ. Sci. Pollut. 2020, 27, 40643–40651. [Google Scholar] [CrossRef]
- Moeini, S.; Karimi, E.; Oskoueian, E. Antiproliferation effects of nanophytosome-loaded phenolic compounds from fruit of Juniperus polycarpos against breast cancer in mice model: Synthesis, characterization and therapeutic effects. Cancer Nano 2022, 13, 20. [Google Scholar] [CrossRef]
- Badria, F.; Mazyed, E. Formulation of Nanospanlastics as a Promising Approach for Improving the Topical Delivery of a Natural Leukotriene Inhibitor (3-Acetyl-11-Keto-β-Boswellic Acid): Statistical Optimization, in vitro Characterization, and ex vivo Permeation Study. Drug Des. Dev. Ther. 2020, 14, 3697–3721. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Hareeri, R.H.; Bazuhair, M.; Alfaleh, M.A.; Alhakamy, N.A.; Fahmy, U.A.; Alamoudi, A.A.; Badr-Eldin, S.M.; Ahmed, O.A.; AlGhamdi, S.A.; et al. Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment. Pharmaceutics 2022, 14, 2627. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Khalifa, M.; Shawky, S.; Bani-Ali, A.; Eassa, H. Zolmitriptan Intranasal spanlastics for enhanced migraine treatment; Formulation parameters optimized via Quality by Design Approach. Sci. Pharm. 2021, 89, 24. [Google Scholar] [CrossRef]
- Abdelmonem, R.; El Nabarawi, M.; Attia, A. Development of novel bioadhesive granisetron hydrochloride spanlastic gel and insert for brain targeting and study their effects on rats. Drug Deliv. 2018, 25, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yassin, G.E.; Amer, R.I.; Fayez, A.M. Carbamazepine Loaded Vesicular Structures for Enhanced Brain Targeting via Intranasal Route: Optimization, in Vitro Evaluation, and in Vivo Study. Int. J. Appl. Pharm. 2019, 11, 264–274. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, G.; Guan, H.; Li, M.; Liu, Y.; Tian, B.; He, Z.; Fu, Q. Naringenin nanocrystals for improving anti-rheumatoid arthritis activity. Asian J. Pharm. Sci. 2021, 16, 816–825. [Google Scholar] [CrossRef]
- Kola, P.K.; Akula, A.; NissankaraRao, L.S.; Danduga, R.C.S.R. Protective effect of naringin on pentylenetetrazole (PTZ)-induced kindling; possible mechanisms of antikindling, memory improvement, and neuroprotection. Epilepsy Behav. 2017, 75, 114–126. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.; Yu, W.; Cao, J.; Li, X. Rhein attenuates PTZ-induced epilepsy and exerts neuroprotective activity via inhibition of the TLR4-NFκB signaling pathway. Neurosci. Lett. 2021, 758, 136002. [Google Scholar] [CrossRef] [PubMed]
- Saleha, R.; Bushra, N.; Amaan, J.; Tahira, K.; Ashif, I.; Mohammad, J.A.; Sanjula, B.; Javed, A. Unraveling enhanced brain delivery of paliperidone-loaded lipid nanoconstructs: Pharmacokinetic, behavioral, biochemical, and histological aspects. Drug Deliv. 2020, 29, 1409–1422. [Google Scholar] [CrossRef]
- Shafaei, N.; Barkhordar, S.M.A.; Rahmani, F.; Nabi, S.; Idliki, R.B.; Alimirzaei, M.; Karimi, E.; Oskoueian, E. Protective Effects of Anethum graveolens Seed’s Oil Nanoemulsion Against Cadmium-Induced Oxidative Stress in Mice. Biol. Trace Elem. Res. 2020, 198, 583–591. [Google Scholar] [CrossRef] [PubMed]







| Variables | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Independent variables | |||
| A: phospholipon 90G (mg) | 50 | 60 | 70 |
| B: span 60 (mg) | 10 | 15 | 20 |
| C: Sodium cholate (mg) | 10 | 15 | 20 |
| Dependent Variable | |||
| Y1: PDI Y2: Vesicle size (nm) Y3: Entrapment efficiency (%) | |||
| Formulation | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | Y3 | |
| 1 | 60 | 15 | 15 | 0.1118 ± 0.007 | 152.4 ± 4.09 | 72.93 ± 1.34 |
| 2 | 70 | 20 | 15 | 0.1575 ± 0.003 | 136.12 ± 3.92 | 66.89 ± 1.68 |
| 3 | 50 | 15 | 10 | 0.1192 ± 0.009 | 168.81 ± 2.98 | 74.18 ± 2.09 |
| 4 | 70 | 15 | 20 | 0.1241 ± 0.009 | 144.71 ± 2.98 | 63.32 ± 2.09 |
| 5 | 60 | 15 | 15 | 0.1112 ± 0.003 | 152.1 ± 3.92 | 72.56 ± 1.68 |
| 6 | 60 | 15 | 15 | 0.1123 ± 0.003 | 151.9 ± 3.92 | 73.03 ± 1.68 |
| 7 | 50 | 15 | 20 | 0.1423 ± 0.003 | 119.21 ± 4.03 | 56.1 ± 2.98 |
| 8 | 60 | 15 | 15 | 0.1116 ± 0.012 | 152.7 ± 4.26 | 73.07 ± 1.62 |
| 9 | 60 | 20 | 10 | 0.1156 ± 0.005 | 148.29 ± 4.36 | 70.26 ± 1.72 |
| 10 | 70 | 10 | 15 | 0.2789 ± 0.003 | 203.24 ± 4.67 | 78.12 ± 1.87 |
| 11 | 60 | 15 | 15 | 0.1108 ± 0.009 | 152.04 ± 2.98 | 72.89 ± 2.09 |
| 12 | 60 | 10 | 10 | 0.2074 ± 0.004 | 217.12 ± 4.23 | 79.62 ± 1.62 |
| 13 | 50 | 20 | 15 | 0.1408 ± 0.003 | 113.43 ± 3.92 | 53.12 ± 1.68 |
| 14 | 70 | 15 | 10 | 0.2734 ± 0.002 | 192.43 ± 2.09 | 79.51 ± 1.76 |
| 15 | 60 | 10 | 20 | 0.1207 ± 0.004 | 166.52 ± 2.32 | 71.03 ± 1.23 |
| 16 | 60 | 20 | 20 | 0.0792 ± 0.008 | 99.92 ± 3.07 | 42.41 ± 2.09 |
| 17 | 50 | 10 | 15 | 0.1532 ± 0.003 | 176.69 ± 3.92 | 79.54 ± 1.68 |
| Quadratic model | R2 | Adjusted R2 | Predicted R2 | S.D. | %CV | |
| Response (Y1) | 0.9998 | 0.9997 | 0.9980 | 0.0010 | 0.7202 | |
| Response (Y2) | 0.9998 | 0.9996 | 0.9979 | 0.5750 | 0.3688 | |
| Response (Y3) | 0.9995 | 0.9990 | 0.9941 | 0.3316 | 0.4783 | |
| Release Kinetics | R2 | Equation | X-Axis | Y-Axis |
|---|---|---|---|---|
| Korsmeyer–Peppas | 0.989 | Mt/M∞ = Ktn | Log fraction of drug released | Log time |
| Higuchi | 0.952 | Mt = M0 + kht1/2 | Fraction of drug released | √time |
| Zero-order release | 0.971 | Mt = M0 + k0 t | Fraction of drug released | time |
| First-order release | 0.976 | ln Mt = ln M0 + k1 t | Log % drug remaining | time |
| Pharmacokinetic Parameters | PIP-SPL Oral (In Blood) | PIP-SPL Nasal (In Blood) |
| Cmax | 3.46 ± 1.91 a µg/mL | 7.72 ± 0.51 b µg/mL |
| Tmax | 1 a h | 1 b h |
| T1/2 | 9 a h | 12 b h |
| AUC0–24h | 22.68 ± 9.76 a µg·h/mL | 38.96 ± 7.90 b µg·h/mL |
| Pharmacokinetic Parameters | PIP-SPL Oral (In Brain) | PIP-SPL Nasal (In Brain) |
| Cmax | 5.34 ± 1.12 a µg/mL | 10.24 ± 1.21 b µg/mL |
| Tmax | 1 a h | 1 b h |
| T1/2 | 9 a h | 12 b h |
| AUC0–24h | 49.68 ± 5.63 a µg·h/mL | 61.76 ± 2.36 b µg·h/mL |
| Latency of Myoclonic Jerks (s) | Latency of GTSs (s) | Duration of GTSs (min) | % of Animals Kindled | % Protection | No. of Seizures | Seizure Score | |
|---|---|---|---|---|---|---|---|
| PTZ | 57.6 ± 2.92 a | 252.23 ± 3.07 a | 35.33 ± 2.01 a | 100 a % | 0 a % | 5 a | 5 a |
| Diazepam | Not observed | Not observed | Not observed | 0 b % | 100 b % | 0 b | 0 b |
| PIP suspension | 474 ± 4.75 c | 971.65 ± 6.23 c | 17.23 ± 1.23 c | 50 c % | 50 c % | 2 c | 2 c |
| PIP-SPL formulation | 1263 ± 9.23 d | Not observed | Not observed | 0 d % | 100 d % | 0 d | 0 d |
| Stay Time for Each Rat on Rotarod (in Seconds) | |
|---|---|
| Control | 500 ± 5 a seconds |
| PTZ | 28 ± 2 b seconds |
| Diazepam | 412 ± 3 c seconds |
| PIP suspension | 376 ± 2 d seconds |
| PIP-SPL formulation | 452 ± 4 e seconds |
| MDA (nmol/mg Wet Tissue) | Glutathione (ugmol/gm Wet Tissue) | Catalase (umol/min/g Wet Tissue) | SOD (Unit/mg of Tissue) | |
|---|---|---|---|---|
| Control | 28 ± 2.01 a | 190 ± 5.76 b | 14 ± 1.06 c | 13 ± 0.98 d |
| PTZ | 92 ± 3.92 a | 92 ± 3.07 b | 7 ± 0.21 c | 3.2 ± 0.06 d |
| Diazepam | 36 ± 1.96 a | 180 ± 4.21 b | 12 ± 0.96 c | 10.1 ± 1.23 d |
| PIP suspension | 55 ± 1.75 a | 145 ± 3.23 b | 9 ± 1.23 c | 9.1 ± 0.92 d |
| PIP-SPL formulation | 30 ± 2.23 a | 188 ± 4.76 b | 13 ± 1.87 c | 13.2 ± 1.07 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, I.; Adin, S.N.; Rashid, M.A.; Alhamhoom, Y.; Aqil, M.; Mujeeb, M. Spanlastics as a Potential Approach for Enhancing the Nose-To-Brain Delivery of Piperine: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Management of Epilepsy. Pharmaceutics 2023, 15, 641. https://doi.org/10.3390/pharmaceutics15020641
Gupta I, Adin SN, Rashid MA, Alhamhoom Y, Aqil M, Mujeeb M. Spanlastics as a Potential Approach for Enhancing the Nose-To-Brain Delivery of Piperine: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Management of Epilepsy. Pharmaceutics. 2023; 15(2):641. https://doi.org/10.3390/pharmaceutics15020641
Chicago/Turabian StyleGupta, Isha, Syeda Nashvia Adin, Md Abdur Rashid, Yahya Alhamhoom, Mohd. Aqil, and Mohd. Mujeeb. 2023. "Spanlastics as a Potential Approach for Enhancing the Nose-To-Brain Delivery of Piperine: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Management of Epilepsy" Pharmaceutics 15, no. 2: 641. https://doi.org/10.3390/pharmaceutics15020641