Spray-Dried Powder Containing Cannabigerol: A New Extemporaneous Emulgel for Topical Administration
Abstract
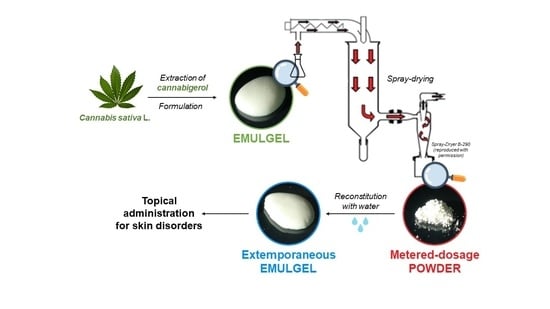
:1. Introduction
2. Materials and Methods
2.1. Cannabinoids Extraction, Isolation and Characterization
2.2. Pre-Formulation Studies
2.2.1. Preparation of and Characterization of CBs-MβCD Binary Systems
2.2.2. Antioxidant Activity
2.2.3. Permeation Study of CBs Solutions
2.3. Formulation Study
2.3.1. Preparation of Emulgels
2.3.2. Spray-Drying of Emulgels
2.3.3. Reconstitution of the Spray-Dried Powders
2.4. Characterization of the Emulgels
2.4.1. Appearance, pH, Drug Content and Viscosity
2.4.2. Drug Content Determination
2.5. Characterization of Powders
2.5.1. Spray-Drying Process Yield
2.5.2. Physical-Chemical Properties
2.5.3. Dynamic Angle of Repose Measurement
2.5.4. Particle Size Distribution
2.6. Statistical Analysis
3. Results and Discussion
3.1. CBs-MβCD Solid–Solid Interaction
3.2. Antioxidant Activity
3.3. Permeation Results
3.4. Appearance, pH, Drug Content and Viscosity of Emulgels
3.5. Spray-Drying Process Yield and Moisture Content
3.6. Drug Content, TGA, ATR-FTIR and XRPD of Powders
3.7. Dynamic Angle of Repose and Particle Size Distribution of Powders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis Sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. A New Formulation of Cannabidiol in Cream Shows Therapeutic Effects in a Mouse Model of Experimental Autoimmune Encephalomyelitis. DARU J Pharm. Sci. 2015, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The Potential Role of Cannabinoids in Dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis Sativa and Skin Health: Dissecting the Role of Phytocannabinoids. Planta Med. 2022, 88, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The Medicinal Natural Products of Cannabis Sativa Linn.: A Review. Molecules 2022, 27, 1689. [Google Scholar] [CrossRef] [PubMed]
- Maioli, C.; Mattoteia, D.; Amin, H.I.M.; Minassi, A.; Caprioglio, D. Cannabinol: History, Syntheses, and Biological Profile of the Greatest “Minor” Cannabinoid. Plants 2022, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Río, C.D.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The Endocannabinoid System of the Skin. A Potential Approach for the Treatment of Skin Disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef]
- Filipiuc, S.-I.; Neagu, A.-N.; Uritu, C.M.; Tamba, B.-I.; Filipiuc, L.-E.; Tudorancea, I.M.; Boca, A.N.; Hâncu, M.F.; Porumb, V.; Bild, W. The Skin and Natural Cannabinoids–Topical and Transdermal Applications. Pharmaceuticals 2023, 16, 1049. [Google Scholar] [CrossRef]
- Mounessa, J.S.; Siegel, J.A.; Dunnick, C.A.; Dellavalle, R.P. The Role of Cannabinoids in Dermatology. J. Am. Acad. Dermatol. 2017, 77, 188–190. [Google Scholar] [CrossRef]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential Effectiveness of Selected Non-psychotropic Phytocannabinoids on Human Sebocyte Functions Implicates Their Introduction in Dry/Seborrhoeic Skin and Acne Treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef]
- Mnekin, L.; Ripoll, L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics 2021, 8, 85. [Google Scholar] [CrossRef]
- Tijani, A.O.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.I.; Puri, A. Delivering Therapeutic Cannabinoids via Skin: Current State and Future Perspectives. J. Control. Release 2021, 334, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. CCID 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Czech, K.; Luca, S.V. Cannabidiol and Minor Phytocannabinoids: A Preliminary Study to Assess Their Anti-Melanoma, Anti-Melanogenic, and Anti-Tyrosinase Properties. Pharmaceuticals 2023, 16, 648. [Google Scholar] [CrossRef] [PubMed]
- Maor, Y.; Yu, J.; Kuzontkoski, P.M.; Dezube, B.J.; Zhang, X.; Groopman, J.E. Cannabidiol Inhibits Growth and Induces Programmed Cell Death in Kaposi Sarcoma-Associated Herpesvirus-Infected Endothelium. Genes Cancer 2012, 3, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Maghfour, J.; Rundle, C.W.; Rietcheck, H.R.; Dercon, S.; Lio, P.; Mamo, A.; Runion, T.M.; Fernandez, J.; Kahn, J.; Dellavalle, R.P.; et al. Assessing the Effects of Topical Cannabidiol in Patients with Atopic Dermatitis. Dermatol. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol Is an Effective Helper Compound in Combination with Bacitracin to Kill Gram-Positive Bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis Sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, B.; Vadalà, M. A Therapeutic Effect of Cbd-Enriched Ointment in Inflammatory Skin Diseases and Cutaneous Scars. Clin Ter. 2019, 170, 93–99. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids Inhibit Human Keratinocyte Proliferation through a Non-CB1/CB2 Mechanism and Have a Potential Therapeutic Value in the Treatment of Psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Deiana, S. Potential Medical Uses of Cannabigerol: A Brief Overview. In Handbook of Cannabis and Related Pathologies. Biology, Pharmacology, Diagnosis, and Treatment; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 958–967. [Google Scholar] [CrossRef]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic Control of Skin Differentiation Genes by Phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef]
- Jackson, D.K.; Hyatt, K. Silicone and Hyaluronic Acid (HLA) Delivery Systems for Products by Sustainable Processes for Medical Uses Including Wound Management. U.S. Patent 20130184354A1, 18 July 2013. Available online: https://patents.google.com/patent/US20130184354A1/en (accessed on 6 November 2023).
- Skalicky, J.; Husek, J.; Hofbauerova, J.; Dittrich, M. A Composition for the Treatment of Inflammatory Diseases Comprising Boswellic Acids and Cannabidiol. EP Patent 2444081A1, 25 April 2012. Available online: https://patents.google.com/patent/EP2444081A1/en (accessed on 6 November 2023).
- Shemanski, M.E. Formulations of Argan Oil and Cannabidiol for Treating Inflammatory Disorders Including Arthritis. WO Patent 2017160923A1, 21 September 2017. Available online: https://patents.google.com/patent/WO2017160923A1/en (accessed on 6 November 2023).
- Siurkus, J. The Oleo Gel Composition and Delivery System with Active Compounds from Cannabis Sativa and Mentha Arvensis for Reduction of Inflammation and Pain in Deep Tissues. WO Patent 2017178937A1, 19 October 2017. Available online: https://patents.google.com/patent/WO2017178937A1/en (accessed on 6 November 2023).
- Siurkus, J.; Peciura, R. The Topical Composition with Active Compounds from Cannabis Sativa and Calendula Officinalis for Reduction of Skin Lesions. WO Patent 2017175126A1, 12 October 2017. Available online: https://patents.google.com/patent/WO2017175126A1/en (accessed on 6 November 2023).
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering Emulsions Stabilized with Chitosan/Collagen Peptides Nanoparticles as Green Topical Delivery Vehicles for Cannabidiol (CBD). Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127677. [Google Scholar] [CrossRef]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and Optimisation of Biopharmaceutical Properties of a New Microemulgel of Cannabidiol for Locally-Acting Dermatological Delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2022, 14, 1637. [Google Scholar] [CrossRef]
- Freeze, D.C.; Freeze, A. Cannabinoid and terpene-infused topical cream. U.S. Patent 20200197359A1, 25 June 2020. Available online: https://patents.google.com/patent/US20200197359A1/en (accessed on 6 November 2023).
- Changoer, L.; Anastassov, G. Method to treat vitiligo. U.S. Patent 20190060252A1, 28 February 2022. Available online: https://patents.google.com/patent/US20190060252A1/en (accessed on 6 November 2023).
- Changoer, L.; Anastassov, G. Cosmetic and topical compositions comprising cannabigerol and cannabidiol. EP Patent 3258942B1, 13 January 2021. Available online: https://patents.google.com/patent/EP3258942B1/en (accessed on 6 November 2023).
- Yitzhak, Y.B.; Nava, R. Cannabinoid-Containing Skin Care Compositions. WO Patent 2022190098A1, 15 September 2022. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2022190098A1 (accessed on 6 November 2023).
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, Y.C.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J. Pharm. Sci. 2023, 112, 1772–1793. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, D. Hypersensitivity to Preservatives. Dermatol. Ther. 2004, 17, 251–263. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.A.; Alcántara-Nicolás, F.; Melgar-Molero, V.; Pérez-Mesonero, R.; Vergara-Sánchez, A.; Martín-Fuentes, A.; González-Muñoz, P.; De Eusebio-Murillo, E. Preservatives in Personal Hygiene and Cosmetic Products, Topical Medications, and Household Cleaners in Spain. Actas Dermo-Sifiliográficas 2017, 108, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lu, Y.I.; Wu, W. Manufacturing Solid Dosage Forms from Bulk Liquids Using the Fluid-bed Drying Technology. Curr. Pharm. Des. 2015, 21, 2668–2676. [Google Scholar] [CrossRef]
- Costa, C.; Casimiro, T.; Corvo, M.L.; Aguiar-Ricardo, A. Solid Dosage Forms of Biopharmaceuticals in Drug Delivery Systems Using Sustainable Strategies. Molecules 2021, 26, 7653. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, M.-C.; Grisel, M.; Gore, E. Multifunctional Active Ingredient-Based Delivery Systems for Skincare Formulations: A Review. Colloids Surf. B Biointerfaces 2022, 217, 112676. [Google Scholar] [CrossRef]
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced Skin Performance of Emulgel vs. Cream as Systems for Topical Delivery of Herbal Actives (Immortelle Extract and Hemp Oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An Effective Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation and Structure of.DELTA.+− Tetrahydrocannabinol and Other Neutral Cannabinoids from Hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef]
- Pattarino, F.; Giovannelli, L.; Giovenzana, G.B.; Rinaldi, M.; Trotta, M. Inclusion of Methotrexate in Alkyl-Cyclodextrins: Effects of Host Substitutents on the Stability of Complexes. J. Drug Deliv. Sci. Technol. 2005, 15, 465–468. [Google Scholar] [CrossRef]
- Colasanto, A.; Travaglia, F.; Bordiga, M.; Monteduro, S.; Arlorio, M.; Coïsson, J.D.; Locatelli, M. Cooking of Artemide Black Rice: Impact on Proximate Composition and Phenolic Compounds. Foods 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Moroni, S.; Curzi, G.; Aluigi, A.; Tiboni, M.; Vergassola, M.; Saviano, M.; Marchitto, L.; Zucchi, S.; Ragni, L.; Casettari, L. Influence of Permeability Enhancers on in Vitro Peptides Delivery through STRAT-M® Membranes. J. Drug Deliv. Sci. Technol. 2023, 89, 104987. [Google Scholar] [CrossRef]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of Skin Permeation by Chemical Compounds Using the Artificial Membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical Administration of Cannabidiol: Influence of Vehicle-Related Aspects on Skin Permeation Process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.M.; Fuchs, C.S.K.; Philipsen, P.A.; Hædersdal, M.; Agner, T.; Clausen, M.-L. Advancement through Epidermis Using Tape Stripping Technique and Reflectance Confocal Microscopy. Sci. Rep. 2019, 9, 12217. [Google Scholar] [CrossRef]
- Giovannelli, L.; Milanesi, A.; Ugazio, E.; Fracchia, L.; Segale, L. Effect of Methyl–β–Cyclodextrin and Trehalose on the Freeze–Drying and Spray–Drying of Sericin for Cosmetic Purposes. Pharmaceuticals 2021, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Rassu, G.; Gavini, E.; Petretto, G.L.; Bonferoni, M.C.; Giunchedi, P.; Torre, M.L. Sericin/Crocetin Micro/Nanoparticles for Nucleus Pulposus Cells Regeneration: An “Active” Drug Delivery System. Front. Pharmacol. 2023, 14, 1129882. [Google Scholar] [CrossRef]
- Cheng, N.-S.; Zhao, K. Difference between Static and Dynamic Angle of Repose of Uniform Sediment Grains. Int. J. Sediment Res. 2017, 32, 149–154. [Google Scholar] [CrossRef]
- European Pharmacopoeia Supplement 11.2. 2023. Available online: https://pheur.edqm.eu/home (accessed on 6 November 2023).
- Taşoyan, İ.C.; Yolaçaner, E.T.; Öztop, M.H. Physical Characterization of Chocolates Prepared with Various Soybean and Milk Powders. J. Texture Stud. 2023, 54, 334–346. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Meenatchi, V.; Lin, L. Encapsulation of Essential Oil Components with Methyl-β-Cyclodextrin Using Ultrasonication: Solubility, Characterization, DPPH and Antibacterial Assay. Ultrason. Sonochemistry 2020, 64, 104997. [Google Scholar] [CrossRef]
- Mohandoss, S.; Sukanya, R.; Ganesan, S.; Alkallas, F.H.; Ben Gouider Trabelsi, A.; Kusmartsev, F.V.; Sakthi Velu, K.; Stalin, T.; Lo, H.-M.; Rok Lee, Y. SARS-CoV-2 Main Protease (3CLpro) Interaction with Acyclovir Antiviral Drug/Methyl-β-Cyclodextrin Complex: Physiochemical Characterization and Molecular Docking. J. Mol. Liq. 2022, 366, 120292. [Google Scholar] [CrossRef] [PubMed]
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.-W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun Fibers for the Oral Delivery of Cannabinoids. AAPS PharmSciTech 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Monou, P.-K.; Louka, A.; Papaefstathiou, E.; Eleftheriadis, G.K.; Fatouros, D.G. Development of Food Grade 3D Printable Ink Based on Pectin Containing Cannabidiol/Cyclodextrin Inclusion Complexes. Drug Dev. Ind. Pharm. 2020, 46, 1569–1577. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W.; et al. Inclusion Complexes of Cannabidiol with β-Cyclodextrin and Its Derivative: Physicochemical Properties, Water Solubility, and Antioxidant Activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Salau, O.; Bagde, A.; Kalvala, A.; Singh, M. Enhancement of Transdermal Permeation of Cannabinoids and Their Pharmacodynamic Evaluation in Rats. Int. J. Pharm. 2022, 624, 122016. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráðsdóttir, F. Effects of Cyclodextrins on Drug Delivery Through Biological Membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef]
- Dolz, M.; Hernández, M.J.; Delegido, J.; Alfaro, M.C.; Muñoz, J. Influence of Xanthan Gum and Locust Bean Gum upon Flow and Thixotropic Behaviour of Food Emulsions Containing Modified Starch. J. Food Eng. 2007, 81, 179–186. [Google Scholar] [CrossRef]
- Tan, G.; Morton, D.A.V.; Larson, I. On the Methods to Measure Powder Flow. Curr. Pharm. Des. 2015, 21, 5751–5765. [Google Scholar] [CrossRef]











| Formulation | Composition (% w/w) | |||
|---|---|---|---|---|
| Emulgels | Ea | Eb | Ec | Ed |
| Hemp oil | 2.0 | 2.0 | 2.0 | 2.0 |
| Emulsifier and gelling agent | 3.0 | 3.0 | 3.0 | 3.0 |
| MβCD | - | 1.0 | - | 1.0 |
| CBG | - | - | 1.0 | 1.0 |
| Water | 95.0 | 94.0 | 94.0 | 93.0 |
| Formulation | Theoretical Composition (% w/w) | |||
|---|---|---|---|---|
| Spray-Dried Powders | SDEa | SDEb | SDEc | SDEd |
| Hemp oil | 40.0 | 33.3 | 33.3 | 28.6 |
| Emulsifier and gelling agent | 60.0 | 50.0 | 50.0 | 42.8 |
| MβCD | - | 16.7 | - | 14.3 |
| CBG | - | - | 16.7 | 14.3 |
| Sample | T Onset (° C) | Theoretical Weight Loss (%) | Experimental Weight Loss (%) |
|---|---|---|---|
| CBD | 140.8 | 100.0 | 99.8 |
| pmCBD | 143.4 | 50.0 | 53.0 |
| knCBD | 186.6 | 50.0 | 40.8 |
| CBG | 135.7 | 100.0 | 100.0 |
| pmCBG | 152.4 | 50.0 | 51.0 |
| knCBG | 170.7 | 50.0 | 40.2 |
| MβCD | 310.0 | 100.0 | 85.5 |
| CBs | CBDsol (%) | CBD-MβCDsol (%) | CBGsol (%) | CBG-MβCDsol (%) |
|---|---|---|---|---|
| “In donor phase” | 47.3 ± 3.3 a | 49.2 ± 2.5 a | 53.1 ± 1.2 a | 47.5 ± 4.6 a |
| “Total diffused” | 21.9 ± 0.6 A | 20.5 ± 0.6 A | 23.9 ± 2.4 A | 35.1 ± 4.7 B |
| “Retained” | 34.1 ± 1.1 | 48.7 ± 0.2 | 67.1 ± 7.4 | 69.6 ± 7.0 |
| “Washed” | 2.8 ± 2.0 | 1.1 ± 1.2 | 4.6 ± 2.5 | 0.7 ± 0.8 |
| “Stripped” | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| “Permeated” | 63.0 ± 2.6 | 49.7 ± 11.2 | 29.4 ± 4.1 | 28.6 ± 7.6 |
| Spray-Dried Powders | Yields (%) | Moisture Content (%) |
|---|---|---|
| SDEa | 36.0 ± 0.2 b | 5.3 ± 0.7 a |
| SDEb | 54.0 ± 4.0 a | 3.8 ± 0.4 b |
| SDEc | 50.7 ± 6.3 ab | 2.6 ± 0.3 c |
| SDEd | 65.0 ± 6.0 a | 2.0 ± 0.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picco, A.; Segale, L.; Miletto, I.; Pollastro, F.; Aprile, S.; Locatelli, M.; Bari, E.; Torre, M.L.; Giovannelli, L. Spray-Dried Powder Containing Cannabigerol: A New Extemporaneous Emulgel for Topical Administration. Pharmaceutics 2023, 15, 2747. https://doi.org/10.3390/pharmaceutics15122747
Picco A, Segale L, Miletto I, Pollastro F, Aprile S, Locatelli M, Bari E, Torre ML, Giovannelli L. Spray-Dried Powder Containing Cannabigerol: A New Extemporaneous Emulgel for Topical Administration. Pharmaceutics. 2023; 15(12):2747. https://doi.org/10.3390/pharmaceutics15122747
Chicago/Turabian StylePicco, Alice, Lorena Segale, Ivana Miletto, Federica Pollastro, Silvio Aprile, Monica Locatelli, Elia Bari, Maria Luisa Torre, and Lorella Giovannelli. 2023. "Spray-Dried Powder Containing Cannabigerol: A New Extemporaneous Emulgel for Topical Administration" Pharmaceutics 15, no. 12: 2747. https://doi.org/10.3390/pharmaceutics15122747