Theoretical—Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers’ Behavior in Wound Exudate Media
Abstract
:1. Introduction
2. Theoretical Model
- (i).
- Introduction of the two-valuedness of this derivative because of the symmetry breaking of the reflection invariance under the exchange , giving a rise of a complex scale velocity based on this two-valuedness;
- (ii).
- The construction of a new total covariant derivative with respect to the , which can be written as:
- (iii).
- The introduction of a wave function as a re-expression of the action, which is now complex:
- (iv).
- Transformation and integration of the free Newtonian scale-dynamics equation
Dynamics in Scales Space through Riccati Gauge Type
3. Experimental Methods
3.1. Materials
3.2. Synthesis of N-(2-hydroxy)propyl-3-trimethyl Ammonium Chitosan Chloride (Q)
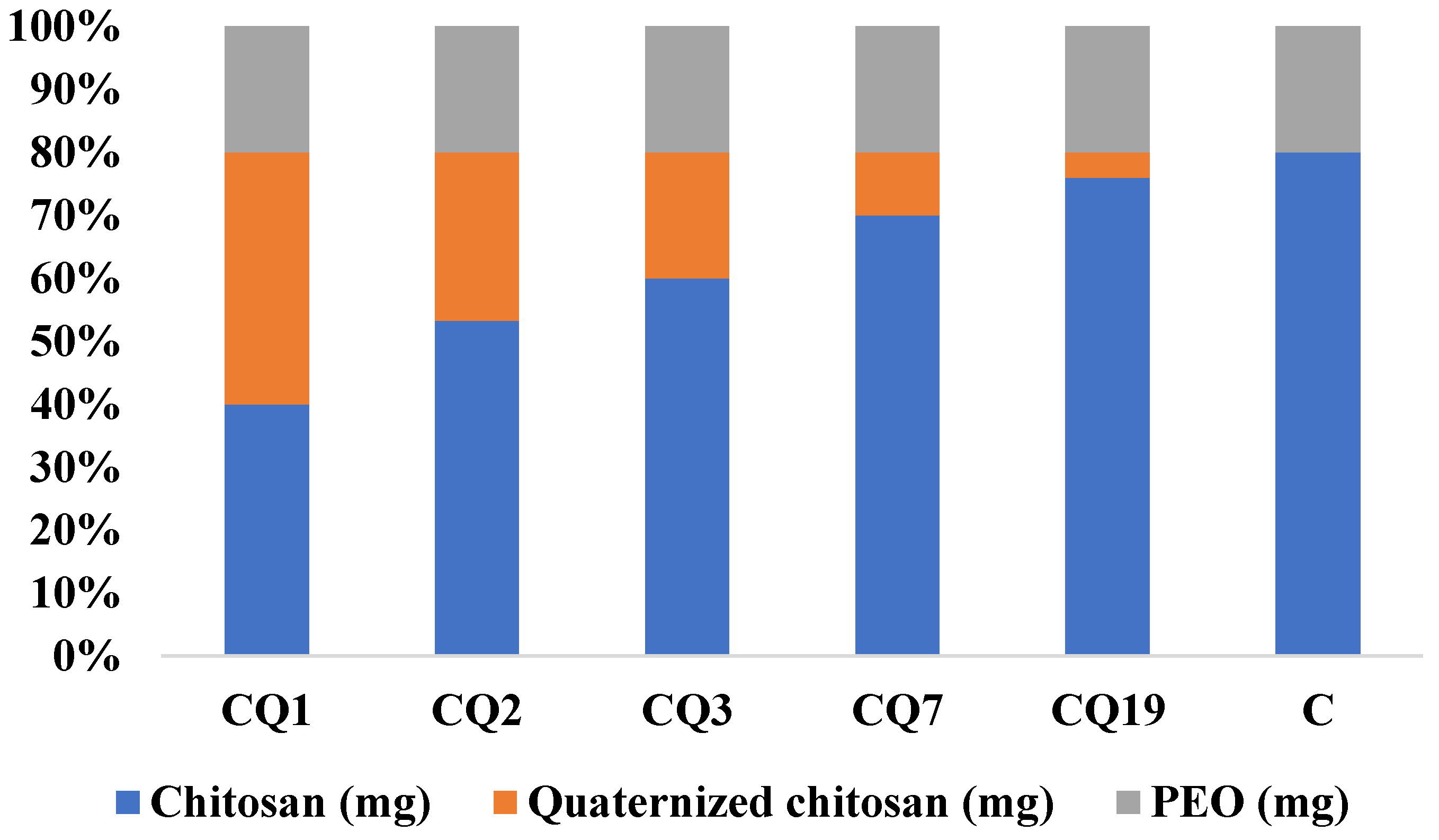
3.3. Preparation of the Chitosan/Quaternized Chitosan Fibers
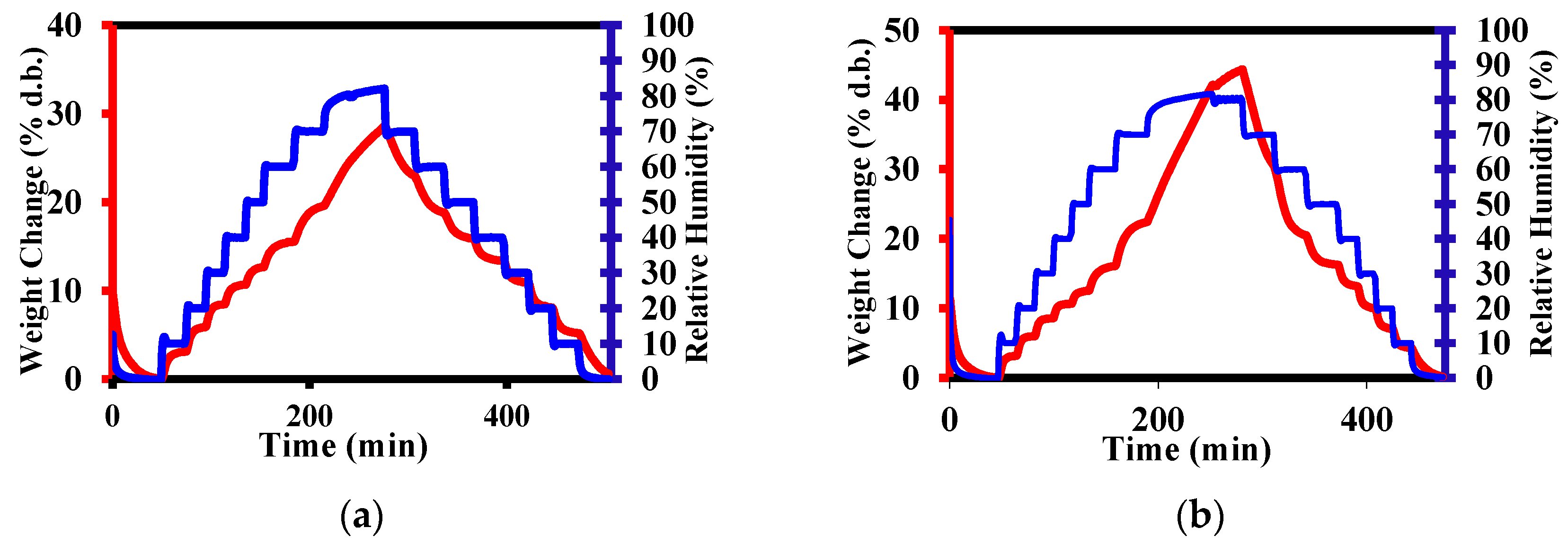
3.4. Methods
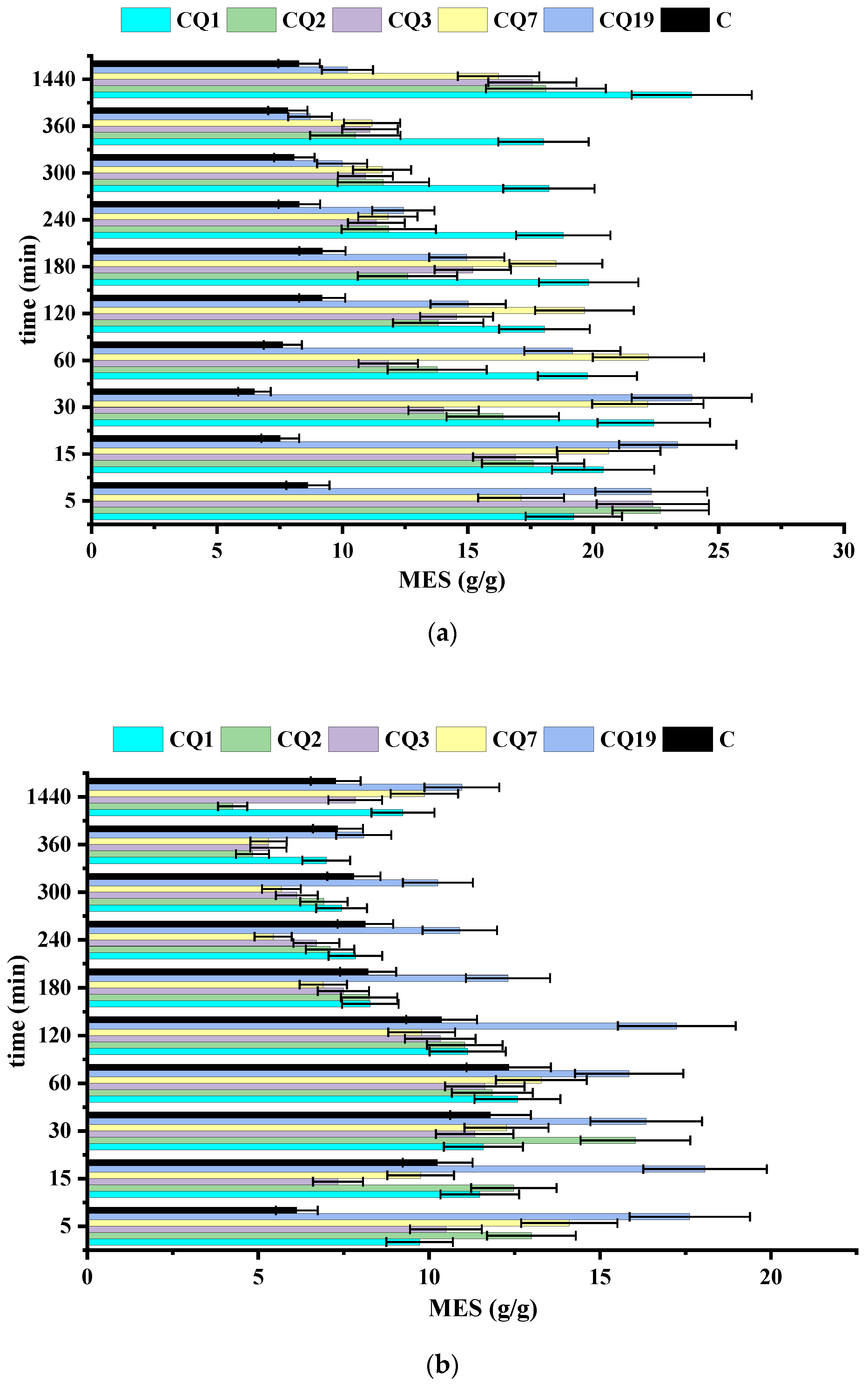
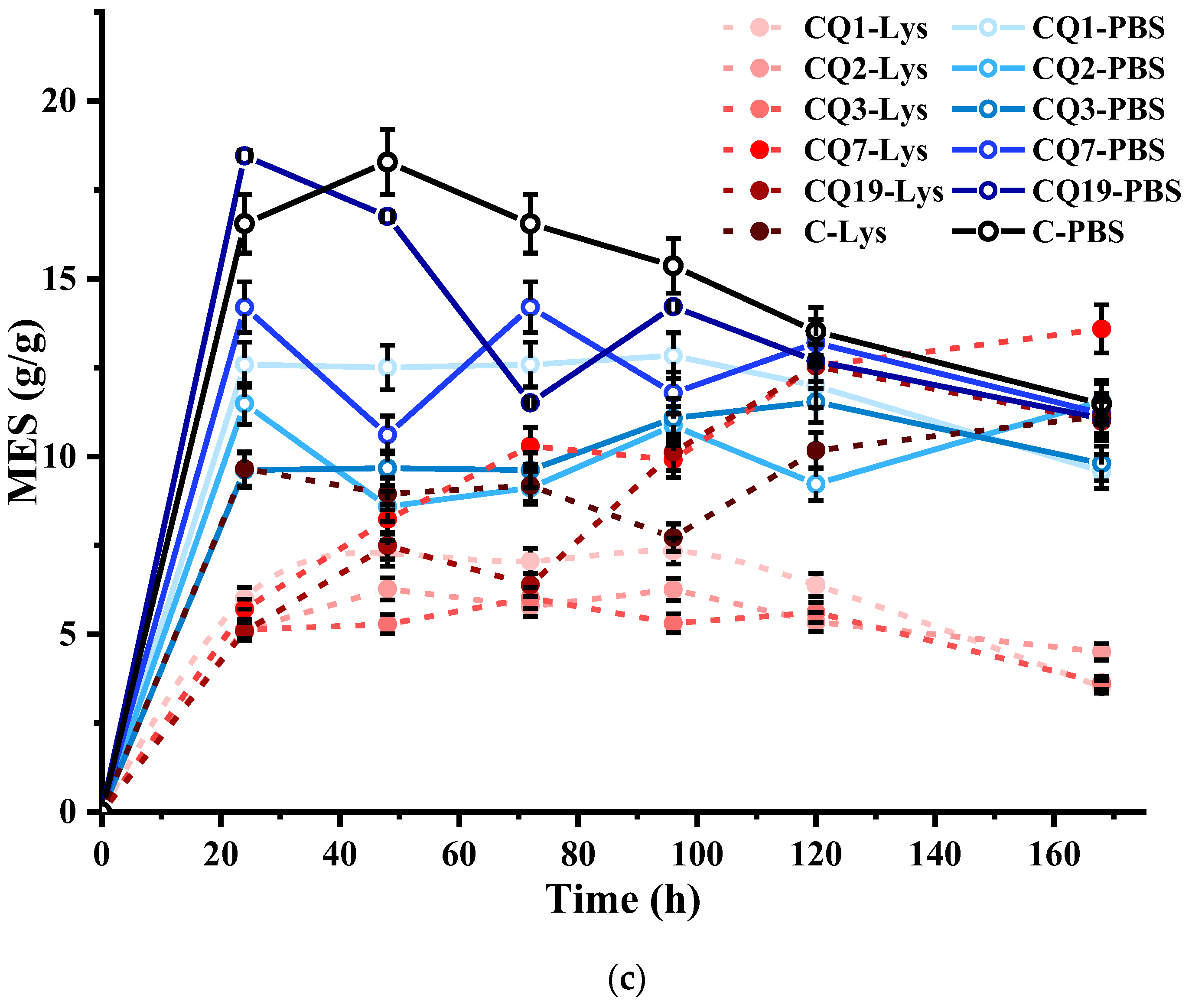
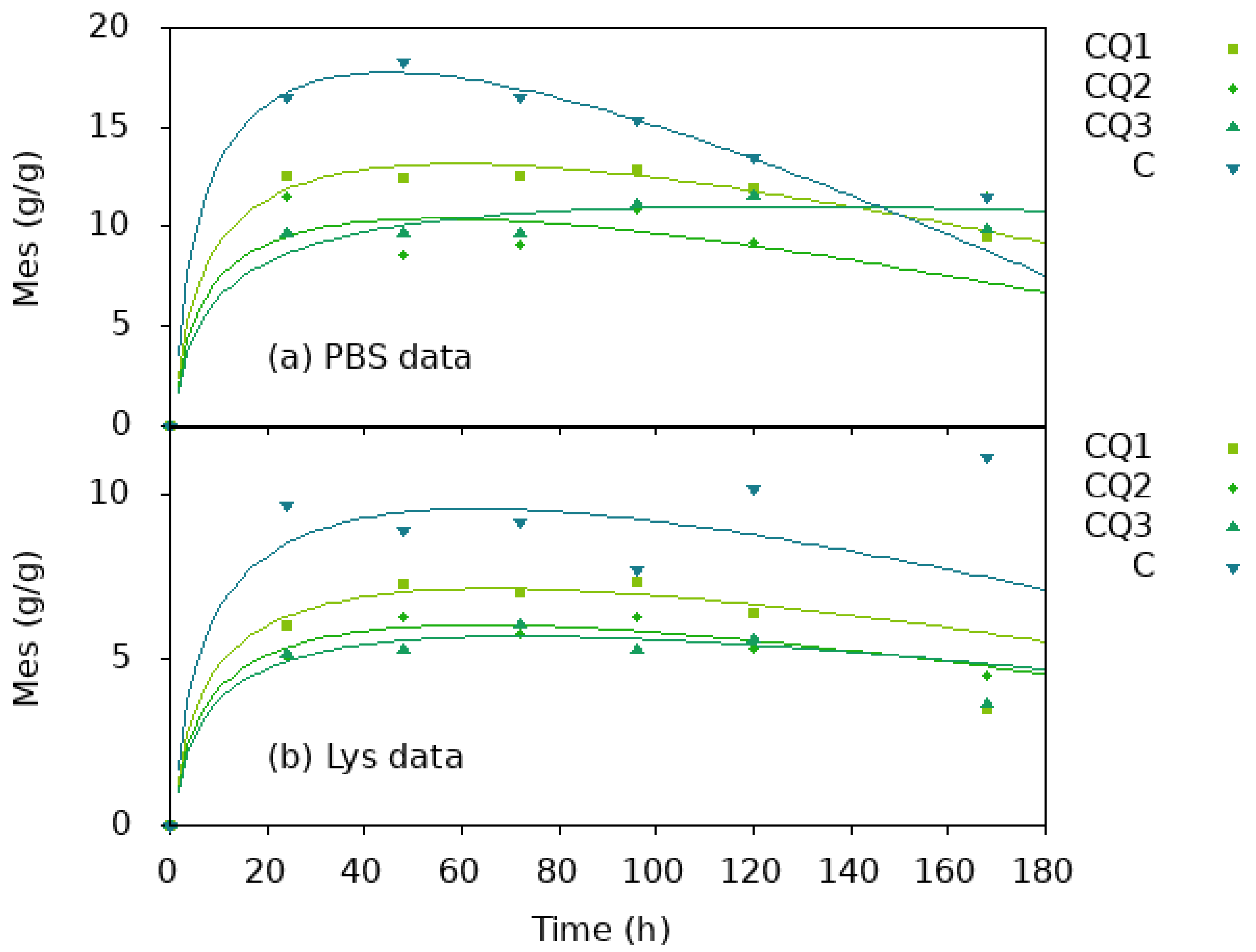
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posnett, J.; Franks, P.J. The Burden of Chronic Wounds in the UK. Nurs. Times 2008, 104, 44–45. [Google Scholar]
- Bardossy, A.C.; Zervos, J.; Zervos, M. Preventing Hospital-Acquired Infections in Low-Income and Middle-Income Countries. Infect. Dis. Clin. N. Am. 2016, 30, 805–818. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Kamińska, M.S.; Cybulska, A.M.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ramezani, H.; Sun, M.; Xie, M.; Nie, J.; Lv, S.; Cai, J.; Fu, J.; He, Y. 3D Printing of High-Strength Chitosan Hydrogel Scaffolds without Any Organic Solvents. Biomater. Sci. 2020, 8, 5020–5028. [Google Scholar] [CrossRef]
- Jung, H.; Yook, S.; Han, C.; Jang, T.; Kim, H.; Koh, Y.; Estrin, Y. Highly Aligned Porous Ti Scaffold Coated with Bone Morphogenetic Protein-loaded Silica/Chitosan Hybrid for Enhanced Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Galek, T.; Szajna, E.; Bogdał, D.; Tupaj, M. Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe Pinnata as Potential Hemostatic Agents—Development and Characterization. Polymers 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef]
- Wasiak, J.; Cleland, H.; Campbell, F.; Spinks, A. Dressings for Superficial and Partial Thickness Burns. Cochrane Database Syst. Rev. 2013, 3, CD002106. [Google Scholar] [CrossRef]
- MacEwan, M.R.; MacEwan, S.; Kovacs, T.R.; Batts, J. What Makes the Optimal Wound Healing Material? A Review of Current Science and Introduction of a Synthetic Nanofabricated Wound Care Scaffold. Cureus 2017, 9, 1736. [Google Scholar] [CrossRef]
- Shi, R.; Niu, Y.; Gong, M.; Ye, J.; Tian, W.; Zhang, L. Antimicrobial Gelatin-Based Elastomer Nanocomposite Membrane Loaded with Ciprofloxacin and Polymyxin B Sulfate in Halloysite Nanotubes for Wound Dressing. Mater. Sci. Eng. C 2018, 87, 128–138. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-Based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction. Angew. Chem. Int. Ed. 2016, 55, 9984–9987. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Lee, M.-K.; Park, C.; Jung, H.-D.; Kim, H.-E.; Jang, T.-S. Fabrication of Strong, Bioactive Vascular Grafts with PCL/Collagen and PCL/Silica Bilayers for Small-Diameter Vascular Applications. Mater. Des. 2019, 181, 108079. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Chen, Y.-C.; Lin, T.-H.; Yang, C.-Y.; Kuo, Y.-W.; Lei, U. Hemostatic Enhancement via Chitosan Is Independent of Classical Clotting Pathways—A Quantitative Study. Polymers 2020, 12, 2391. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Ahmad Ruzaidi, D.A.; Mahat, M.M.; Mohamed Sofian, Z.; Nor Hashim, N.A.; Osman, H.; Nawawi, M.A.; Ramli, R.; Jantan, K.A.; Aizamddin, M.F.; Azman, H.H.; et al. Synthesis and Characterization of Porous, Electro-Conductive Chitosan–Gelatin–Agar-Based PEDOT: PSS Scaffolds for Potential Use in Tissue Engineering. Polymers 2021, 13, 2901. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated Chitin and Chitosan as Novel Biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pemble, O.J.; Bardosova, M.; Povey, I.M.; Pemble, M.E. A Slot-Die Technique for the Preparation of Continuous, High-Area, Chitosan-Based Thin Films. Polymers 2021, 13, 1566. [Google Scholar] [CrossRef]
- Marin, L.; Dragoi, B.; Olaru, N.; Perju, E.; Coroaba, A.; Doroftei, F.; Scavia, G.; Destri, S.; Zappia, S.; Porzio, W. Nanoporous Furfuryl-Imine-Chitosan Fibers as a New Pathway towards Eco-Materials for CO2 Adsorption. Eur. Polym. J. 2019, 120, 109214. [Google Scholar] [CrossRef]
- Lungu, R.; Anisiei, A.; Rosca, I.; Sandu, A.-I.; Ailincai, D.; Marin, L. Double Functionalization of Chitosan Based Nanofibers towards Biomaterials for Wound Healing. React. Funct. Polym. 2021, 167, 105028. [Google Scholar] [CrossRef]
- Anisiei, A.; Rosca, I.; Sandu, A.-I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers—A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary Ammonium Salts of Chitosan. A Critical Overview on the Synthesis and Properties Generated by Quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Ailincai, D.; Cibotaru, S.; Anisiei, A.; Coman, C.G.; Pasca, A.S.; Rosca, I.; Sandu, A.-I.; Mititelu-Tartau, L.; Marin, L. Mesoporous Chitosan Nanofibers Loaded with Norfloxacin and Coated with Phenylboronic Acid Perform as Bioabsorbable Active Dressings to Accelerate the Healing of Burn Wounds. Carbohydr. Polym. 2023, 318, 121135. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, M.; Najeeb, S.; Khurshid, Z.; Shah, A.; Husain, S.; Rehman, I. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Anisiei, A.; Oancea, F.; Marin, L. Electrospinning of Chitosan-Based Nanofibers: From Design to Prospective Applications. Rev. Chem. Eng. 2023, 39, 31–70. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Smagina, V.; Yudaev, P.; Kuskov, A.; Chistyakov, E. Polymeric Gel Systems Cytotoxicity and Drug Release as Key Features for Their Effective Application in Various Fields of Addressed Pharmaceuticals Delivery. Pharmaceutics 2023, 15, 830. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Park, J.-U.; Jung, H.-D.; Song, E.-H.; Choi, T.-H.; Kim, H.-E.; Song, J.; Kim, S. The Accelerating Effect of Chitosan-Silica Hybrid Dressing Materials on the Early Phase of Wound Healing. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. In Polysaccharides I; Springer: Berlin/Heidelberg, Germany, 2005; pp. 151–209. [Google Scholar]
- Muzzarelli, R.A.A. Chitin; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Rinaudo, M. Main Properties and Current Applications of Some Polysaccharides as Biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; Macmillan Education UK: London, UK, 1992; ISBN 978-1-349-11547-1. [Google Scholar]
- Campana-Filho, S.P.; de Britto, D.; Curti, E.; Abreu, F.R.; Cardoso, M.B.; Battisti, M.V.; Sim, P.C.; Goy, R.C.; Signini, R.; Lavall, R.L. Extraction, Structures and Properties of Alpha- and Beta-Chitin. Quim. Nova 2007, 30, 644–650. [Google Scholar] [CrossRef]
- Peter, M.G. Chitin and Chitosan from Animal Sources. In Biopolymers Online; Vandamme, E.J., De Baets, S., Steinbüchel, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic Liquids in the Processing and Chemical Modification of Chitin and Chitosan for Biomedical Applications. Green. Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Ailincai, D.; Sandu, A.-I.; Marin, L. Amphiphilic Chitosan-g-Poly(Trimethylene Carbonate)—A New Approach for Biomaterials Design. Int. J. Biol. Macromol. 2021, 193, 414–424. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Anisiei, A.; Rosca, I.; Sandu, A.-I.; Pasca, A.S.; Tartau, L.M.; Marin, L. Quaternized Chitosan/Chitosan Nanofibrous Mats: An Approach toward Bioactive Materials for Tissue Engineering and Regenerative Medicine. Carbohydr. Polym. 2023, 302, 120431. [Google Scholar] [CrossRef] [PubMed]
- Lungu, R.; Paun, M.-A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.-V.; Paun, V.-A.; Paun, V.-P. Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels 2022, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Narasimhan, B. Mathematical Models in Drug Delivery: How Modeling Has Shaped the Way We Design New Drug Delivery Systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.M.; Dobreci, D.L.; Irimiciuc, S.A.; Agop, M.; Petrescu, T.; Doroftei, B. A Theoretical Mathematical Model for Assessing Diclofenac Release from Chitosan-Based Formulations. Drug Deliv. 2020, 27, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S. Chitin and Chitosan as Novel Biotechnological Materials. Polym. Int. 1999, 48, 732–734. [Google Scholar] [CrossRef]
- Nottale, L. Scale Relativity and Fractal Space-Time: Applications to Quantum Physics, Cosmology and Chaotic Systems. Chaos Solitons Fractals 1996, 7, 877–938. [Google Scholar] [CrossRef]
- Merches, I.; Agop, M. Differentiability and Fractality in Dynamics of Physical Systems; World Scientific: Singapore, 2015; ISBN 978-981-4678-38-4. [Google Scholar]
- Munceanu, G.V.; Paun, V.-P.; Casian-Botez, I.; Agop, M. The Microscopic-Macroscopic Scale Transformation through a Chaos Scenario in the Fractal Space-Time Theory. Int. J. Bifurc. Chaos 2011, 21, 603–618. [Google Scholar] [CrossRef]
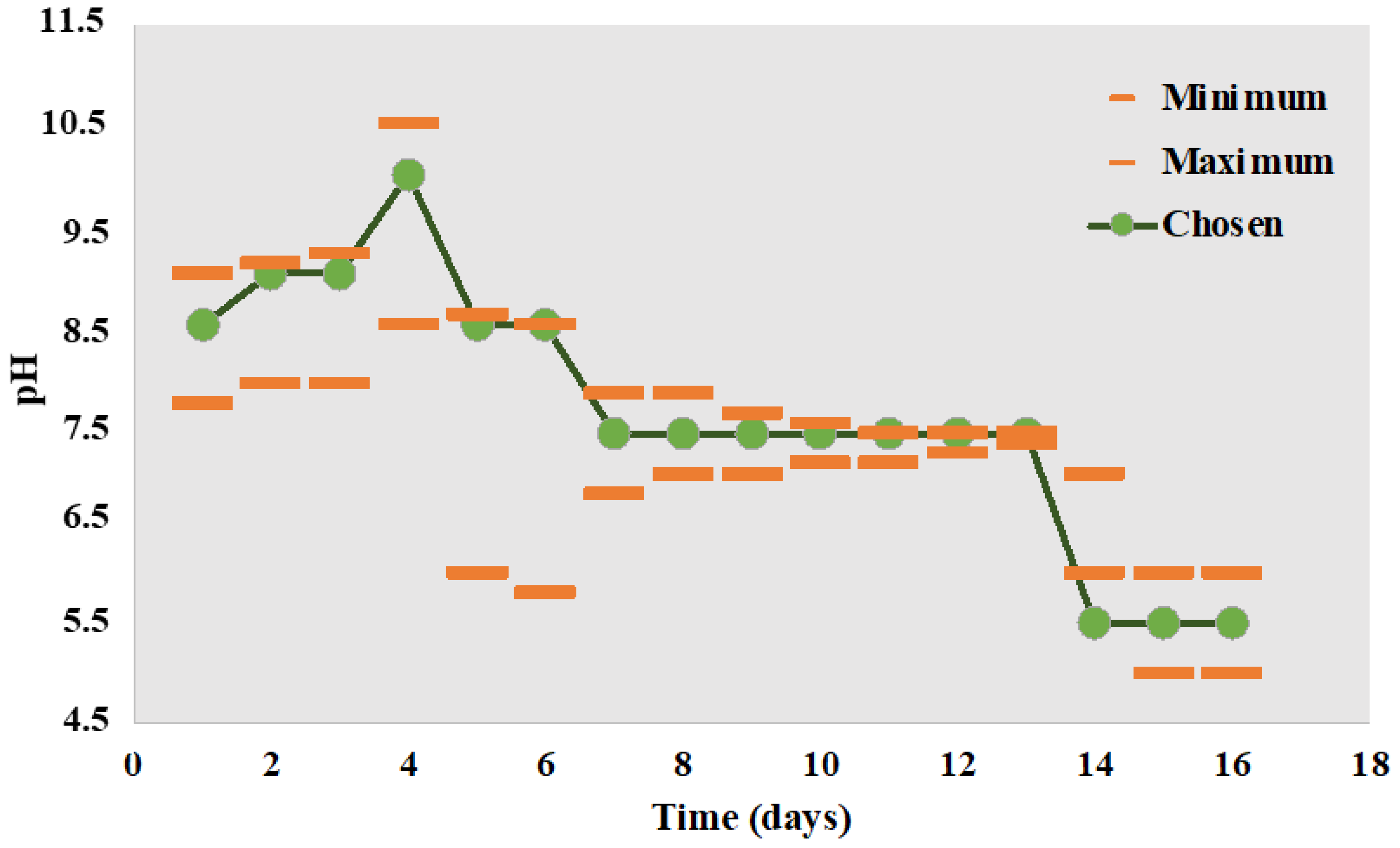
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased Wound PH as an Indicator of Local Wound Infection in Second Degree Burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Loubaki, E.; Ourevitch, M.; Sicsic, S. Chemical Modification of Chitosan by Glycidyl Trimethylammonium Chloride. Characterization of Modified Chitosan by 13C- and 1H-NMR Spectroscopy. Eur. Polym. J. 1991, 27, 311–317. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Equilibrium Swelling Study of Crosslinked Chitosan Membranes in Water, Buffer and Salt Solutions. Prog. Chem. Appl. Chitin Deriv. 2016, 21, 55–62. [Google Scholar] [CrossRef]
- Carrera-Gallissà, E.; Capdevila, X.; Valldeperas, J.; Abril, H. New Approach to Assessing Fabric Drape Based on the Fractal Dimension. Fibres Text. East. Eur. 2015, 23, 66–70. [Google Scholar] [CrossRef]
- Freitas, E.D.; Moura, C.F., Jr.; Kerwald, J.; Beppu, M.M. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers 2020, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Huang, L.; Wang, Y.; Mei, L.; Fan, R.; He, M.; Wang, C.; Tong, A.; Chen, H.; Guo, G. Stereocomplexed Electrospun Nanofibers Containing Poly (Lactic Acid) Modified Quaternized Chitosan for Wound Healing. Carbohydr. Polym. 2020, 247, 116754. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 899760. [Google Scholar] [CrossRef]
- Gonçalves da Costa Sousa, M.; Conceição de Almeida, G.; Martins Mota, D.C.; Andrade da Costa, R.; Dias, S.C.; Limberger, S.N.; Ko, F.; Lin, L.T.; Haney, E.F.; Etayash, H.; et al. Antibiofilm and Immunomodulatory Resorbable Nanofibrous Filing for Dental Pulp Regenerative Procedures. Bioact. Mater. 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef]
- Namgung, S.D.; Song, M.; Sung, T.; Cho, O.H.; Ju, M.; Kim, H.; Lee, Y.; Nam, K.T.; Kwon, J. Tyrosine-Rich Peptide Insulator for Rapidly Dissolving Transient Electronics. Adv. Mater. Technol. 2020, 5, 202000516. [Google Scholar] [CrossRef]
- Kafi, M.A.; Paul, A.; Vilouras, A.; Dahiya, R. Mesoporous Chitosan Based Conformable and Resorbable Biostrip for Dopamine Detection. Biosens. Bioelectron. 2020, 147, 111781. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial Wound Dressings for the Treatment of Exuding Wounds: An in-Depth Physico-Chemical Comparative Study. Burns Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef] [PubMed]



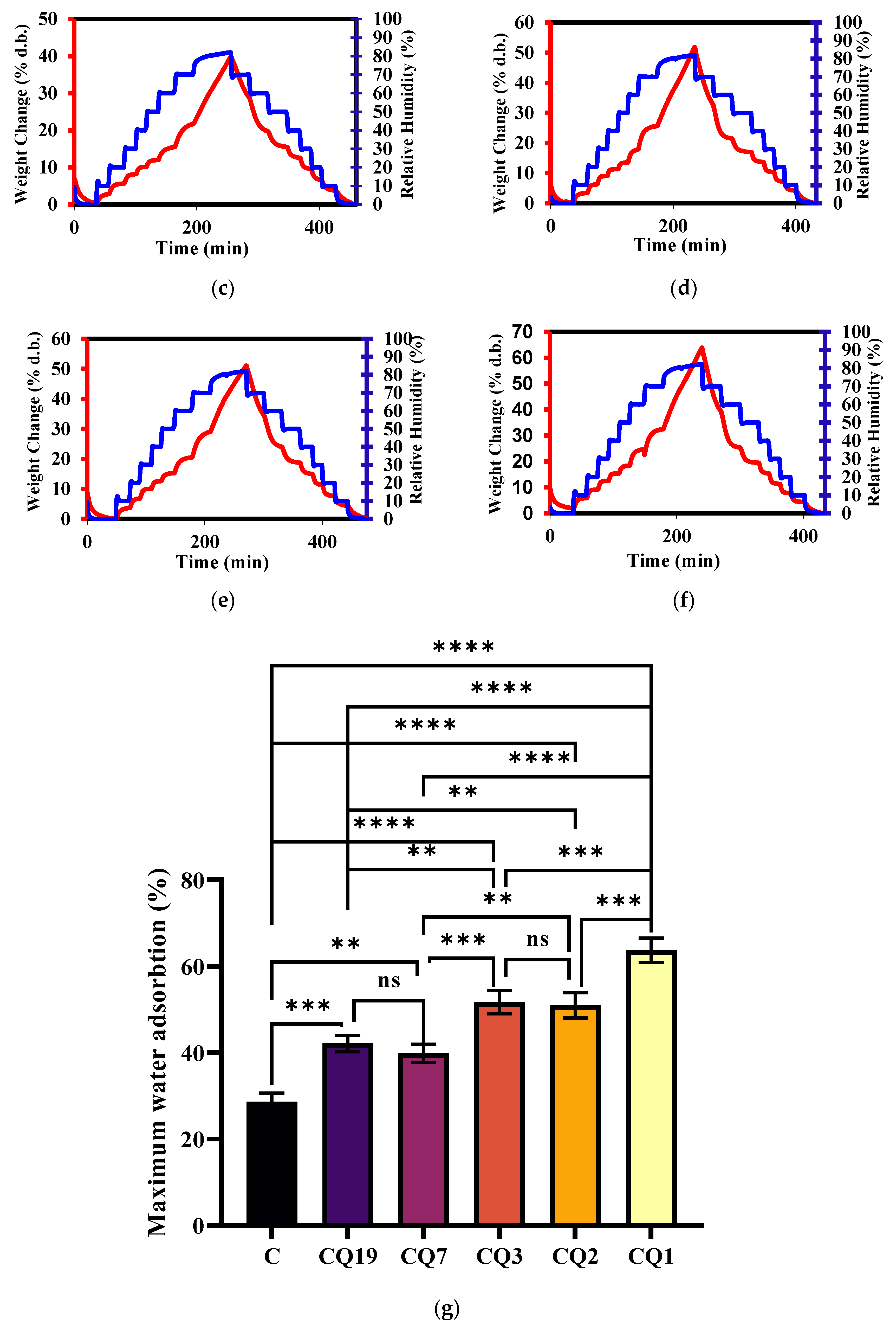
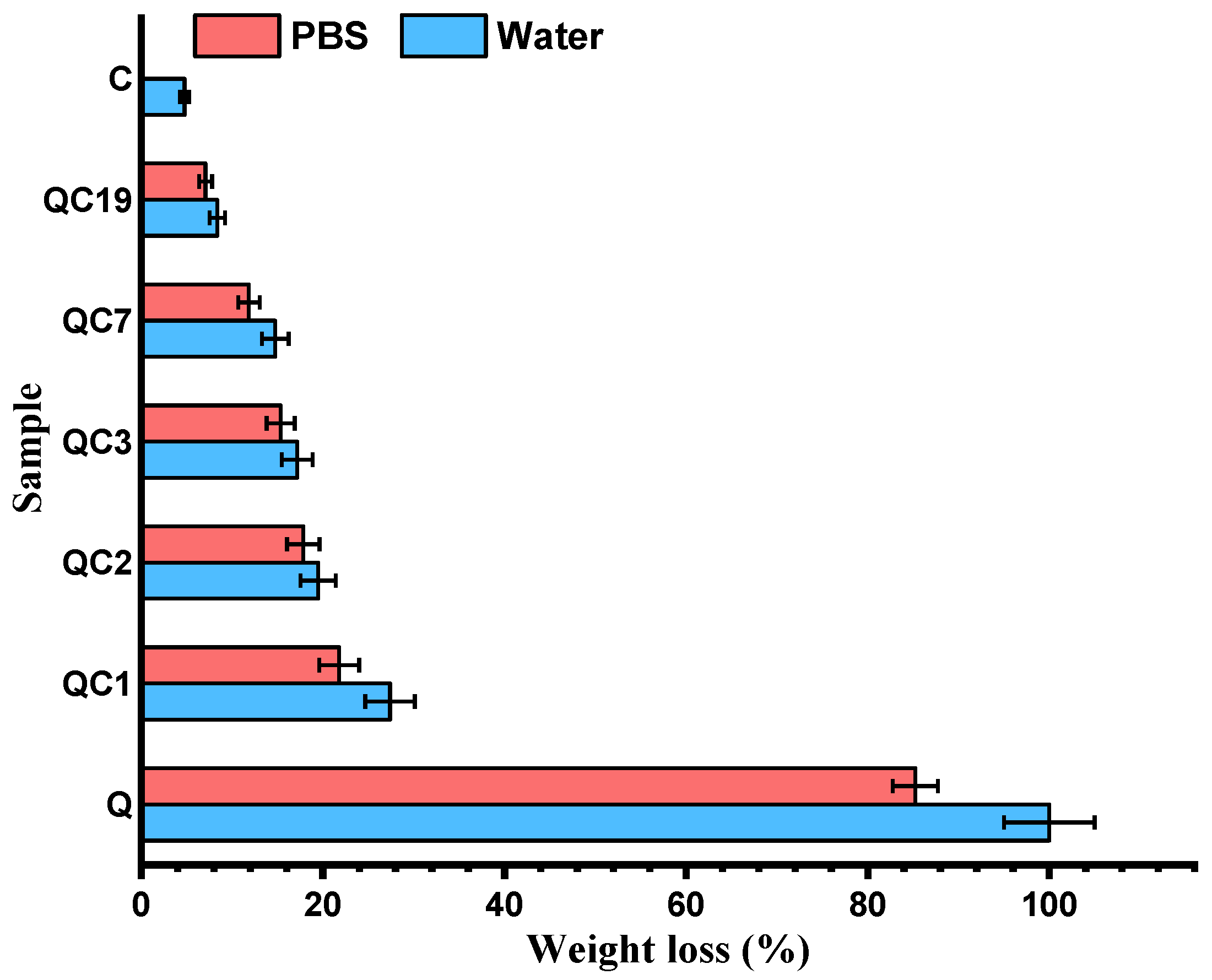
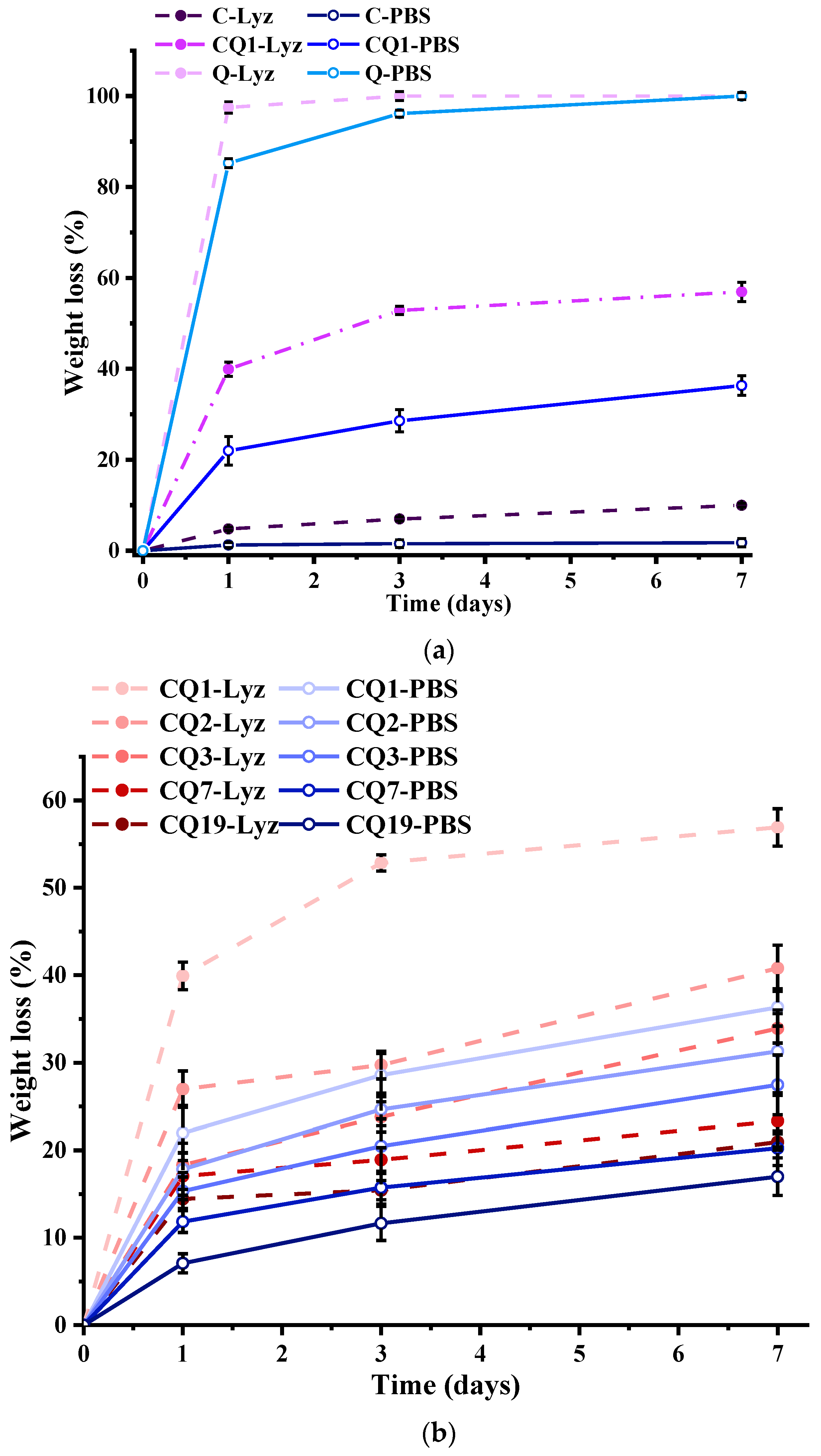
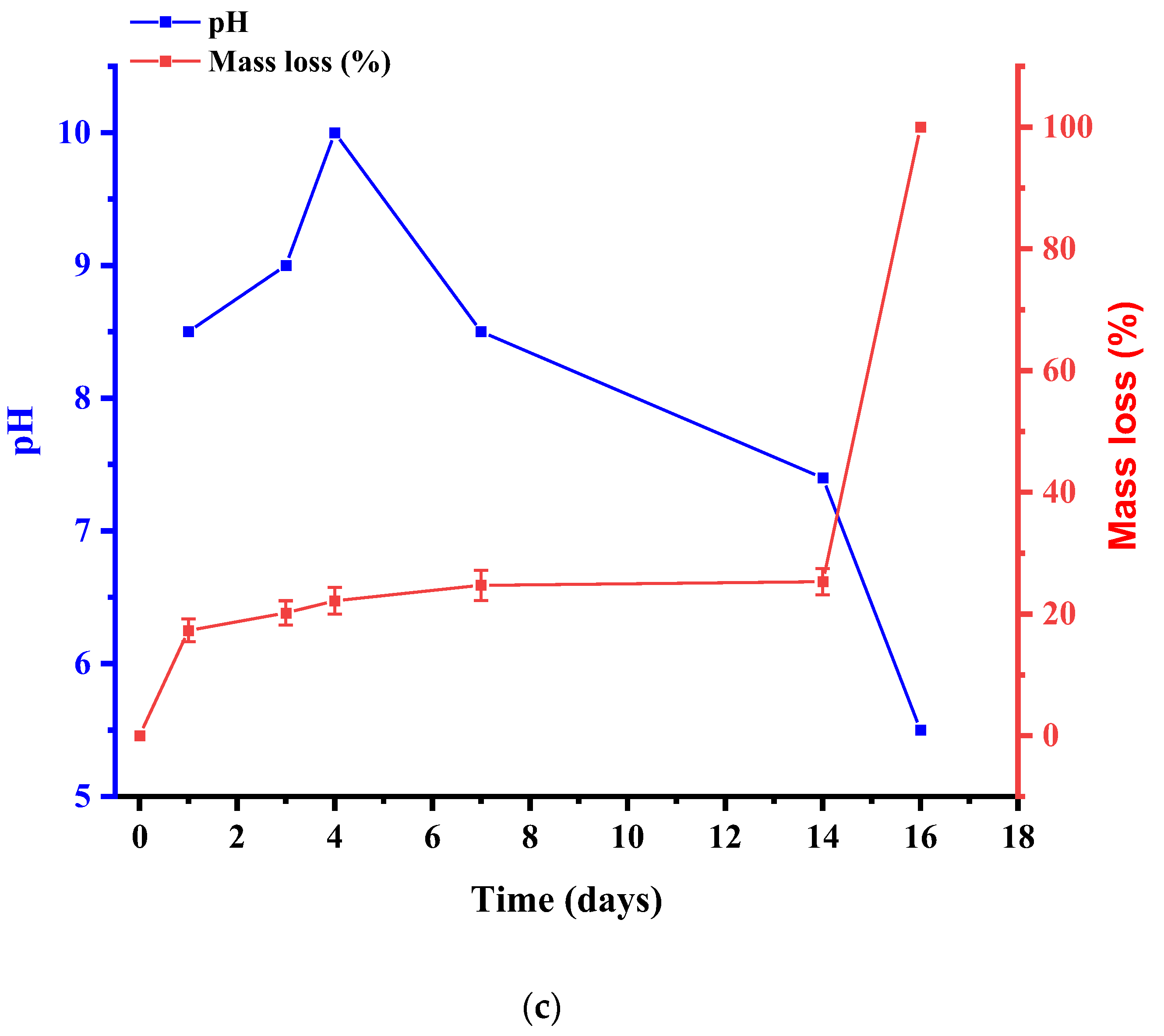
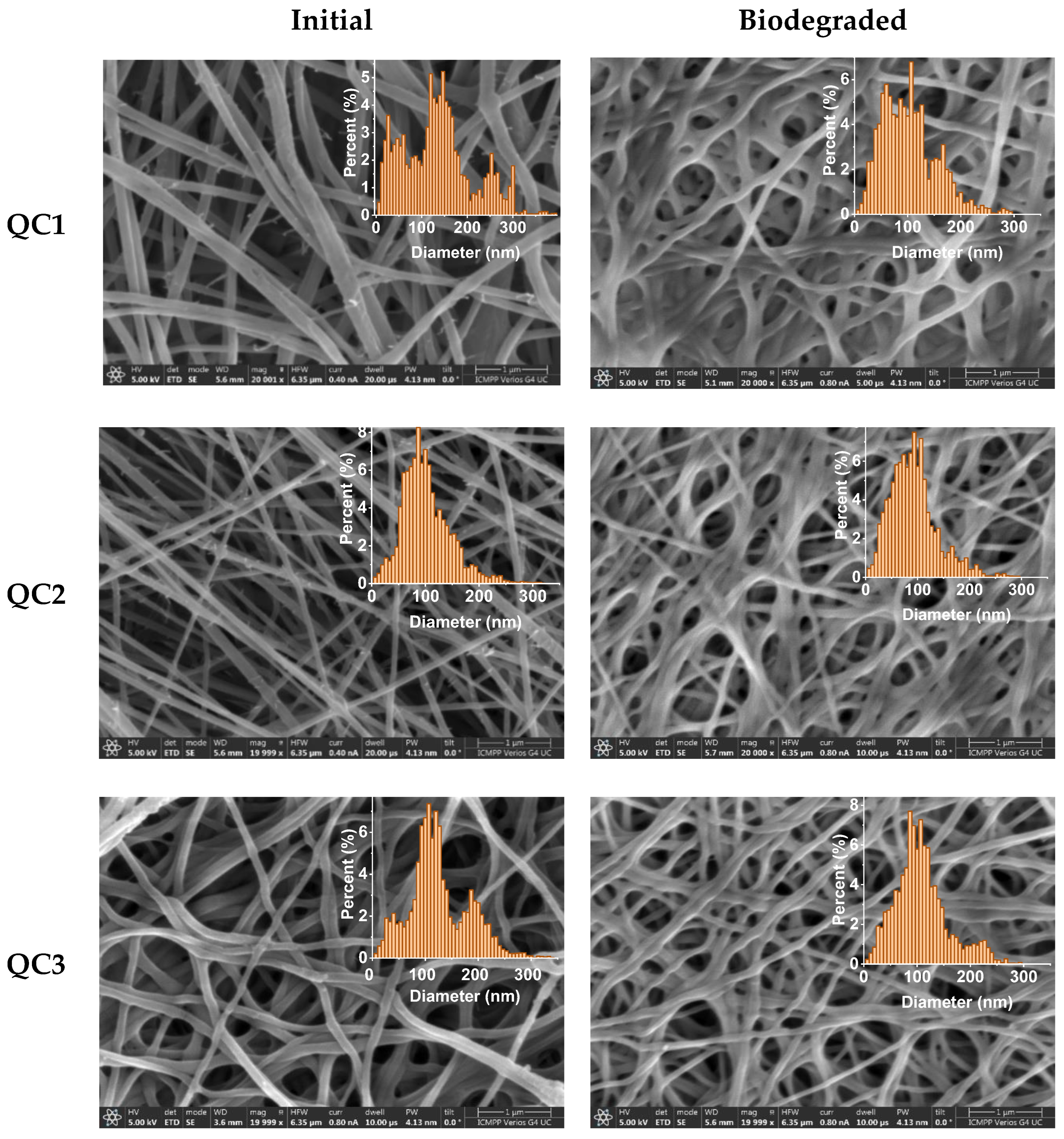
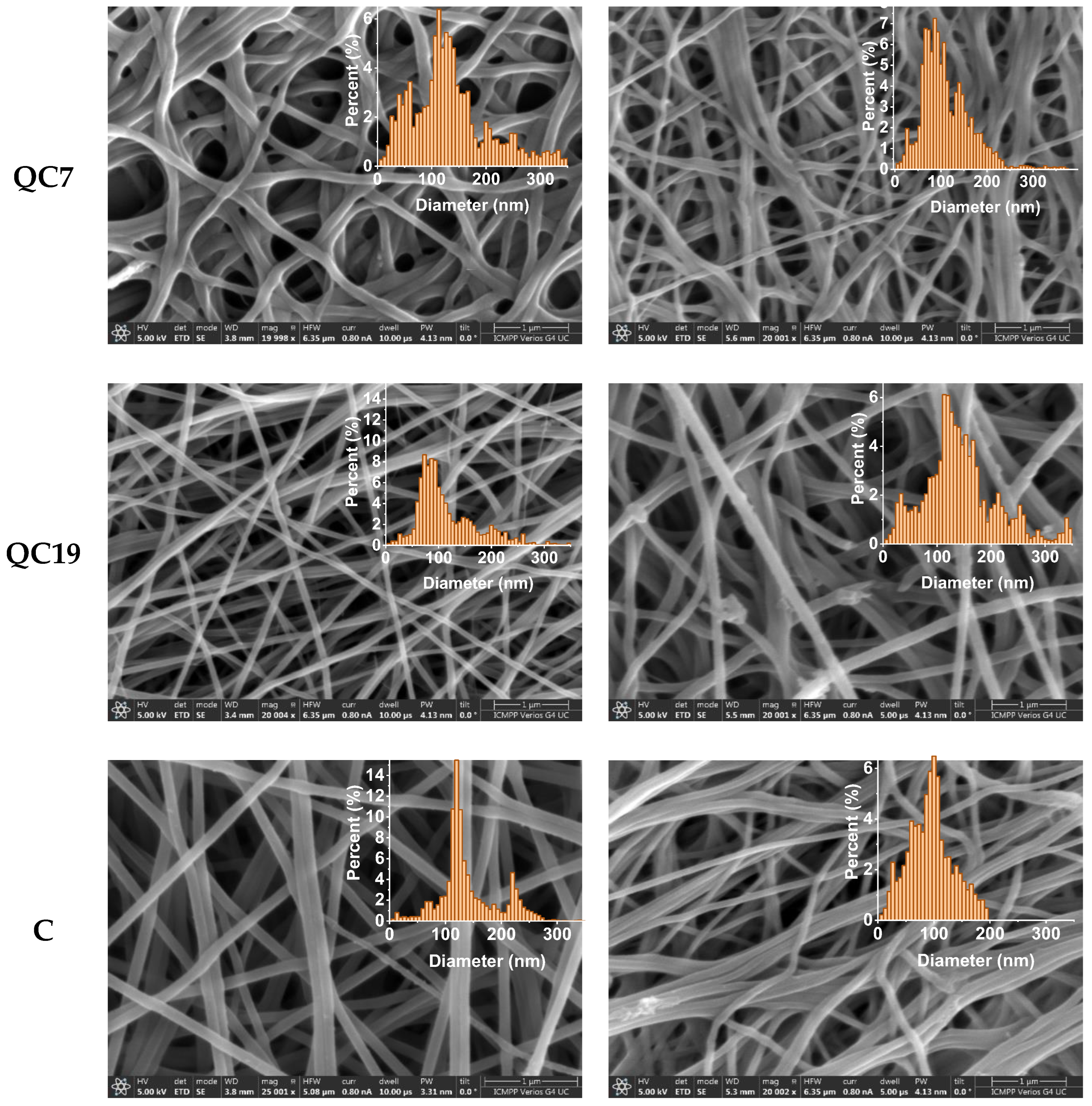
| Initial | After Biodegradation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | QC1 | QC2 | QC3 | QC7 | QC19 | C | QC1 | QC2 | QC3 | QC7 | QC19 | C |
| Mean (nm) | 120.54 | 91.92 | 112.69 | 117.12 | 86.68 | 121.01 | 91.76 | 83.41 | 98.16 | 96.48 | 131.38 | 91.49 |
| SD (nm) | 82.24 | 36.43 | 44.17 | 54.44 | 24.36 | 10.61 | 50.36 | 38.19 | 38.27 | 42.72 | 48.85 | 37.13 |
| HM (nm) | 146.67 | 86.67 | 106.67 | 113.33 | 73.33 | 120.00 | 106.67 | 93.33 | 86.67 | 86.67 | 113.33 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreica, B.-I.; Anisiei, A.; Iftime, M.-M.; Ababei, R.-V.; Ochiuz, L.; Vasincu, D.; Vasilache, I.-A.; Volovat, C.; Boboc, D.; Poroch, V.; et al. Theoretical—Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers’ Behavior in Wound Exudate Media. Pharmaceutics 2023, 15, 2722. https://doi.org/10.3390/pharmaceutics15122722
Andreica B-I, Anisiei A, Iftime M-M, Ababei R-V, Ochiuz L, Vasincu D, Vasilache I-A, Volovat C, Boboc D, Poroch V, et al. Theoretical—Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers’ Behavior in Wound Exudate Media. Pharmaceutics. 2023; 15(12):2722. https://doi.org/10.3390/pharmaceutics15122722
Chicago/Turabian StyleAndreica, Bianca-Iustina, Alexandru Anisiei, Manuela-Maria Iftime, Razvan-Vasile Ababei, Lacramioara Ochiuz, Decebal Vasincu, Ingrid-Andrada Vasilache, Constantin Volovat, Diana Boboc, Vladimir Poroch, and et al. 2023. "Theoretical—Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers’ Behavior in Wound Exudate Media" Pharmaceutics 15, no. 12: 2722. https://doi.org/10.3390/pharmaceutics15122722





