Investigation on Electrospun and Solvent-Casted PCL-PLGA Blends Scaffolds Embedded with Induced Pluripotent Stem Cells for Tissue Engineering
Abstract
:1. Introduction
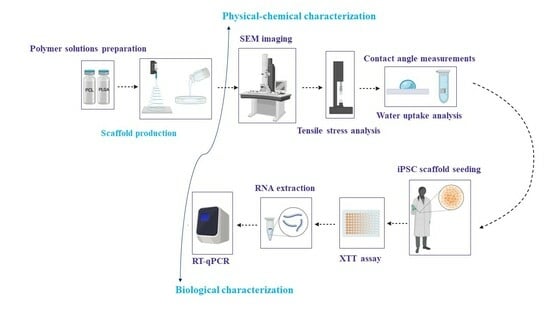
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scaffolds Preparation by Electrospinning
2.2.2. Scaffolds Preparation by Solvent Casting/Porogen Leaching
2.2.3. Scaffolds’ Physicochemical Characterisation
Scanning Electron Microscopy (SEM)
Mechanical Uniaxial Tensile Testing
Contact Angle Measurements and Hydration Assays
2.2.4. Scaffolds’ Preliminary Biological Characterisation
Cell Culture on Electrospun and Solvent Casted/Particulate Leaching Scaffolds
Cell Viability Assay
RNA Extraction and Reverse Transcription-PCR
Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.2.5. Statistical Analysis
3. Results
3.1. Morphometric Characterisation of Electrospun and Solvent-Casted Scaffolds
3.2. Mechanical Properties of Electrospun and Solvent-Casted Scaffolds
3.3. Surface Wettability and Water Uptake of Electrospun and Solvent-Casted Scaffolds
3.4. Rationale of Scaffold Selection for Preliminary Biological Characterisation
3.5. iPS Cell Viability on Electrospun and Solvent-Casted Scaffolds
3.6. iPSC Pluripotency and Adhesion Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Farè, S.; Candiani, G. Chapter 8—Advanced Applications. In Foundations of Biomaterials Engineering, 1st ed.; Tanzi, M.C., Farè, S., Candiani, G., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 471–545. ISBN 978-0-08-101034-1. [Google Scholar]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, M.C.; Farè, S.; Candiani, G. Chapter 1—Organization, Structure, and Properties of Materials. In Foundations of Biomaterials Engineering, 1st ed.; Tanzi, M.C., Farè, S., Candiani, G., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–103. ISBN 978-0-08-101034-1. [Google Scholar]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Viera Rey, D.F.; St-Pierre, J.-P. 6—Fabrication Techniques of Tissue Engineering Scaffolds. In Biomaterials and Scaffold Fabrication Techniques for Tissue Engineering Applications; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 109–125. ISBN 978-0-08-102563-5. [Google Scholar]
- Chimerad, M.; Barazesh, A.; Zandi, M.; Zarkesh, I.; Moghaddam, A.; Borjian, P.; Chimehrad, R.; Asghari, A.; Akbarnejad, Z.; Khonakdar, H.A.; et al. Tissue engineered scaffold fabrication methods for medical applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 1455–1479. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Ahmed, S.; Chauhan, V.M.; Ghaemmaghami, A.M.; Aylott, J.W. New Generation of Bioreactors That Advance Extracellular Matrix Modelling and Tissue Engineering. Biotechnol. Lett. 2019, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Polák, Š.; Danišovič, L. Toxicity Testing and Drug Screening Using IPSC-Derived Hepatocytes, Cardiomyocytes, and Neural Cells. Can. J. Physiol. Pharmacol. 2016, 94, 687–694. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.-M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Publisher Correction: Human Heart-Forming Organoids Recapitulate Early Heart and Foregut Development. Nat. Biotechnol. 2021, 39, 775. [Google Scholar] [CrossRef]
- Marini, V.; Marino, F.; Aliberti, F.; Giarratana, N.; Pozzo, E.; Duelen, R.; Cortés Calabuig, Á.; La Rovere, R.; Vervliet, T.; Torella, D.; et al. Long-Term Culture of Patient-Derived Cardiac Organoids Recapitulated Duchenne Muscular Dystrophy Cardiomyopathy and Disease Progression. Front. Cell Dev. Biol. 2022, 10, 878311. [Google Scholar] [CrossRef]
- Noh, H.; Shao, Z.; Coyle, J.T.; Chung, S. Modeling Schizophrenia Pathogenesis Using Patient-Derived Induced Pluripotent Stem Cells (IPSCs). Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Ogawa, S.; Bear, C.E.; Ahmadi, S.; Chin, S.; Li, B.; Grompe, M.; Keller, G.; Kamath, B.M.; Ghanekar, A. Directed Differentiation of Cholangiocytes from Human Pluripotent Stem Cells. Nat. Biotechnol. 2015, 33, 853–861. [Google Scholar] [CrossRef]
- Pang, L. Toxicity Testing in the Era of Induced Pluripotent Stem Cells: A Perspective Regarding the Use of Patient-Specific Induced Pluripotent Stem Cell–Derived Cardiomyocytes for Cardiac Safety Evaluation. Curr. Opin. Toxicol. 2020, 23–24, 50–55. [Google Scholar] [CrossRef]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s Disease with IPSC-Derived Brain Cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Wiegand, C.; Banerjee, I. Recent Advances in the Applications of IPSC Technology. Curr. Opin. Biotechnol. 2019, 60, 250–258. [Google Scholar] [CrossRef]
- Nakamura, S.; Sugimoto, N.; Eto, K. Ex Vivo Generation of Platelet Products from Human IPS Cells. Inflamm. Regen. 2020, 40, 30. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized IPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human IPSC-Derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef]
- Joanne, P.; Kitsara, M.; Boitard, S.-E.; Naemetalla, H.; Vanneaux, V.; Pernot, M.; Larghero, J.; Forest, P.; Chen, Y.; Menasché, P.; et al. Nanofibrous Clinical-Grade Collagen Scaffolds Seeded with Human Cardiomyocytes Induces Cardiac Remodeling in Dilated Cardiomyopathy. Biomaterials 2016, 80, 157–168. [Google Scholar] [CrossRef]
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.-H.; Lessmann, K.; Stolen, T.; et al. Cardiac Repair in Guinea Pigs with Human Engineered Heart Tissue from Induced Pluripotent Stem Cells. Sci. Transl. Med. 2016, 8, 363ra148. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a Target for Cancer Treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Rasti, A.; Mehrazma, M.; Madjd, Z.; Abolhasani, M.; Saeednejad Zanjani, L.; Asgari, M. Co-Expression of Cancer Stem Cell Markers OCT4 and NANOG Predicts Poor Prognosis in Renal Cell Carcinomas. Sci. Rep. 2018, 8, 11739. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of IPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Farè, S.; Candiani, G. Chapter 2—Mechanical Properties of Materials. In Foundations of Biomaterials Engineering, 1st ed.; Tanzi, M.C., Farè, S., Candiani, G., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 105–136. ISBN 978-0-08-101034-1. [Google Scholar]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-Invasive Microneedle-Based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Genta, I.; Modena, T.; Conti, B. Effect of Porogen on the Physico-Chemical Properties and Degradation Performance of PLGA Scaffolds. Polym. Degrad. Stab. 2010, 95, 694–701. [Google Scholar] [CrossRef]
- Rosalia, M.; Grisoli, P.; Dorati, R.; Chiesa, E.; Pisani, S.; Bruni, G.; Genta, I.; Conti, B. Influence of Electrospun Fibre Secondary Morphology on Antibiotic Release Kinetic and Its Impact on Antimicrobic Efficacy. Int. J. Mol. Sci. 2023, 24, 12108. [Google Scholar] [CrossRef]
- Pisani, S.; Genta, I.; Dorati, R.; Modena, T.; Chiesa, E.; Bruni, G.; Benazzo, M.; Conti, B. A Design of Experiment (DOE) Approach to Correlate PLA-PCL Electrospun Fibers Diameter and Mechanical Properties for Soft Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol. 2022, 68, 103060. [Google Scholar] [CrossRef]
- Rosalia, M.; Ravipati, P.; Grisoli, P.; Dorati, R.; Genta, I.; Chiesa, E.; Bruni, G.; Conti, B. Tobramycin Supplemented Small-Diameter Vascular Grafts for Local Antibiotic Delivery: A Preliminary Formulation Study. Int. J. Mol. Sci. 2021, 22, 13557. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Chiesa, E.; Rosalia, M.; Pisani, S.; Genta, I.; Bruni, G.; Modena, T.; Conti, B. Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization. Polymers 2021, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release Profile of Gentamicin Sulfate from Polylactide-Co-Polycaprolactone Electrospun Nanofiber Matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef]
- Radisic, M.; Malda, J.; Epping, E.; Geng, W.; Langer, R.; Vunjak-Novakovic, G. Oxygen Gradients Correlate with Cell Density and Cell Viability in Engineered Cardiac Tissue. Biotechnol. Bioeng. 2006, 93, 332–343. [Google Scholar] [CrossRef]
- Chiesa, E.; Tottoli, E.M.; Giglio, A.; Conti, B.; Rosalia, M.; Rizzi, L.G.; Dorati, R.; Genta, I. Graphene Nanoplatelets-Based Textured Polymeric Fibrous Fabrics for the Next-Generation Devices. Polymers 2022, 14, 5415. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL Electrospun Membranes of Tailored Fibres Diameter as Drug Delivery Systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Performance Evaluation of Electrospun Nanofibrous Mats of Polylactic Acid (PLA)/Poly (ε-Caprolactone) (PCL) Blends. Mater. Today Proc. 2019, 19, 188–195. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(Lactic-Co-Glycolic Acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- McKeen, L. Chapter 11—The Effect of Heat Aging on the Properties of Sustainable Polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers, 2nd ed.; McKeen, L., Ed.; William Andrew Publishing: Norwich, NY, USA, 2021; pp. 313–332. ISBN 978-0-323-85436-8. [Google Scholar]
- Croisier, F.; Duwez, A.-S.; Jérôme, C.; Léonard, A.F.; van der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical Testing of Electrospun PCL Fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, J.; Łukowicz, K.; Cholewa-Kowalska, K.; Osyczka, A.M. New Insights into the PLGA and PCL Blending: Physico-Mechanical Properties and Cell Response. Mater. Res. Express 2019, 6, 085344. [Google Scholar] [CrossRef]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The Effect of Molecular Weight and Fiber Diameter on the Mechanical Properties of Single, Electrospun PCL Nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Morel, A.; Domaschke, S.; Urundolil Kumaran, V.; Alexeev, D.; Sadeghpour, A.; Ramakrishna, S.N.; Ferguson, S.J.; Rossi, R.M.; Mazza, E.; Ehret, A.E.; et al. Correlating Diameter, Mechanical and Structural Properties of Poly(l-Lactide) Fibres from Needleless Electrospinning. Acta Biomater. 2018, 81, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Camasão, D.B.; Mantovani, D. The Mechanical Characterization of Blood Vessels and Their Substitutes in the Continuous Quest for Physiological-Relevant Performances. A Critical Review. Mater. Today Bio 2021, 10, 100106. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Huang, C.-H.; Cheng, C.-K.; Chan, K.-K.; Chang, T.-K. The Effect of Graft Strength on Knee Laxity and Graft In-Situ Forces after Posterior Cruciate Ligament Reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Mechanical Properties of Whole-Body Soft Human Tissues: A Review. Biomed. Mater. 2021, 16, 62004. [Google Scholar] [CrossRef]
- Wciślik, S.; Mukherjee, S. Evaluation of Three Methods of Static Contact Angle Measurements for TiO2 Nanofluid Droplets during Evaporation. Phys. Fluids 2022, 34, 62006. [Google Scholar] [CrossRef]
- Sadeghi, P.; Tavanai, H.; Khoddami, A. Hydrophobicity of Fluorocarbon-Finished Electrospun Poly (Acrylonitrile) Nanofibrous Webs. J. Text. Inst. 2017, 108, 189–195. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact Angle Measurement on Porous Substrates: Effect of Liquid Absorption and Drop Size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Wulf, K.; Senz, V.; Eickner, T.; Illner, S. Water Uptake of Various Electrospun Nonwovens. Curr. Dir. Biomed. Eng. 2020, 6, 155–158. [Google Scholar] [CrossRef]
- Wang, A.Y.L.; Loh, C.Y.Y. Episomal Induced Pluripotent Stem Cells: Functional and Potential Therapeutic Applications. Cell Transpl. 2019, 28, 112S–131S. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Gerardo, H.; Lima, A.; Carvalho, J.; Ramos, J.R.D.; Couceiro, S.; Travasso, R.D.M.; Pires das Neves, R.; Grãos, M. Soft Culture Substrates Favor Stem-like Cellular Phenotype and Facilitate Reprogramming of Human Mesenchymal Stem/Stromal Cells (HMSCs) through Mechanotransduction. Sci. Rep. 2019, 9, 9086. [Google Scholar] [CrossRef]
- Santoro, R.; Perrucci, G.L.; Gowran, A.; Pompilio, G. Unchain My Heart: Integrins at the Basis of IPSC Cardiomyocyte Differentiation. Stem Cells Int. 2019, 2019, 8203950. [Google Scholar] [CrossRef]
- Diederichs, S.; Klampfleuthner, F.A.M.; Moradi, B.; Richter, W. Chondral Differentiation of Induced Pluripotent Stem Cells Without Progression into the Endochondral Pathway. Front. Cell Dev. Biol. 2019, 7, 270. [Google Scholar] [CrossRef]
- Zhu, H.; Kimura, T.; Swami, S.; Wu, J.Y. Pluripotent Stem Cells as a Source of Osteoblasts for Bone Tissue Regeneration. Biomaterials 2019, 196, 31–45. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.-J.; Park, J.-H.; Lee, S.; Park, B.-W.; Lee, S.M.; Hwang, J.-W.; Kim, J.-J.; Kang, B.; Sim, W.-S.; et al. Enhancement Strategy for Effective Vascular Regeneration Following Myocardial Infarction through a Dual Stem Cell Approach. Exp. Mol. Med. 2022, 54, 1165–1178. [Google Scholar] [CrossRef]








| Batch | Polymer Solution Composition | |
|---|---|---|
| PCL (w/w) | PLGA (w/w) | |
| ES1 | 100% | - |
| ES2 | 95% | 5% |
| ES3 | 85% | 15% |
| ES4 | - | 100% |
| ES5 | 5% | 95% |
| ES6 | 15% | 85% |
| SC1 | 100% | - |
| SC2 | 95% | 5% |
| SC3 | 85% | 15% |
| SC4 | - | 100% |
| SC5 | 5% | 95% |
| SC6 | 15% | 85% |
| Gene Name | Sequence Name | Primer Sequence |
|---|---|---|
| GAPDH | GAPDH RV | 5′-ACCAGGAAATGAGCTTGACAAA-3′ |
| GAPDH | GAPDH FW | 5′-TCAAGAAGGTGGTGAAGCAGG-3′ |
| NANOG | NANOG RV | 5′-TTCCAGGTCTGGTTGCTCCACATT-3′ |
| NANOG | NANOG FW | 5′-TGGCCGAAGAATAGCAATGGTGTG-3′ |
| OCT4 | OCT4 RV | 5′-GCCGCAGCTTACACATGTTCTTGA-3′ |
| OCT4 | OCT4 FW | 5′-CGAGCAATTTGCCAAGCTCCTGAA-3′ |
| ITGα6 | Integrin Alpha 6 RV | 5′-TTCCTGCTTCGTATTAACATGCT-3′ |
| ITGα6 | Integrin Alpha 6 FW | 5′-ATGCACGCGGATCGAGTTT-3′ |
| ITGβ1 | Integrin Beta 1 RV | 5′-TCCCCTGATCTTAATCGCAAAAC-3′ |
| ITGβ1 | Integrin Beta 1 FW | 5′-GTAACCAACCGTAGCAAAGGA-3′ |
| ITGβ5 | Integrin Beta 5 RV | 5′-TGGCGAACCTGTAGCTGGA-3′ |
| ITGβ5 | Integrin Beta 5 FW | 5′-TCTCGGTGTGATCTGAGGG-3′ |
| ITGαV | Integrin Alpha V RV | 5′-TCTGCTCGCCAGTAAAATTGT-3′ |
| ITGαV | Integrin Alpha V FW | 5′-GCTGTCGGAGATTTCAATGGT-3′ |
| Batch | Polymer Blend Composition (w/w) | Fibre Diameter (µm) | Pore Diameter (µm) | Porosity (%) |
|---|---|---|---|---|
| * ES1 | PCL 100% | 0.992 ± 0.228 | 3.15 ± 2.55 | 37.58 |
| ES2 | PCL 95% + PLGA 5% | 1.085 ± 0.360 | 3.47 ± 3.32 | 40.44 |
| ES3 | PCL 85% + PLGA 15% | 1.076 ± 0.537 | 3.15 ± 2.70 | 33.46 |
| ES4 | PLGA 100% | 0.607 ± 0.107 | 3.62 ± 3.04 | 51.60 |
| ES5 | PLGA 95% + PCL 5% | 0.740 ± 0.181 | 3.59 ± 2.74 | 48.25 |
| ES6 | PLGA 85% + PCL 15% | 0.969 ± 0.195 | 3.51 ± 2.81 | 40.85 |
| ** SC1 | PCL 100% | *** NA | 3.10 ±11.10 | 61.42 |
| SC2 | PCL 95% + PLGA 5% | NA | 3.13 ± 9.91 | 48.50 |
| SC3 | PCL 85% + PLGA 15% | NA | 3.67 ± 8.87 | 41.92 |
| SC4 | PLGA 100% | NA | NA | NA |
| SC5 | PLGA 95% + PCL 5% | NA | 2.85 ± 7.94 | 32.64 |
| SC6 | PLGA 85% + PCL 15% | NA | 2.88 ± 6.62 | 23.01 |
| Batch | Polymer Blend Composition (w/w) | Young Modulus (MPa) | Contact Angle (°) |
|---|---|---|---|
| * ES1 | PCL 100% | 3.25 ± 0.52 | 96.73 ± 1.30 |
| ES2 | PCL 95% + PLGA 5% | 3.43 ± 0.46 | 96.63 ± 1.72 |
| ES3 | PCL 85% + PLGA 15% | 3.11 ± 0.83 | 106.83 ± 2.35 |
| ES4 | PLGA 100% | 22.02 ± 1.93 | 102.47 ± 1.26 |
| ES5 | PLGA 95% + PCL 5% | 20.56 ± 1.15 | 101.70 ± 0.98 |
| ES6 | PLGA 85% + PCL 15% | 9.80 ± 2.09 | 105.80 ± 0.78 |
| ** SC1 | PCL 100% | 12.48 ± 2.56 | 108.60 ± 7.23 |
| SC2 | PCL 95% + PLGA 5% | 4.81 ± 0.494 | 113.23 ± 6.23 |
| SC3 | PCL 85% + PLGA 15% | 8.03 ± 2.57 | 87.47 ± 0.55 |
| SC4 | PLGA 100% | *** NA | NA |
| SC5 | PLGA 95% + PCL 5% | 20.23 ± 7.68 | 76.60 ± 4.19 |
| SC6 | PLGA 85% + PCL 15% | 12.83 ± 2.08 | 74.47 ± 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosalia, M.; Giacomini, M.; Tottoli, E.M.; Dorati, R.; Bruni, G.; Genta, I.; Chiesa, E.; Pisani, S.; Sampaolesi, M.; Conti, B. Investigation on Electrospun and Solvent-Casted PCL-PLGA Blends Scaffolds Embedded with Induced Pluripotent Stem Cells for Tissue Engineering. Pharmaceutics 2023, 15, 2736. https://doi.org/10.3390/pharmaceutics15122736
Rosalia M, Giacomini M, Tottoli EM, Dorati R, Bruni G, Genta I, Chiesa E, Pisani S, Sampaolesi M, Conti B. Investigation on Electrospun and Solvent-Casted PCL-PLGA Blends Scaffolds Embedded with Induced Pluripotent Stem Cells for Tissue Engineering. Pharmaceutics. 2023; 15(12):2736. https://doi.org/10.3390/pharmaceutics15122736
Chicago/Turabian StyleRosalia, Mariella, Martina Giacomini, Erika Maria Tottoli, Rossella Dorati, Giovanna Bruni, Ida Genta, Enrica Chiesa, Silvia Pisani, Maurilio Sampaolesi, and Bice Conti. 2023. "Investigation on Electrospun and Solvent-Casted PCL-PLGA Blends Scaffolds Embedded with Induced Pluripotent Stem Cells for Tissue Engineering" Pharmaceutics 15, no. 12: 2736. https://doi.org/10.3390/pharmaceutics15122736