An In Vitro Assessment Method for Chemotherapy-Induced Peripheral Neurotoxicity Caused by Anti-Cancer Drugs Based on Electrical Measurement of Impedance Value and Spontaneous Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Primary DRG Neurons
2.2. Extracellular Recording
2.3. Immunocytochemistry
2.4. Statistics
3. Results
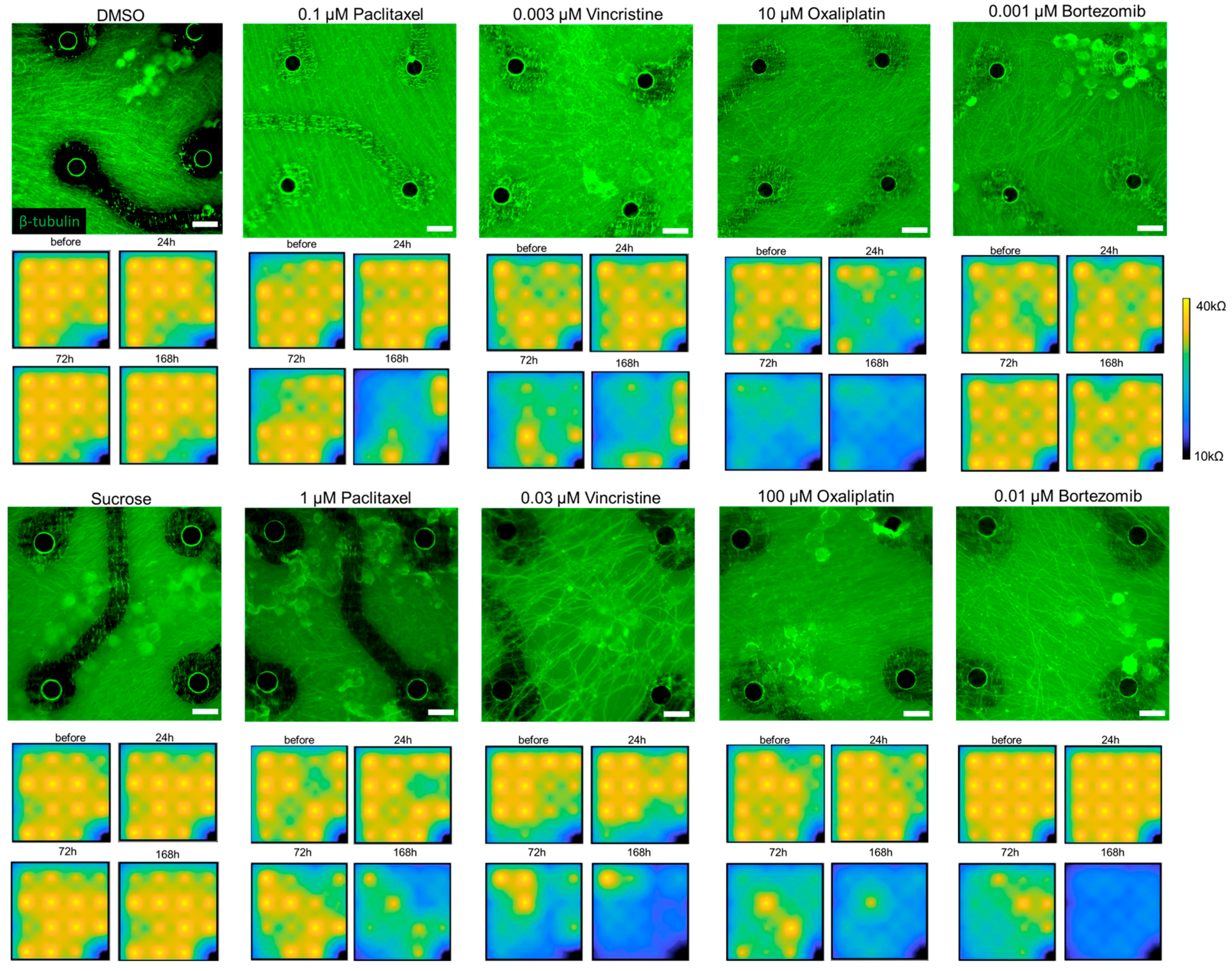
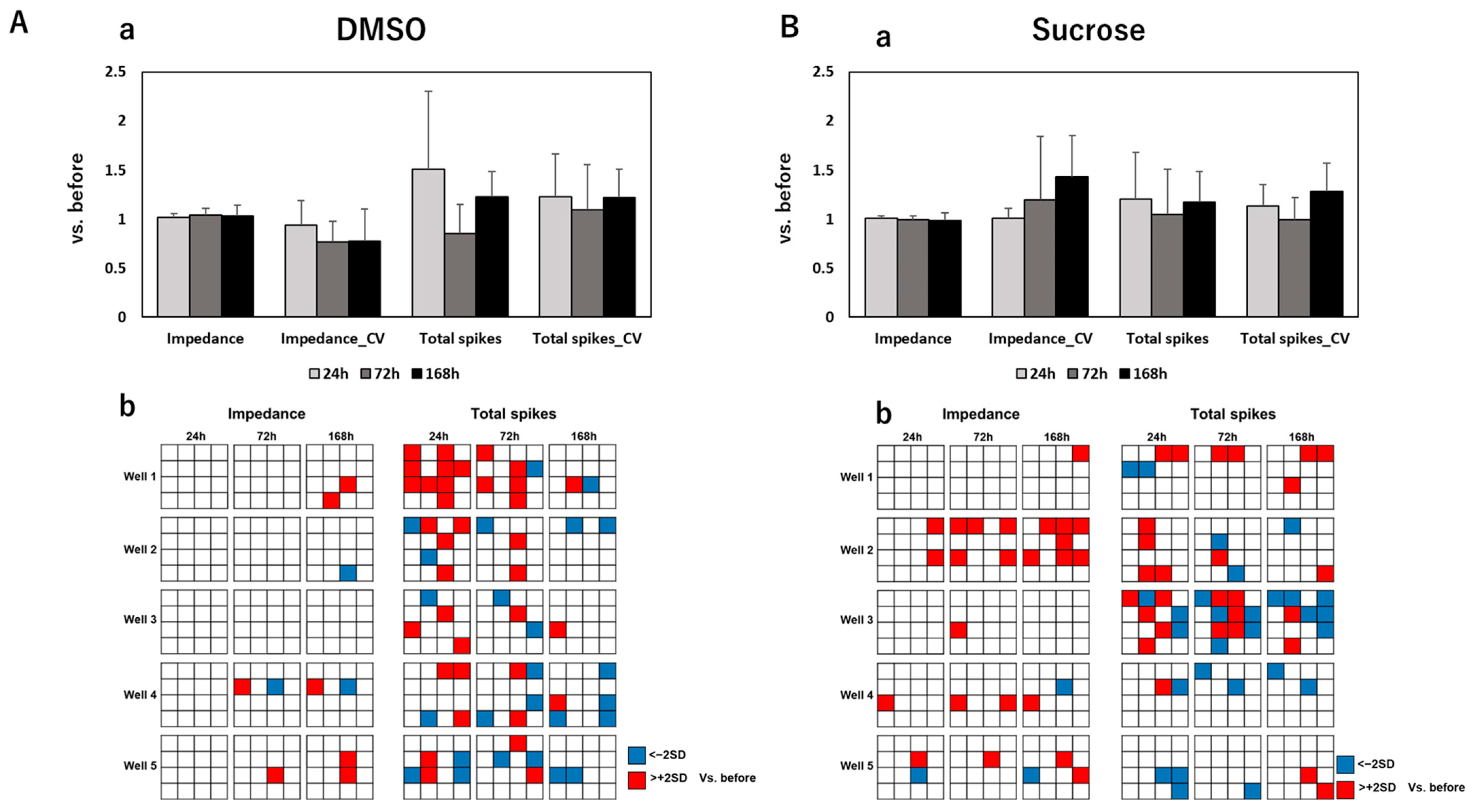
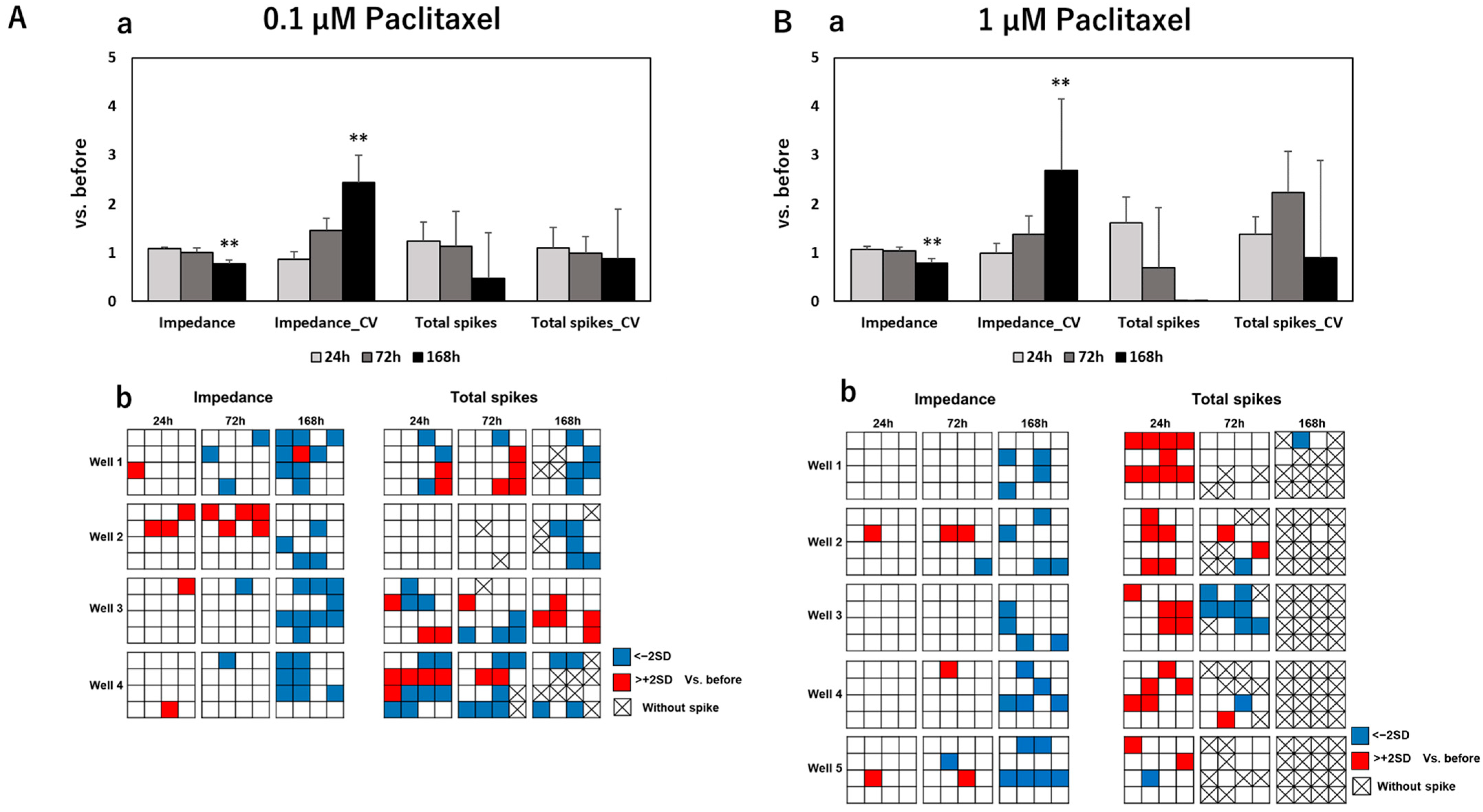
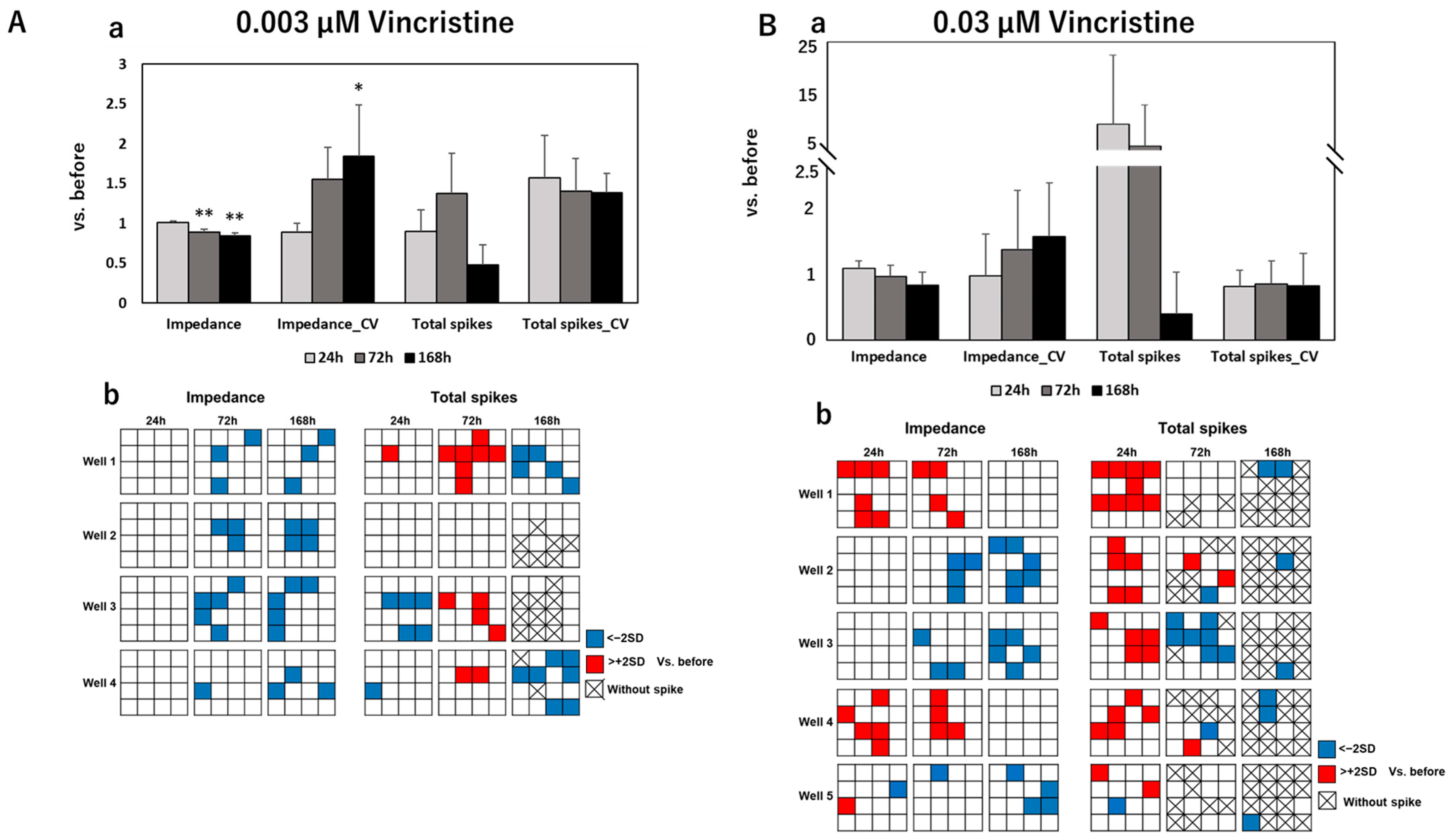
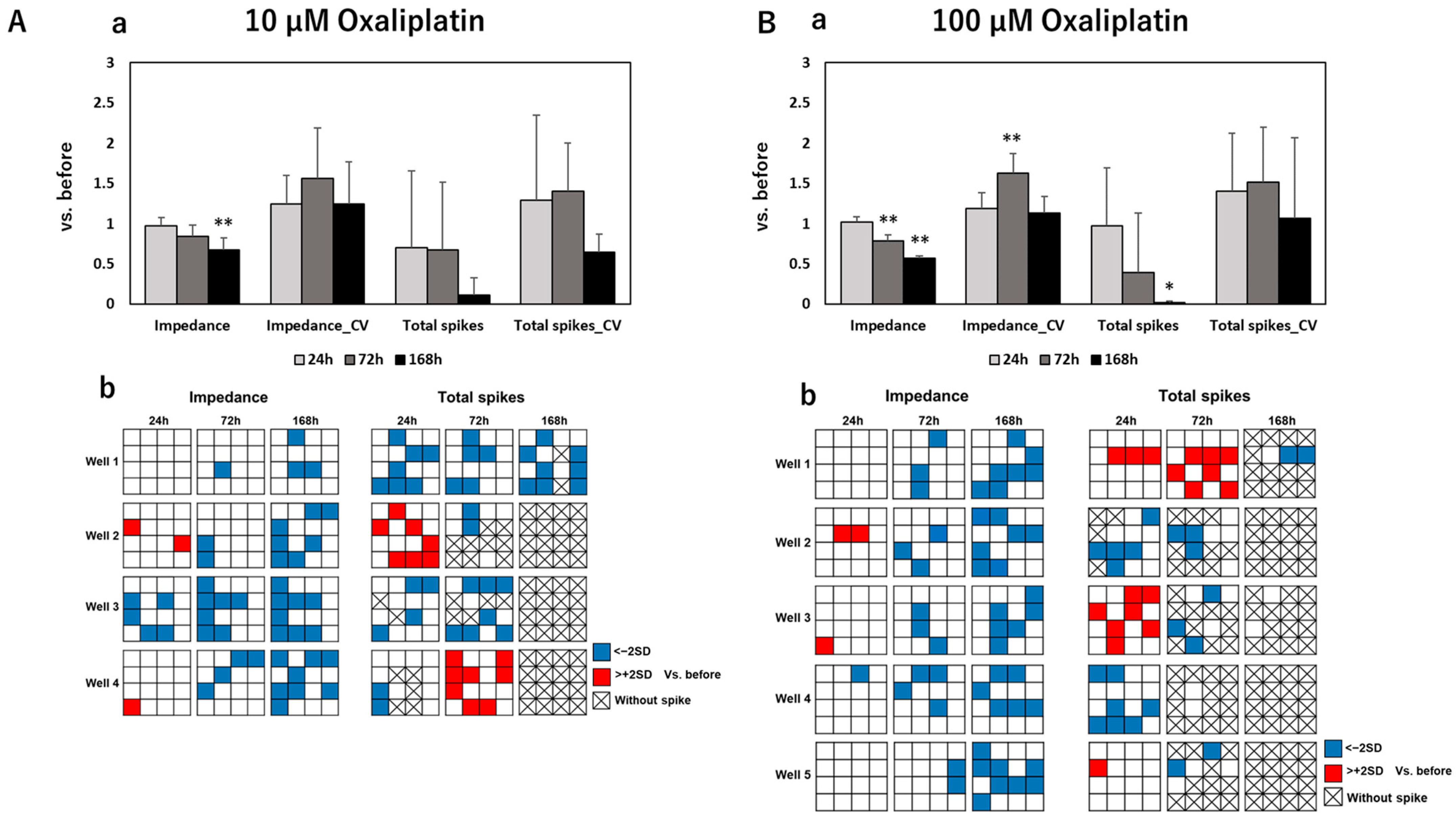
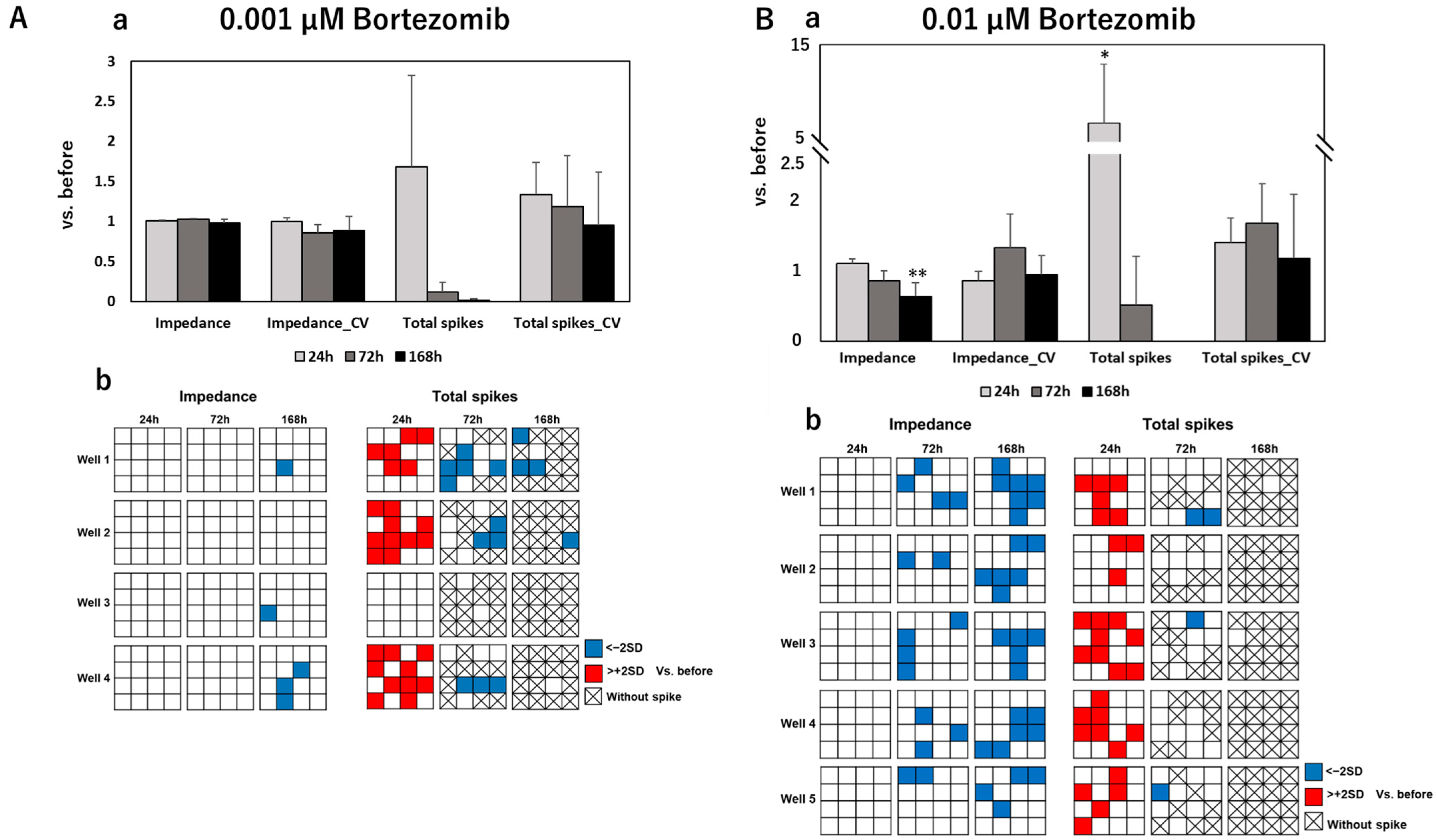
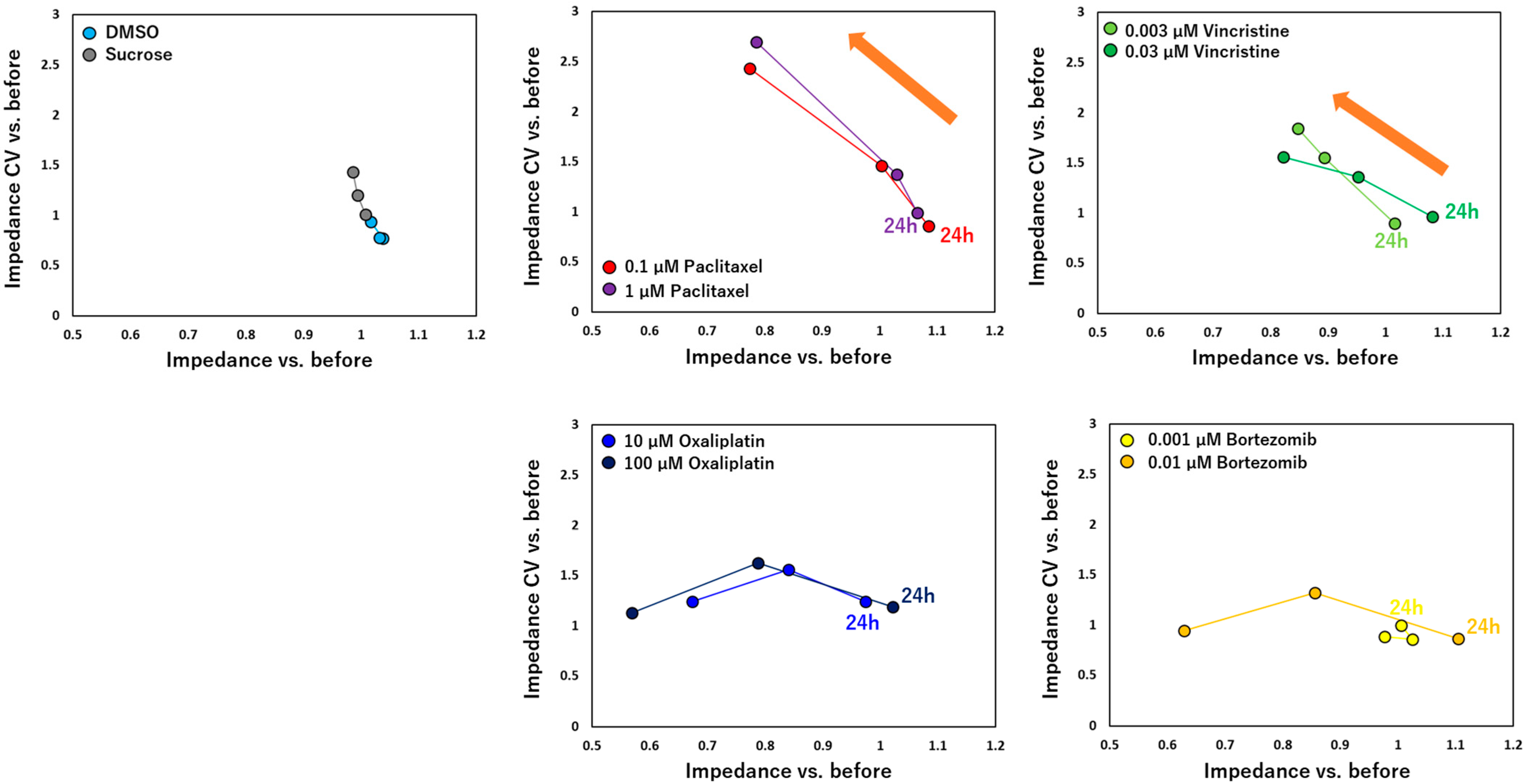
3.1. Morphological and Impedance Changes of Cultured DRG Responding to Anti-Cancer Drugs
3.2. Impedance and Spontaneous Measurements Reflect DRG Neurons’ Response to Anti-Cancer Drugs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhagra, A.; Rao, R.D. Chemotherapy-induced neuropathy. Curr. Oncol. Rep. 2007, 9, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Frigeni, B.; Piatti, M.; Susani, E. Chemotherapy-Induced Neuropathy. Curr. Treat. Options Neurol. 2010, 13, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.; Guo, L.; Hamre, J. A Comparative Review of Chemotherapy-Induced Peripheral Neuropathy in In Vivo and In Vitro Models. Toxicol. Pathol. 2019, 48, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol. 2020, 145, 102831. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, A.; Für, C.S.; Bölcskei, H.; Ilkei, V.; Keglevich, P.; Hazai, L. Results in Chemistry of Natural Organic Compounds. Synthesis of New Anticancer Vinca Alkaloids and Flavone Alkaloids. Chemistry 2020, 2, 714–726. [Google Scholar] [CrossRef]
- Yao, H.; Wang, F.; Chong, H.; Wang, J.; Bai, Y.; Du, M.; Yuan, X.; Yang, X.; Wu, M.; Li, Y.; et al. A Curcumin-Modified Coordination Polymers with ROS Scavenging and Macrophage Phenotype Regulating Properties for Efficient Ulcerative Colitis Treatment. Adv. Sci. 2023, 10, e2300601. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Y.; Gao, Y.; Meng, Z.; Sun, Y.; Bai, Y.; Suen, N.; Chen, H.; Pi, Y.; Pang, H. 2D MOF-assisted Pyrolysis-displacement-alloying Synthesis of High-entropy Alloy Nanoparticles Library for Efficient Electrocatalytic Hydrogen Oxidation. Angew. Chem. Int. Ed. 2023, 62, e202306881. [Google Scholar] [CrossRef]
- Rodwin, R.L.; Siddiq, N.Z.; Ehrlich, B.E.; Lustberg, M.B. Biomarkers of Chemotherapy-Induced Peripheral Neuropathy: Current Status and Future Directions. Front. Pain Res. 2022, 3, 864910. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Malgrange, B.; Delrée, P.; Rigo, J.; Baron, H.; Moonen, G. Image analysis of neuritic regeneration by adult rat dorsal root ganglion neurons in culture: Quantification of the neurotoxicity of anticancer agents and of its prevention by nerve growth factor or basic fibroblast growth factor but not brain-derived neurotrophic factor or neurotrophin-3. J. Neurosci. Methods 1994, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.H.; Siddique, R.; Hosmane, S.; Thakor, N.; Höke, A. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp. Neurol. 2009, 218, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hamre, J.; Eldridge, S.; Behrsing, H.P.; Cutuli, F.M.; Mussio, J.; Davis, M. Multiparametric Image Analysis of Rat Dorsal Root Ganglion Cultures to Evaluate Peripheral Neuropathy-Inducing Chemotherapeutics. Toxicol. Sci. 2017, 156, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Li, Y.; Segal, R.A. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Doan, R.A.; Cheng, G.C.; Cetinkaya-Fisgin, A.; Huang, S.X.; Höke, A.; Milbrandt, J.; DiAntonio, A. Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program. J. Clin. Investig. 2019, 4, e129920. [Google Scholar] [CrossRef]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum–DNA binding. NeuroToxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef]
- Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 1980, 2, 19–31. [Google Scholar] [CrossRef]
- Hofmann, F.; Bading, H. Long term recordings with microelectrode arrays: Studies of transcription-dependent neuronal plasticity and axonal regeneration. J. Physiol. 2006, 99, 125–132. [Google Scholar] [CrossRef]
- Enright, H.A.; Felix, S.H.; Fischer, N.O.; Mukerjee, E.V.; Soscia, D.; Mcnerney, M.; Kulp, K.; Zhang, J.; Page, G.; Miller, P.; et al. Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform. Analyst 2016, 141, 5346–5357. [Google Scholar] [CrossRef] [PubMed]
- Black, B.J.; Atmaramani, R.; Kumaraju, R.; Plagens, S.; Romero-Ortega, M.; Dussor, G.; Price, T.J.; Campbell, Z.T.; Pancrazio, J.J. Adult mouse sensory neurons on microelectrode arrays exhibit increased spontaneous and stimulus-evoked activity in the presence of interleukin-6. J. Neurophysiol. 2018, 120, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Odawara, A.; Shibata, M.; Ishibashi, Y.; Nagafuku, N.; Matsuda, N.; Suzuki, I. In Vitro Pain Assay Using Human iPSC-Derived Sensory Neurons and Microelectrode Array. Toxicol. Sci. 2022, 188, 131–141. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Renna, J.M.; Stukel, J.M.; Willits, R.K.; Engeberg, E.D. Dorsal root ganglia neurite outgrowth measured as a function of changes in microelectrode array resistance. PLoS ONE 2017, 12, e0175550. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Nicolini, G.; Miloso, M.; Bossi, M.; Cavaletti, G.; Windebank, A.J.; Tredici, G. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. 2006, 26, 1065–1070. [Google Scholar] [PubMed]
- Zhang, H.; Dougherty, P.M. Enhanced Excitability of Primary Sensory Neurons and Altered Gene Expression of Neuronal Ion Channels in Dorsal Root Ganglion in Paclitaxel-induced Peripheral Neuropathy. Anesthesiology 2014, 120, 1463–1475. [Google Scholar] [CrossRef]
- Wing, C.; Komatsu, M.; Delaney, S.M.; Krause, M.; Wheeler, H.E.; Dolan, M.E. Application of stem cell derived neuronal cells to evaluate neurotoxic chemotherapy. Stem Cell Res. 2017, 22, 79–88. [Google Scholar] [CrossRef]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef]
- Wei, G.; Gu, Z.; Gu, J.; Yu, J.; Huang, X.; Qin, F.; Li, L.; Ding, R.; Huo, J. Platinum accumulation in oxaliplatin-induced peripheral neuropathy. J. Peripher. Nerv. Syst. 2021, 26, 35–42. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Rossi, G.; Pelizzari, A.M.; Buzio, S.; Tentorio, M.; Broglio, L.; Mancuso, M.; Rinaldi, M.; Scarpelli, M.; Padovani, A. A high-dose bortezomib neuropathy with sensory ataxia and myelin involvement. J. Neurol. Sci. 2007, 263, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Jang, S.Y.; Lee, H.K.; Jung, J.; Suh, D.J.; Seo, S.-Y.; Park, H.T. Pathological adaptive responses of Schwann cells to endoplasmic reticulum stress in bortezomib-induced peripheral neuropathy. Glia 2010, 58, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Egashira, N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Nagayasu, K.; Shirakawa, H.; Kaneko, S. Specific Cellular Effects of Low Bortezomib Concentrations on Purified Cultures of Schwann Cells, Satellite Glial Cells, Macrophages, and Dorsal Root Ganglion Neurons. Biol. Pharm. Bull. 2023, 46, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Nguyen, H.T.; Jacobs, E.; McCoy, L.; Curley, J.L.; Sharma, A.D.; Moore, M.J. Modeling chemotherapy-induced peripheral neuropathy using a Nerve-on-a-chip microphysiological system. Altex 2020, 37, 350–364. [Google Scholar] [CrossRef]
- van de Velde, M.E.; Kaspers, G.L.; Abbink, F.C.; Wilhelm, A.J.; Ket, J.C.F.; van den Berg, M.H. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit. Rev. Oncol. 2017, 114, 114–130. [Google Scholar] [CrossRef]
- Li, G.-Z.; Hu, Y.-H.; Li, D.-Y.; Zhang, Y.; Guo, H.-L.; Li, Y.-M.; Chen, F.; Xu, J. Vincristine-induced peripheral neuropathy: A mini-review. NeuroToxicology 2020, 81, 161–171. [Google Scholar] [CrossRef]
- Zille, M.; Palumbo, A. Approaches to quantify axonal morphology for the analysis of axonal degeneration. Neural Regen. Res. 2022, 18, 309–310. [Google Scholar] [CrossRef]
- Matsuda, K.; Han, X.; Matsuda, N.; Yamanaka, M.; Suzuki, I. Development of an In Vitro Assessment Method for Chemotherapy-Induced Peripheral Neuropathy (CIPN) by Integrating a Microphysiological System (MPS) with Morphological Deep Learning of Soma and Axonal Images. Toxics 2023, 11, 848. [Google Scholar] [CrossRef]
| Compounds Administration Conditions | vs. DMSO | vs. Sucrose | |||||
|---|---|---|---|---|---|---|---|
| 24 h | 72 h | 168 h | 24 h | 72 h | 168 h | ||
| DMSO | 24 h | – | p = 0.54 | p = 0.61 | p = 0.86 | p = 0.68 | p = 0.14 |
| 72 h | p = 0.54 | – | p = 0.99 | p = 0.15 | p = 0.40 | * p = 0.043 | |
| 168 h | p = 0.61 | p = 0.99 | – | p = 0.28 | p = 0.51 | p = 0.09 | |
| Sucrose | 24 h | p = 0.86 | p = 0.15 | p = 0.28 | – | p = 0.77 | p = 0.11 |
| 72 h | p = 0.68 | p = 0.40 | p = 0.51 | p = 0.77 | – | p = 0.81 | |
| 168 h | p = 0.14 | * p = 0.043 | p = 0.09 | p = 0.11 | p = 0.81 | – | |
| 0.1 µM of paclitaxel | 24 h | p = 0.086 | p = 0.29 | p = 0.33 | ** p = 0.0063 | * p = 0.027 | * p = 0.016 |
| 72 h | ** p = 0.0071 | ** p = 0.0025 | ** p = 0.0057 | ** p = 0.0039 | p = 0.64 | p = 0.95 | |
| 168 h | ** p = 0.0024 | ** p = 0.0024 | ** p = 0.0058 | ** p = 0.0012 | ** p = 0.0037 | ** p = 0.0097 | |
| 1 µM of paclitaxel | 24 h | p = 0.23 | p = 0.12 | p = 0.15 | p = 0.27 | p = 0.22 | p = 0.075 |
| 72 h | p = 0.13 | * p = 0.049 | p = 0.063 | p = 0.16 | p = 0.54 | p = 0.71 | |
| 168 h | ** p = 0.0010 | ** p = 0.0014 | ** p = 0.0054 | ** p = 0.58 × 10−4 | ** p = 0.0025 | ** p = 0.0059 | |
| 0.003 µM of vincristine | 24 h | p = 0.84 | p = 0.63 | p = 0.78 | p = 0.34 | p = 0.59 | p = 0.073 |
| 72 h | ** p = 0.0076 | * p = 0.025 | p = 0.066 | ** p = 0.0031 | * p = 0.012 | p = 0.21 | |
| 168 h | ** p = 0.0030 | ** p = 0.0092 | * p = 0.036 | ** p = 0.69 × 10−4 | ** p = 0.0032 | ** p = 0.049 | |
| 0.03 µM of vincristine | 24 h | ** p = 0.0070 | p = 0.075 | p = 0.12 | * p = 0.018 | p = 0.18 | p = 0.31 |
| 72 h | p = 0.61 | p = 0.39 | p = 0.39 | p = 0.70 | p = 0.88 | p = 0.81 | |
| 168 h | p = 0.16 | p = 0.10 | p = 0.14 | p = 0.18 | p = 0.27 | p = 0.34 | |
| 10 µM of oxaliplatin | 24 h | p = 0.41 | p = 0.12 | p = 0.17 | p = 0.46 | p = 0.92 | p = 0.77 |
| 72 h | p = 0.11 | p = 0.079 | p = 0.12 | p = 0.11 | p = 0.15 | p = 0.26 | |
| 168 h | ** p = 0.0077 | * p = 0.010 | * p = 0.019 | ** p = 0.0079 | ** p = 0.0091 | * p = 0.021 | |
| 100 µM of oxaliplatin | 24 h | p = 0.11 | * p = 0.023 | * p = 0.043 | p = 0.14 | p = 0.70 | p = 0.41 |
| 72 h | ** p = 0.0013 | ** p = 0.81 × 10−5 | ** p = 0.0056 | ** p = 0.24 × 10−5 | ** p = 0.0047 | * p = 0.010 | |
| 168 h | ** p = 2.71 × 10−7 | ** p = 1.01 × 10−5 | ** p = 6.16 × 10−5 | ** p = 1.01 × 10−7 | ** p = 3.44 × 10−7 | ** p = 5.45 × 10−5 | |
| 0.001 µM of bortezomib | 24 h | p = 0.90 | p = 0.21 | p = 0.39 | p = 0.95 | p = 0.82 | p = 0.14 |
| 72 h | p = 0.84 | p = 0.79 | p = 0.88 | p = 0.11 | p = 0.40 | * p = 0.048 | |
| 168 h | p = 0.32 | p = 0.45 | p = 0.69 | p = 0.30 | p = 0.48 | p = 0.14 | |
| 0.01 µM of bortezomib | 24 h | p = 0.10 | p = 0.17 | p = 0.19 | * p = 0.019 | * p = 0.039 | ** p = 0.0082 |
| 72 h | p = 0.12 | p = 0.11 | p = 0.17 | p = 0.13 | p = 0.15 | p = 0.20 | |
| 168 h | * p = 0.013 | ** p = 0.0090 | * p = 0.019 | * p = 0.012 | * p = 0.019 | * p = 0.025 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Matsuda, N.; Ishibashi, Y.; Shibata, M.; Suzuki, I. An In Vitro Assessment Method for Chemotherapy-Induced Peripheral Neurotoxicity Caused by Anti-Cancer Drugs Based on Electrical Measurement of Impedance Value and Spontaneous Activity. Pharmaceutics 2023, 15, 2788. https://doi.org/10.3390/pharmaceutics15122788
Han X, Matsuda N, Ishibashi Y, Shibata M, Suzuki I. An In Vitro Assessment Method for Chemotherapy-Induced Peripheral Neurotoxicity Caused by Anti-Cancer Drugs Based on Electrical Measurement of Impedance Value and Spontaneous Activity. Pharmaceutics. 2023; 15(12):2788. https://doi.org/10.3390/pharmaceutics15122788
Chicago/Turabian StyleHan, Xiaobo, Naoki Matsuda, Yuto Ishibashi, Mikako Shibata, and Ikuro Suzuki. 2023. "An In Vitro Assessment Method for Chemotherapy-Induced Peripheral Neurotoxicity Caused by Anti-Cancer Drugs Based on Electrical Measurement of Impedance Value and Spontaneous Activity" Pharmaceutics 15, no. 12: 2788. https://doi.org/10.3390/pharmaceutics15122788





