Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches
Abstract
:1. Introduction
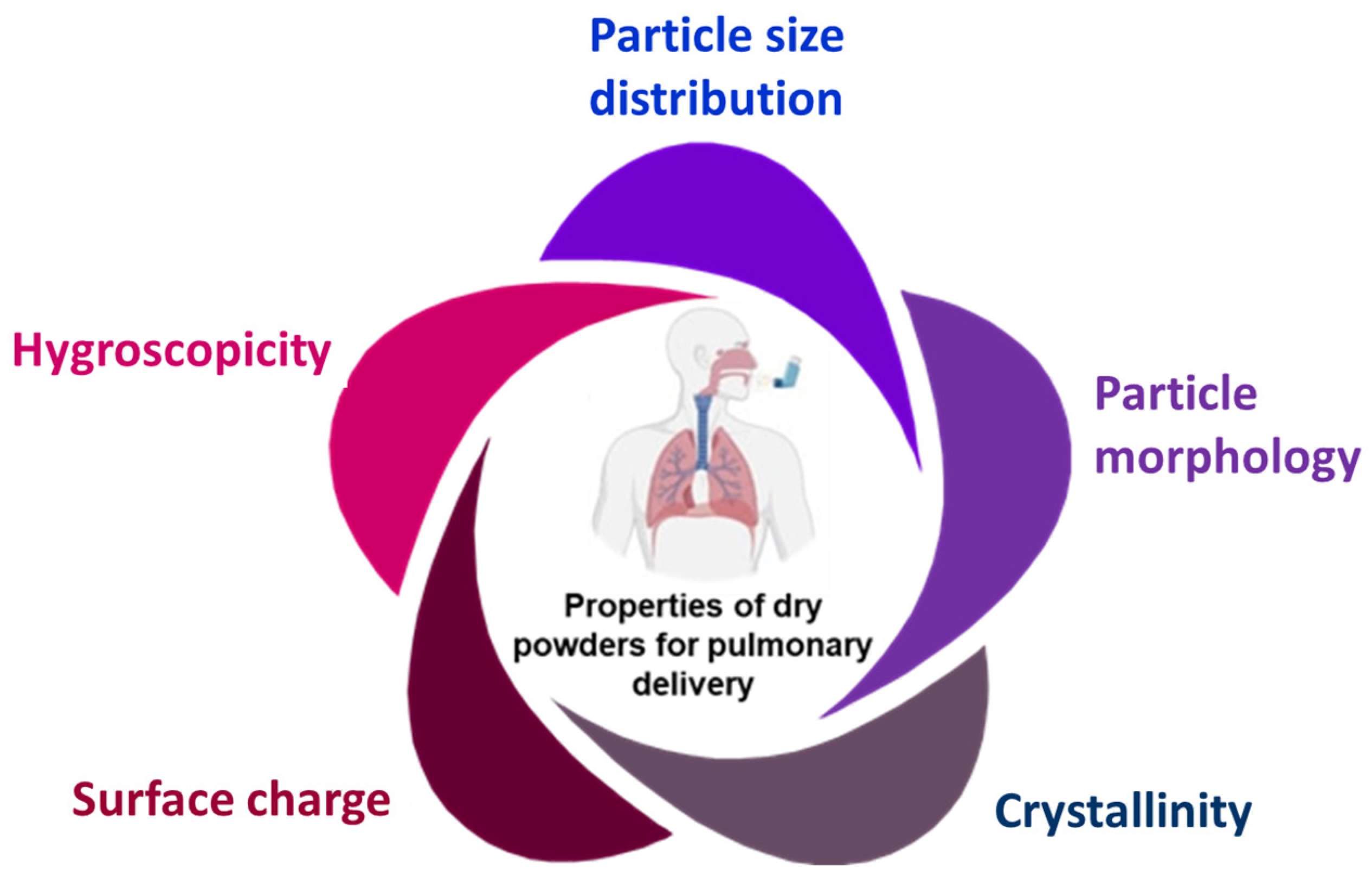
2. Physicochemical Properties of Inhaled Therapeutic Ingredients
2.1. Particle Size and Distribution
2.2. Particle Morphology
2.3. Crystallinity
2.4. Hygroscopicity
2.5. Surface Charge
3. Carriers and Excipients Used for Inhaled Dry Powder Formulations
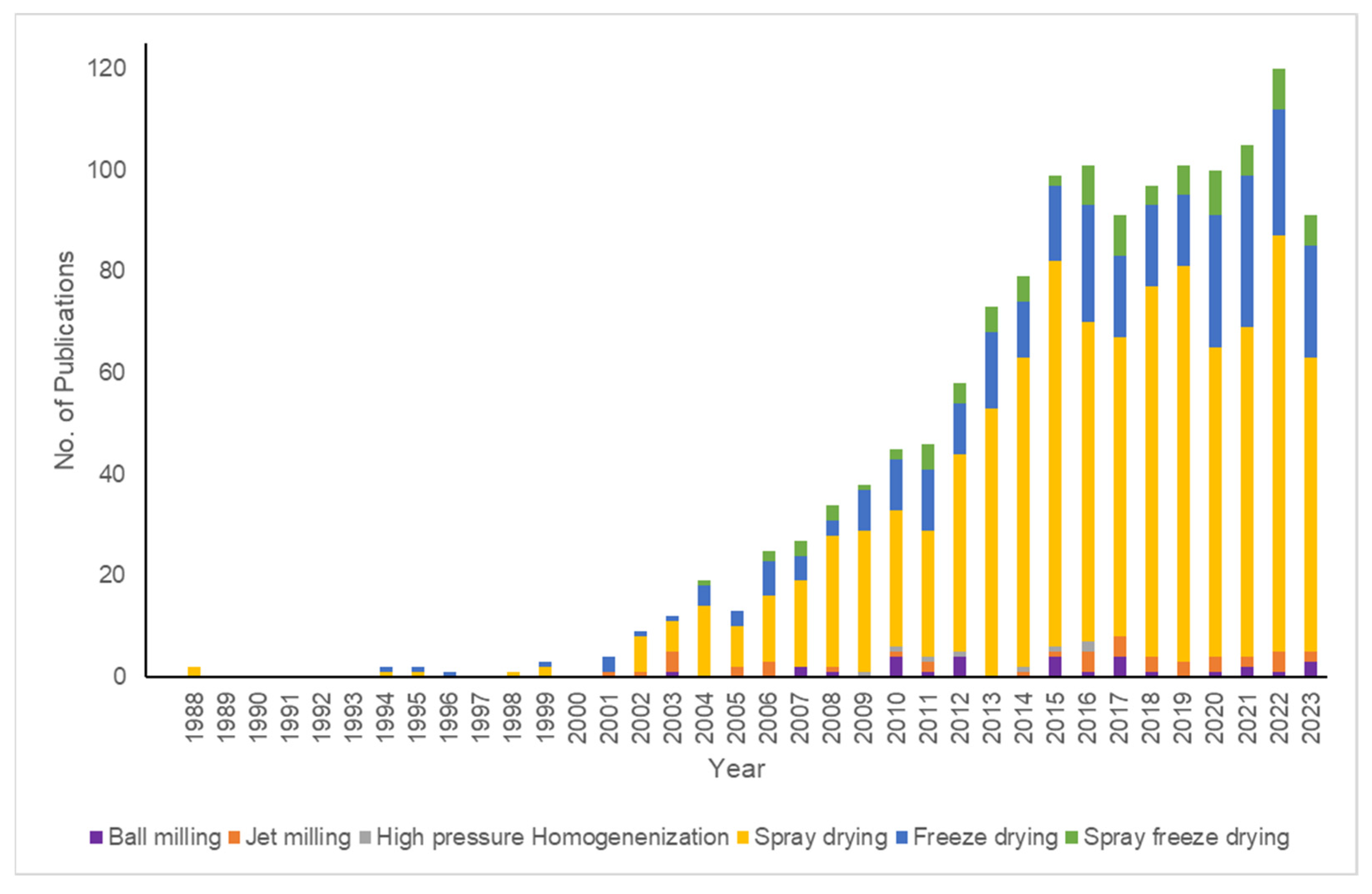
4. Approaches for Particle Engineering
4.1. Top-Down Approaches
4.1.1. Ball Milling
4.1.2. Media Milling
4.1.3. Jet Milling
4.1.4. High Pressure Homogenization (HPH)
4.2. Bottom-Up Approaches
4.2.1. Spray Drying (SD)
Nanospray Drying
4.2.2. Freeze Drying (FD)/Lyophilization
4.2.3. Spray Freeze Drying (SFD)
4.2.4. Supercritical Fluid Technology (SCF)
4.2.5. Electrohydrodynamic Approaches
4.3. Hybrid Techniques
5. Other Emerging Approaches in Inhaled Therapeutic Ingredient Preparation
6. Clinical Trials for the Assessment of Inhaled Dry Powder Formulations
7. Prospects of Dry Powder Formulations for Inhalation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavanya, M.N.; Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Aerosol-Based Pulmonary Delivery of Therapeutic Molecules from Food Sources: Delivery Mechanism, Research Trends, and the Way Forward. Food Rev. Int. 2022, 38, 753–788. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Brunel, J.M. Scope and Limitations on Aerosol Drug Delivery for the Treatment of Infectious Respiratory Diseases. J. Control. Release 2020, 325, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J. Emerging Trends in Inhaled Drug Delivery. Adv. Drug Deliv. Rev. 2020, 157, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ma, Y.; Zhu, J. The Future of Dry Powder Inhaled Therapy: Promising or Discouraging for Systemic Disorders? Int. J. Pharm. 2022, 614, 121457. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Spray-Freeze-Dried Dry Powder Inhalation of Insulin-Loaded Liposomes for Enhanced Pulmonary Delivery. J. Drug Target. 2008, 16, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Bothiraja, C.; Kadam, S.; Pawar, A. Potential of Dry Powder Inhalers for Tuberculosis Therapy: Facts, Fidelity and Future. Artif. Cells Nanomed. Biotechnol. 2018, 46, S791–S806. [Google Scholar] [CrossRef] [PubMed]
- Sou, T.; Meeusen, E.N.; de Veer, M.; Morton, D.A.V.; Kaminskas, L.M.; McIntosh, M.P. New Developments in Dry Powder Pulmonary Vaccine Delivery. Trends Biotechnol. 2011, 29, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Salar-Behzadi, S. Oral Inhalation for Delivery of Proteins and Peptides to the Lungs. Eur. J. Pharm. Biopharm. 2021, 163, 198–211. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef]
- De Pablo, E.; Fernández-García, R.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Nebulised Antibiotherapy: Conventional versus Nanotechnology-Based Approaches, Is Targeting at a Nano Scale a Difficult Subject? Ann. Transl. Med. 2017, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wong, J.; Qu, L.; Chan, H.; Zhou, Q.T. Powder Production and Particle Engineering for Dry Powder Inhaler Formulations. Curr. Pharm. Des. 2015, 21, 3902–3916. [Google Scholar] [CrossRef] [PubMed]
- Courrier, H.M.; Butz, N.; Vandamme, T.F. Pulmonary Drug Delivery Systems: Recent Developments and Prospects. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.Y.K.; Bagster, D.F.; Chan, H.-K. Effect of Particle Size, Air Flow and Inhaler Device on the Aerosolisation of Disodium Cromoglycate Powders. Int. J. Pharm. 2000, 206, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Lau, R.W.M. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- French, D.L.; Edwards, D.A.; Niven, R.W. The Influence of Formulation on Emission, Deaggregation and Deposition of Dry Powders for Inhalation. J. Aerosol Sci. 1996, 27, 769–783. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Nguyen, P.-A.; Andya, J.D.; Dasovich, N.; Sweeney, T.D.; Shire, S.J.; Hsu, C.C. Effect of Spray Drying and Subsequent Processing Conditions on Residual Moisture Content and Physical/Biochemical Stability of Protein Inhalation Powders. Pharm. Res. 1998, 15, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.Y.K.; Chan, H.-K. The Role of Particle Properties in Pharmaceutical Powder Inhalation Formulations. J. Aerosol Med. 2002, 15, 325–330. [Google Scholar] [CrossRef]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical Stability of Dry Powder Inhaler Formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cheng, J.; Chen, H.; Xu, J.; Liu, Z.; Shi, Q.; Zhang, C. Recent Advances in the Application of Characterization Techniques for Studying Physical Stability of Amorphous Pharmaceutical Solids. Crystals 2021, 11, 1440. [Google Scholar] [CrossRef]
- Zhu, K.; Tan, R.B.H.; Chen, F.; Ong, K.H.; Heng, P.W.S. Influence of Particle Wall Adhesion on Particle Electrification in Mixers. Int. J. Pharm. 2007, 328, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gengenbach, T.; Denman, J.A.; Yu, H.H.; Li, J.; Chan, H.K. Synergistic Antibiotic Combination Powders of Colistin and Rifampicin Provide High Aerosolization Efficiency and Moisture Protection. AAPS J. 2014, 16, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of Moist Pharmaceutical Powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Shariare, M.H.; De Matas, M.; York, P. Effect of Crystallisation Conditions and Feedstock Morphology on the Aerosolization Performance of Micronised Salbutamol Sulphate. Int. J. Pharm. 2011, 415, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W.; Hussain, T.; Alhalaweh, A.; Nokhodchi, A. Towards a More Desirable Dry Powder Inhaler Formulation: Large Spray-Dried Mannitol Microspheres Outperform Small Microspheres. Pharm. Res. 2014, 31, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J. Pharmaceutical Inhalation Aerosol Technology; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0429223463. [Google Scholar]
- Zillen, D.; Beugeling, M.; Hinrichs, W.L.J.; Frijlink, H.W.; Grasmeijer, F. Natural and Bioinspired Excipients for Dry Powder Inhalation Formulations. Curr. Opin. Colloid Interface Sci. 2021, 56, 101497. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation Strategy and Use of Excipients in Pulmonary Drug Delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry Powders for Oral Inhalation Free of Lactose Carrier Particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Steckel, H.; Rasenack, N.; Villax, P.; Müller, B.W. In Vitro Characterization of Jet-Milled and in-Situ-Micronized Fluticasone-17-Propionate. Int. J. Pharm. 2003, 258, 65–75. [Google Scholar] [CrossRef]
- Luinstra, M.; Grasmeijer, F.; Hagedoorn, P.; Moes, J.R.; Frijlink, H.W.; De Boer, A.H. A Levodopa Dry Powder Inhaler for the Treatment of Parkinson’s Disease Patients in off Periods. Eur. J. Pharm. Biopharm. 2015, 97, 22–29. [Google Scholar] [CrossRef]
- Yazdi, A.K.; Smyth, H.D.C. Hollow Crystalline Straws of Diclofenac for High-Dose and Carrier-Free Dry Powder Inhaler Formulations. Int. J. Pharm. 2016, 502, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tulbah, A.S.; Ong, H.X.; Morgan, L.; Colombo, P.; Young, P.M.; Traini, D. Dry Powder Formulation of Simvastatin. Expert Opin. Drug Deliv. 2015, 12, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Wauthoz, N.; Sebti, T.; Vanderbist, F.; Amighi, K. New Inhalation-Optimized Itraconazole Nanoparticle-Based Dry Powders for the Treatment of Invasive Pulmonary Aspergillosis. Int. J. Nanomed. 2012, 7, 5475–5489. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, T.; Liu, P.; Rahikkala, A.; Peltonen, L.; Kauppinen, E.I.; Hirvonen, J.; Järvinen, K.; Raula, J. Intact Nanoparticulate Indomethacin in Fast-Dissolving Carrier Particles by Combined Wet Milling and Aerosol Flow Reactor Methods. Pharm. Res. 2011, 28, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Mangal, S.; Park, H.; Wang, S.; Cavallaro, A.; Zhou, Q.T. Simultaneous Particle Size Reduction and Homogeneous Mixing to Produce Combinational Powder Formulations for Inhalation by the Single-Step Co-Jet Milling. J. Pharm. Sci. 2019, 108, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Ourique, A.F.; dos Santos Chaves, P.; Souto, G.D.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Redispersible Liposomal-N-Acetylcysteine Powder for Pulmonary Administration: Development, in Vitro Characterization and Antioxidant Activity. Eur. J. Pharm. Sci. 2014, 65, 174–182. [Google Scholar] [CrossRef]
- Manca, M.L.; Valenti, D.; Sales, O.D.; Nacher, A.; Fadda, A.M.; Manconi, M. Fabrication of Polyelectrolyte Multilayered Vesicles as Inhalable Dry Powder for Lung Administration of Rifampicin. Int. J. Pharm. 2014, 472, 102–109. [Google Scholar] [CrossRef]
- Chan, J.G.Y.; Duke, C.C.; Ong, H.X.; Chan, J.C.Y.; Tyne, A.S.; Chan, H.-K.; Britton, W.J.; Young, P.M.; Traini, D. A Novel Inhalable Form of Rifapentine. J. Pharm. Sci. 2014, 103, 1411–1421. [Google Scholar] [CrossRef]
- Rojanarat, W.; Changsan, N.; Tawithong, E.; Pinsuwan, S.; Chan, H.-K.; Srichana, T. Isoniazid Proliposome Powders for Inhalation—Preparation, Characterization and Cell Culture Studies. Int. J. Mol. Sci. 2011, 12, 4414–4434. [Google Scholar] [CrossRef]
- Khatib, I.; Khanal, D.; Ruan, J.; Cipolla, D.; Dayton, F.; Blanchard, J.D.; Chan, H.-K. Ciprofloxacin Nanocrystals Liposomal Powders for Controlled Drug Release via Inhalation. Int. J. Pharm. 2019, 566, 641–651. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D. Inhalable Dry Powder Prepared from Folic Acid-Conjugated Docetaxel Liposomes Alters Pharmacodynamic and Pharmacokinetic Properties Relevant to Lung Cancer Chemotherapy. Pulm. Pharmacol. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Osman, R.; Al-Jamal, K.T.; Holayel, S.M.; Geneidi, A.-S. Enhanced Antitubercular Activity, Alveolar Deposition and Macrophages Uptake of Mannosylated Stable Nanoliposomes. J. Drug Deliv. Sci. Technol. 2019, 51, 513–523. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Lu, X.; Jiang, L.; Xi, X.; Liu, J.; Zhu, J. Development and Evaluation of a Dry Powder Formulation of Liposome-Encapsulated Oseltamivir Phosphate for Inhalation. Drug Deliv. 2015, 22, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Sinsuebpol, C.; Chatchawalsaisin, J.; Kulvanich, P. Preparation and in Vivo Absorption Evaluation of Spray Dried Powders Containing Salmon Calcitonin Loaded Chitosan Nanoparticles for Pulmonary Delivery. Drug Des. Devel. Ther. 2013, 7, 861–873. [Google Scholar] [PubMed]
- Li, X.; Vogt, F.G.; Hayes, D., Jr.; Mansour, H.M. Physicochemical Characterization and Aerosol Dispersion Performance of Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Antibiotic Dry Powders of Tobramycin and Azithromycin for Pulmonary Inhalation Aerosol Delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, in Vitro Aerosol Dispersion, and Cellular Studies. AAPS PharmSciTech 2014, 15, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; di Villa Bianca, R.d.E.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry Powders Based on PLGA Nanoparticles for Pulmonary Delivery of Antibiotics: Modulation of Encapsulation Efficiency, Release Rate and Lung Deposition Pattern by Hydrophilic Polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an Inhaled Dry-Powder Formulation of Tobramycin Using PulmoSphereTM Technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Cai, X.; Yang, Y.; Xie, X.; Yu, F.; Yang, Y.; Yang, Z.; Zhang, T.; Mei, X. Preparation, Characterization and Pulmonary Pharmacokinetics of a New Inhalable Zanamivir Dry Powder. Drug Deliv. 2016, 23, 1962–1971. [Google Scholar] [CrossRef]
- Chvatal, A.; Ambrus, R.; Party, P.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Fattal, E.; Tsapis, N. Formulation and Comparison of Spray Dried Non-Porous and Large Porous Particles Containing Meloxicam for Pulmonary Drug Delivery. Int. J. Pharm. 2019, 559, 68–75. [Google Scholar] [CrossRef]
- N’Guessan, A.; Fattal, E.; Chapron, D.; Gueutin, C.; Koffi, A.; Tsapis, N. Dexamethasone Palmitate Large Porous Particles: A Controlled Release Formulation for Lung Delivery of Corticosteroids. Eur. J. Pharm. Sci. 2018, 113, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Hattori, Y.; Otsuka, M.; Ashizawa, K. Application of Spray Freeze Drying to Theophylline-Oxalic Acid Cocrystal Engineering for Inhaled Dry Powder Technology. Drug Dev. Ind. Pharm. 2020, 46, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A Comparison between Spray Drying and Spray Freeze Drying for Dry Powder Inhaler Formulation of Drug-Loaded Lipid–Polymer Hybrid Nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chan, A.Y.L.; Chow, M.Y.T.; Lo, F.F.K.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K.W. Spray Freeze Drying of Small Nucleic Acids as Inhaled Powder for Pulmonary Delivery. Asian J. Pharm. Sci. 2018, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yip, L.; Chow, M.Y.T.; Chow, S.F.; Chan, H.-K.; Kwok, P.C.L.; Lam, J.K.W. Porous and Highly Dispersible Voriconazole Dry Powders Produced by Spray Freeze Drying for Pulmonary Delivery with Efficient Lung Deposition. Int. J. Pharm. 2019, 560, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Li, L.; Huang, Y.; Singh, V.; Zhu, C.; Pan, X.; Quan, G.; Wu, C. Fragmented Particles Containing Octreotide Acetate Prepared by Spray Drying Technique for Dry Powder Inhalation. Drug Deliv. Transl. Res. 2018, 8, 693–701. [Google Scholar] [CrossRef]
- Pouya, M.A.; Daneshmand, B.; Aghababaie, S.; Faghihi, H.; Vatanara, A. Spray-Freeze Drying: A Suitable Method for Aerosol Delivery of Antibodies in the Presence of Trehalose and Cyclodextrins. AAPS PharmSciTech 2018, 19, 2247–2254. [Google Scholar] [CrossRef]
- Okuda, T.; Suzuki, Y.; Kobayashi, Y.; Ishii, T.; Uchida, S.; Itaka, K.; Kataoka, K.; Okamoto, H. Development of Biodegradable Polycation-Based Inhalable Dry Gene Powders by Spray Freeze Drying. Pharmaceutics 2015, 7, 233–254. [Google Scholar] [CrossRef]
- Chunhachaichana, C.; Sritharadol, R.; Sawatdee, S.; Heng, P.W.S.; Srichana, T. Development of Nanodispersion-Based Sildenafil Metered-Dose Inhalers Stabilized by Poloxamer 188: A Potential Candidate for the Treatment of Pulmonary Arterial Hypertension. Pharm. Dev. Technol. 2019, 24, 1218–1228. [Google Scholar] [CrossRef]
- Cho, W.; Kim, M.-S.; Jung, M.-S.; Park, J.; Cha, K.-H.; Kim, J.-S.; Park, H.J.; Alhalaweh, A.; Velaga, S.P.; Hwang, S.-J. Design of Salmon Calcitonin Particles for Nasal Delivery Using Spray-Drying and Novel Supercritical Fluid-Assisted Spray-Drying Processes. Int. J. Pharm. 2015, 478, 288–296. [Google Scholar] [CrossRef]
- Cabral, R.P.; Sousa, A.M.L.; Silva, A.S.; Paninho, A.I.; Temtem, M.; Costa, E.; Casimiro, T.; Aguiar-Ricardo, A. Design of Experiments Approach on the Preparation of Dry Inhaler Chitosan Composite Formulations by Supercritical CO2-Assisted Spray-Drying. J. Supercrit. Fluids 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Mayo, A.S.; Ambati, B.K.; Kompella, U.B. Gene Delivery Nanoparticles Fabricated by Supercritical Fluid Extraction of Emulsions. Int. J. Pharm. 2010, 387, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kito, D.; Oiwa, A.; Fukushima, M.; Hira, D.; Okamoto, H. Gene Silencing in a Mouse Lung Metastasis Model by an Inhalable Dry Small Interfering RNA Powder Prepared Using the Supercritical Carbon Dioxide Technique. Biol. Pharm. Bull. 2013, 36, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, P.; Najafabadi, A.R.; Haririan, I.; Vatanara, A.; Yamini, Y.; Darabi, M.; Gilani, K. Preparation of 5-Fluorouracil Nanoparticles by Supercritical Antisolvents for Pulmonary Delivery. Int. J. Nanomed. 2010, 5, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Kurniawansyah, F.; Duong, H.T.T.; Luu, T.D.; Mammucari, R.; Vittorio, O.; Boyer, C.; Foster, N. Inhalable Curcumin Formulations: Micronization and Bioassay. Chem. Eng. J. 2015, 279, 799–808. [Google Scholar] [CrossRef]
- Yue, P.; Zhou, W.; Huang, G.; Lei, F.; Chen, Y.; Ma, Z.; Chen, L.; Yang, M. Nanocrystals Based Pulmonary Inhalation Delivery System: Advance and Challenge. Drug Deliv. 2022, 29, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Kumar, A.; Wan, P.; Heng, S. ScienceDirect Overview of Milling Techniques for Improving the Solubility of Poorly Water-Soluble Drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef]
- Choi, J.C.; Kang, J.H.; Kim, D.W.; Park, C.W. Preparation and Evaluation of Inhalable Amifostine Microparticles Using Wet Ball Milling. Pharmaceutics 2023, 15, 1696. [Google Scholar] [CrossRef]
- Morton, D. Dry powder inhaler formu-lations com-prising sur-face-modified particles with anti-adherent additives. Patent US20110236492A1, 12 April 2011. [Google Scholar]
- Saleem, I.Y.; Smyth, H.D.C. Micronization of a Soft Material: Air-Jet and Micro-Ball Milling. AAPS PharmSciTech 2010, 11, 1642–1649. [Google Scholar] [CrossRef]
- MacKenzie, B.; Gopal, V.; Fan, L.; Maier, E.; Zhang, Y.; Wasnick, R.; Günther, A.; Williams, R., 3rd; Shetty, S. Late Breaking Abstract-Caveolin-1 Derived Peptide LTI-03 Promotes Epithelial Cell Survival and Attenuates Pulmonary Fibrosis. Eur. Respir. J. 2019, 54, PA1299. [Google Scholar]
- Zhang, Y.; MacKenzie, B.; Koleng, J.J.; Maier, E.; Warnken, Z.N.; Williams, R.O., III. Development of an Excipient-Free Peptide Dry Powder Inhalation for the Treatment of Pulmonary Fibrosis. Mol. Pharm. 2020, 17, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.; Chang, K.; Kim, H. Advancements in Particle Engineering for Inhalation Delivery of Small Molecules and Biotherapeutics. Pharm. Res. 2022, 39, 3047–3061. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Chan, H.; Watanabe, W. Formation, Characterization, and Fate of Inhaled Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.-H. High-Pressure Homogenization. Part I. Liquid-Liquid Dispersion in Turbulence Fields of High Energy Density. J. Food Eng. 1987, 6, 177–186. [Google Scholar] [CrossRef]
- Pandolfe, W.D. Effect of Dispersed and Continuous Phase Viscosity on Droplet Size of Emulsions Generated by Homogenization. J. Dispers. Sci. Technol. 1981, 2, 459–474. [Google Scholar] [CrossRef]
- Sinswat, P.; Overhoff, K.A.; McConville, J.T.; Johnston, K.P.; Williams, R.O., III. Nebulization of Nanoparticulate Amorphous or Crystalline Tacrolimus–Single-Dose Pharmacokinetics Study in Mice. Eur. J. Pharm. Biopharm. 2008, 69, 1057–1066. [Google Scholar] [CrossRef]
- Jacobs, C.; Müller, R.H. Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Keller, M.; Jauernig, J.; Lintz, F.C.; Knoch, M. Nebulizer Nanosuspensions: Important Device and Formulation Interactions. In Respiratory Drug Delivery VIII; The Aerosol Society, Ed.; The Aerosol Society: Lancaster, UK, 2002; pp. 197–206. [Google Scholar]
- Luangkhot, J.; Jauernig, J.; Balcke, A.; Lintz, F.C.; Liening-Ewert, R.; Stangl, R.; Keller, M. Characterisation of Salbutamol Solution Compared to Budesonide Suspensions Consisting of Submicron and Micrometer Particles in the PARI LC STAR and a New PARI Electronic Nebuliser (e-Flow). In Proceedings of the Drug Deliv Lungs XI, London, UK, 11–12 December 2000; pp. 14–17. [Google Scholar]
- El-Gendy, N.; Pornputtapitak, W.; Berkland, C. Nanoparticle Agglomerates of Fluticasone Propionate in Combination with Albuterol Sulfate as Dry Powder Aerosols. Eur. J. Pharm. Sci. 2011, 44, 522–533. [Google Scholar] [CrossRef]
- Bhavna; Ahmad, F.J.; Khar, R.K.; Sultana, S.; Bhatnagar, A. Techniques to Develop and Characterize Nanosized Formulation for Salbutamol Sulfate. J. Mater. Sci. Mater. Med. 2009, 20, 71–76. [Google Scholar] [CrossRef]
- James, J.; Crean, B.; Davies, M.; Toon, R.; Jinks, P.; Roberts, C.J. The Surface Characterisation and Comparison of Two Potential Sub-Micron, Sugar Bulking Excipients for Use in Low-Dose, Suspension Formulations in Metered Dose Inhalers. Int. J. Pharm. 2008, 361, 209–221. [Google Scholar] [CrossRef]
- Hadiwinoto, G.D.; Lip Kwok, P.C.; Lakerveld, R. A Review on Recent Technologies for the Manufacture of Pulmonary Drugs. Ther. Deliv. 2018, 9, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Li, S.; Hadinoto, K. Spray Drying Formulation of Hollow Spherical Aggregates of Silica Nanoparticles by Experimental Design. Chem. Eng. Res. Des. 2010, 88, 673–685. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.A.; Kumar, D.; Bolás, F.; Ballesteros, M.P.; et al. Targeting Lung Macrophages for Fungal and Parasitic Pulmonary Infections with Innovative Amphotericin B Dry Powder Inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Nobre, B.; Matos, A.S.; Silva, A.S.; Casimiro, T.; Corvo, M.L.; Aguiar-Ricardo, A. Inhalable Hydrophilic Molecule-Loaded Liposomal Dry Powder Formulations Using Supercritical CO2–Assisted Spray-Drying. J. CO2 Util. 2021, 53, 101709. [Google Scholar] [CrossRef]
- Rodrigues, S.; da Costa, A.M.R.; Flórez-Fernández, N.; Torres, M.D.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable Spray-Dried Chondroitin Sulphate Microparticles: Effect of Different Solvents on Particle Properties and Drug Activity. Polymers 2020, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Aldhubaib, B.; Nair, A.; Attimarad, M.; Venugopala, K.; Sa, K. Pharmacokinetics and Tissue Distribution of Microspheres Prepared by Spray Drying Technique: Targeted Drug Delivery. Biomed. Res. 2017, 28, 3387–3396. [Google Scholar]
- Lavanya, M.N.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Development of β-Carotene Aerosol Formulations Using a Modified Spray Dryer. J. Food Process Eng. 2020, 43, e13233. [Google Scholar] [CrossRef]
- Hamedani, S.; Yaqoubi, S.; Safdari, R.; Hamishehkar, H.; Nokhodchi, A. A Novel Particle Engineering Method for the Production of Inhalable Cromolyn Sodium Powders by a Combination of Spray Drier and Nebulizer. J. Drug Deliv. Sci. Technol. 2022, 78, 103958. [Google Scholar] [CrossRef]
- Weers, J.G.; Miller, D.P.; Tarara, T.E. Spray-Dried PulmoSphereTM Formulations for Inhalation Comprising Crystalline Drug Particles. AAPS PharmSciTech 2019, 20, 103. [Google Scholar] [CrossRef]
- Weers, J.; Tarara, T. The PulmoSphereTM Platform for Pulmonary Drug Delivery. Ther. Deliv. 2014, 5, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; López-iglesias, C.; Szewczyk, P.K.; Stachewicz, U.; Barros, J.; Alvarez-lorenzo, C.; Alnaief, M. A Pathway from Porous Particle Technology Toward Tailoring Aerogels for Pulmonary Drug Administration. Front. Bioeng. Biotechnol. 2021, 9, 671381. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Muenster, U.; Zhao, J.; Zhang, L.; Becker-Pelster, E.-M.; Rosenbruch, M.; Mao, S. Exploring Polyvinylpyrrolidone in the Engineering of Large Porous PLGA Microparticles via Single Emulsion Method with Tunable Sustained Release in the Lung: In Vitro and in Vivo Characterization. J. Control. Release 2017, 249, 11–22. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, R.; Zhou, J.; Mao, S. Recent Advances in Controlled Pulmonary Drug Delivery. Drug Discov. Today 2015, 20, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Shiehzadeh, F.; Tafaghodi, M.; Dehghani, M.-L.; Mashhoori, F.; Bazzaz, B.S.F.; Imenshahidi, M. Preparation and Characterization of a Dry Powder Inhaler Composed of PLGA Large Porous Particles Encapsulating Gentamicin Sulfate. Adv. Pharm. Bull. 2019, 9, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Jimenez-Shahed, J. Profile of Inhaled Levodopa and Its Potential in the Treatment of Parkinson’s Disease: Evidence to Date. Neuropsychiatr. Dis. Treat. 2018, 14, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Hohlfeld, J.M.; Grant, S.; Jain, M.; Cabanski, M.; Pertel, P.; Boulet, L.-P.; Cockcroft, D.W.; Davis, B.; Fitzgerald, J.M. Efficacy and Safety of an Inhaled Anti-TSLP Antibody Fragment in Adults with Mild Atopic Asthma. In B93. Late Breaking Clinical Trials in Airway Diseases; American Thoracic Society: New York, NY, USA, 2020; p. A4207. ISBN 1073-449X. [Google Scholar]
- Shepard, K.B.; Pluntze, A.M.; Vodak, D.T. Simultaneous Spray Drying for Combination Dry Powder Inhaler Formulations. Pharmaceutics 2022, 14, 1130. [Google Scholar] [CrossRef]
- Mcaffer, I.C.G.; Tasko, P.E.; Swift, G.J.; Gianfrancesco, S. Dry powder inhaler formulations. Patent WO2011018531A1, 17 February 2011. [Google Scholar]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Wan, K.Y.; Weng, J.; Wong, S.N.; Kwok, P.C.L.; Chow, S.F.; Chow, A.H.L. Converting Nanosuspension into Inhalable and Redispersible Nanoparticles by Combined In-Situ Thermal Gelation and Spray Drying. Eur. J. Pharm. Biopharm. 2020, 149, 238–247. [Google Scholar] [CrossRef]
- Bürki, K.; Jeon, I.; Arpagaus, C.; Betz, G. New Insights into Respirable Protein Powder Preparation Using a Nano Spray Dryer. Int. J. Pharm. 2011, 408, 248–256. [Google Scholar] [CrossRef]
- Schoubben, A.; Giovagnoli, S.; Tiralti, M.C.; Blasi, P.; Ricci, M. Capreomycin Inhalable Powders Prepared with an Innovative Spray-Drying Technique. Int. J. Pharm. 2014, 469, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.-F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.-F.; Wang, C.-H. Study on Formability of Solid Nanosuspensions during Nanodispersion and Solidification: I. Novel Role of Stabilizer/Drug Property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Lam, I.C.H.; Lin, H.H.S.; Wan, L.T.L.; Lo, J.C.K.; Tai, W.; Kwok, P.C.L.; Lam, J.K.W. Effect of Formulation and Inhaler Parameters on the Dispersion of Spray Freeze Dried Voriconazole Particles. Int. J. Pharm. 2020, 584, 119444. [Google Scholar] [CrossRef] [PubMed]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray Freeze Drying: Emerging Applications in Drug Delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Lamprecht, A. Spray Freeze Drying for Dry Powder Inhalation of Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Teo, J.; Chew, J.W.; Hadinoto, K. Dry Powder Inhaler Formulation of High-Payload Antibiotic Nanoparticle Complex Intended for Bronchiectasis Therapy: Spray Drying versus Spray Freeze Drying Preparation. Int. J. Pharm. 2016, 499, 38–46. [Google Scholar] [CrossRef]
- Lavanya, M.N.; Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Production of Bromelain Aerosols Using Spray-Freeze-Drying Technique for Pulmonary Supplementation. Dry. Technol. 2020, 39, 358–370. [Google Scholar] [CrossRef]
- Di, A.; Zhang, S.; Liu, X.; Tong, Z.; Sun, S.; Tang, Z.; Chen, X.D.; Wu, W.D. Microfluidic Spray Dried and Spray Freeze Dried Uniform Microparticles Potentially for Intranasal Drug Delivery and Controlled Release. Powder Technol. 2021, 379, 144–153. [Google Scholar] [CrossRef]
- Lucas, D.; Kožák, J.; Rautenberg, A.; Chrétien, C.; Pellequer, Y.; Lamprecht, A. Designing Highly Porous Amorphous Celecoxib Particles by Spray Freeze Drying Leads to Accelerated Drug Absorption In-Vivo. Eur. J. Pharm. Biopharm. 2022, 174, 20–28. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical Spray Freeze Drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef]
- Lam, K.W.; Pan, H.W.; Lo, C.K.J. Inhaled powder formulations for respiratory delivery of antibodies. Patent WO-2022111547-A1, 24 November 2023. [Google Scholar]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Expert Review Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Sousa, A.M.; Cabral, R.P.; Silva, M.C.; Costa, C.; Miguel, S.P.; Bonifácio, V.D.B.; Casimiro, T.; Correia, I.J.; Aguiar-Ricardo, A. Aerosolizable Gold Nano-in-Micro Dry Powder Formulations for Theragnosis and Lung Delivery. Int. J. Pharm. 2017, 519, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Restani, R.B.; Conde, J.; Pires, R.F.; Martins, P.; Fernandes, A.R.; Baptista, P.V.; Bonifácio, V.D.B.; Aguiar-Ricardo, A. POxylated Polyurea Dendrimers: Smart Core-Shell Vectors with IC50 Lowering Capacity. Macromol. Biosci. 2015, 15, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-Z.; Zhao, C.; Wang, S.-B.; Liu, Y.-G.; Lin, D.-L. Generation of Porous Poly-L-Lactide Microspheres by Emulsion-Combined Precipitation with a Compressed CO2 Antisolvent Process. J. Mater. Chem. B 2013, 1, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kankala, R.K.; Tang, N.; Xu, P.; Hao, L.; Yang, D.; Wang, S.; Zhang, Y.S.; Chen, A. Supercritical Fluid-assisted Porous Microspheres for Efficient Delivery of Insulin and Inhalation Therapy of Diabetes. Adv. Healthc. Mater. 2019, 8, 1800910. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Stephanie, C.; Xinyue, C.; Yayi, Z.; Henry, Z.; Yee, H.; Shing, T.; Chow, F. Inhalable Nanoparticle-Based Dry Powder Formulations for Respiratory Diseases: Challenges and Strategies for Translational Research. AAPS PharmSciTech 2023, 24, 98. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Electrospraying and Electrospinning Techniques for Nanoencapsulation. In Techniques for Nanoencapsulation of Food Ingredients; Springer: New York, NY, USA, 2014; pp. 43–49. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Nikolaou, M.; Krasia-Christoforou, T. Electrohydrodynamic Methods for the Development of Pulmonary Drug Delivery Systems. Eur. J. Pharm. Sci. 2018, 113, 29–40. [Google Scholar] [CrossRef]
- Arauzo, B.; Lobera, M.P.; Monzon, A.; Santamaria, J. Dry Powder Formulation for Pulmonary Infections: Ciprofloxacin Loaded in Chitosan Sub-Micron Particles Generated by Electrospray. Carbohydr. Polym. 2021, 273, 118543. [Google Scholar] [CrossRef]
- Patil, S.; Mahadik, A.; Nalawade, P.; More, P. Crystal Engineering of Lactose Using Electrospray Technology: Carrier for Pulmonary Drug Delivery. Drug Dev. Ind. Pharm. 2017, 43, 2085–2091. [Google Scholar] [CrossRef]
- Jahangiri, A.; Nokhodchi, A.; Asare-Addo, K.; Salehzadeh, E.; Emami, S.; Yaqoubi, S.; Hamishehkar, H. Carrier-Free Inhalable Dry Microparticles of Celecoxib: Use of the Electrospraying Technique. Biomedicines 2023, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Yaqoubi, S.; Adibkia, K.; Nokhodchi, A.; Emami, S.; Alizadeh, A.A.; Hamishehkar, H.; Barzegar-Jalali, M. Co-Electrospraying Technology as a Novel Approach for Dry Powder Inhalation Formulation of Montelukast and Budesonide for Pulmonary Co-Delivery. Int. J. Pharm. 2020, 591, 119970. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous Solid Dispersions and Nanocrystal Technologies for Poorly Water-Soluble Drug Delivery–an Update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Müller, R.H.; Möschwitzer, J.P. Systematical Investigation of a Combinative Particle Size Reduction Technology for Production of Resveratrol Nanosuspensions. AAPS PharmSciTech 2017, 18, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Carling, C.J.; Brülls, M. Milling of Poorly Soluble Crystalline Drug Compounds to Generate Appropriate Particle Sizes for Inhaled Sustained Drug Delivery. Int. J. Pharm. 2021, 593, 120116. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and in Vitro and in Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, S.; Zhang, S.; Yin, Q.; Chen, X.D.; Wu, W.D. Micro-Fluidic Spray Freeze Dried Ciprofloxacin Hydrochloride-Embedded Dry Powder for Inhalation. AAPS PharmSciTech 2022, 23, 211. [Google Scholar] [CrossRef]
- Zendehdel Baher, S.; Yaqoubi, S.; Asare-Addo, K.; Hamishehkar, H.; Nokhodchi, A. Dry Powder Formulation of Simvastatin Nanoparticles for Potential Application in Pulmonary Arterial Hypertension. Pharmaceutics 2022, 14, 895. [Google Scholar] [CrossRef]
- Garcia, A.; Mack, P.; Williams, S.; Fromen, C.; Shen, T.; Tully, J.; Pillai, J.; Kuehl, P.; Napier, M.; DeSimone, J.M. Microfabricated Engineered Particle Systems for Respiratory Drug Delivery and Other Pharmaceutical Applications. J. Drug Deliv. 2012, 2012, 941243. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the Printer to the Lungs: Inkjet-Printed Aerogel Particles for Pulmonary Delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Ma, X.; Su, Y.; Koleng, J.J.; Dolocan, A.; Williams, R.O., III. Using Thin Film Freezing to Minimize Excipients in Inhalable Tacrolimus Dry Powder Formulations. Int. J. Pharm. 2020, 586, 119490. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Watts, A.B.; Lu, X.; Su, Y.; Williams, R.O., III. Enhanced Aerosolization of High Potency Nanoaggregates of Voriconazole by Dry Powder Inhalation. Mol. Pharm. 2019, 16, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.R.; Kole, E.B.; Kapare, H.S.; Chandankar, S.M.; Shinde, P.J.; Boisa, G.S.; Salgaonkar, S.S.; Giram, P.S.; More, M.P.; Kolimi, P.; et al. Progress on Thin Film Freezing Technology for Dry Powder Inhalation Formulations. Pharmaceutics 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Ledford, J.G.; Mansour, H.M. Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection. Pharmaceutics 2021, 13, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Christensen, D.J.; Williams, R.O. Development of Remdesivir as a Dry Powder for Inhalation by Thin Film Freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of Fine Solid-Crystal Suspension with Enhanced Solubility, Stability, and Aerosolization Performance for Dry Powder Inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef]
- Silva, A.S.; Tavares, M.T.; Aguiar-Ricardo, A. Sustainable Strategies for Nano-in-Micro Particle Engineering for Pulmonary Delivery. J. Nanopart. Res. 2014, 16, 2602. [Google Scholar] [CrossRef]
- Agarkhedkar, S.; Kulkarni, P.S.; Winston, S.; Sievers, R.; Dhere, R.M.; Gunale, B.; Powell, K.; Rota, P.A.; Papania, M.; Cape, S.; et al. Safety and Immunogenicity of Dry Powder Measles Vaccine Administered by Inhalation: A Randomized Controlled Phase I Clinical Trial. Vaccine 2014, 32, 6791–6797. [Google Scholar] [CrossRef]
- Bilton, D.; Daviskas, E.; Anderson, S.D.; Kolbe, J.; King, G.; Stirling, R.G.; Thompson, B.R.; Milne, D.; Charlton, B. Phase 3 Randomized Study of the Efficacy and Safety of Inhaled Dry Powder Mannitol for the Symptomatic Treatment of Non-Cystic Fibrosis Bronchiectasis. Chest 2013, 144, 215–225. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled Budesonide in the Treatment of Early COVID-19 (STOIC): A Phase 2, Open-Label, Randomised Controlled Trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.P.; et al. Safety and Efficacy of Inhaled Nebulised Interferon Beta-1a (SNG001) for Treatment of SARS-CoV-2 Infection: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef] [PubMed]


| Technique | Therapeutic Ingredient/API | Additives | Particle Properties | References |
|---|---|---|---|---|
| Top-down approaches | ||||
| Jet milling and in situ micronization | Beclomethasone dipropionate | NA | ~5 µm, FPF 40% | [30] |
| Micronization | Levodopa | L-Leucine | <5 µm Co-microionization with 2% leucine | [31] |
| Jet milling | Diclofenac | NA | 2.36 µm, hollow crystal with different deposition patterns in NGI, jet-milled DF shows the best aerodynamic performance | [32] |
| Dry jet milling | Simvastatin | NA | 2.2 µm, in vitro study with 60 L/min at MLI stage 3 filters with aerodynamic diameter < 6.8 µm | [33] |
| HPH | Itraconazole | Mannitol and sodium taurocholate | 5.91 µm, with FPF 46.2 to 63.2% and increased solubility to 96 ng/mL | [34] |
| Combined wet milling with aerosol flow reactor | Indomethacin | Mannitol and L-leucine | 0.96 µm | [35] |
| Single-step co-jet milling | Ciprofloxacin HCl and Colistin sulfate | NA | <5.4 µm, FPF 57.5 and 80.2% therapeutic ingredients, respectively | [36] |
| Bottom-up approaches | ||||
| SD | N-acetylcysteine | Soya phosphatidylcholine, Cholesterol, Polysorbate 80 | 7 µm MMAD, yield 71%, respirable fraction 30% | [37] |
| Rifampicin | Soya phosphatidylcholine, Cholesterol, Hydrogenated soybean phosphatidylcholine | ~2 µm, 70% loading of therapeutic ingredient, FPF 50% | [38] | |
| Rifapentine | NA | 1.92 µm, FPF 83% | [39] | |
| Isoniazide | L-α-soybean phosphatidylcholine, Cholesterol, Mannitol | 4.92 µm, FPF 15–35% with encapsulation of therapeutic ingredient 18–30% | [40] | |
| Freeze-thaw followed by SD | Ciprofloxacine | Magnesium stearate and isoleucine | ~1 µm, FPF 66–70%, encapsulation efficiency 79% | [41] |
| Co-SD | Docetaxel | Phosphatidylcholine, Cholesterol, Mannitol, Leucine | 3.1 µm | [42] |
| Moxifloxacin | Phosphatidylcholine, Cholesterol, Dextran | <5 µm, FPF 75% with deep lung deposition in rats | [43] | |
| Oseltamivir phosphate | Ovelecithin, Cholesterol, Leucine | ~3.5 µm, FPF 35%, deposition studies show therapeutic ingredient release by twin-stage impinger | [44] | |
| Salmon calcitonin | Sodium tripolyphosphate, Chitosan, Mannitol | 2.5–4.7 μm, FPF 63.5% with ACI | [45] | |
| Azethromycin | Not mentioned | 1.6 µm | [46] | |
| Paclitaxel | Dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylglycerol | 2.3 µm, powder deposition in all stages of NGI, with a higher dose at the lower stages | [47] | |
| Tobramycin | Poly(lactic-co-glycolic acid), Poly(vinyl alcohol) | 3.3 µm | [48] | |
| Tobramycin (PulmoSphere™) | Distearoylphosphatidlcholin, perflurooctyl bromide | ~5 µm | [49] | |
| Zanamivir (Relenza® Glaxo) | Mannitol, L-leucine, Poloxamer 188 | 2.3 µm, in vitro deposition of 58%, and 116% bioavailability relative to Relenza® | [50] | |
| Meloxicam | L-leucin, ammonium bicarbonate, sodium hyaluronate | In carrier-free formulations with 2.5 µm, the fine particle fraction and emitted fraction were higher for large porous particles than in non-porous formulations | [51] | |
| Dexamethasone palmitate (Pro-therapeutic ingredient of dexamethasone) | 1,2-Dipalmitoyl-sn- Glycero-3- Phosphocholine (DPPC) and Hyaluronic Acid (HA) | Around 13 μm MMAD with a tap density of 0.05 g/cm3 and FPF of 40%. Large porous particles show sustained release, and the MMAD varies with therapeutic ingredient concentration. | [52] | |
| SFD | Theophylline anhydrate and oxalic acid | NA | 3.0 µm Geometric mean diameter of 7.20 µm | [53] |
| Levofloxacin | Polycaprolactone, L-leucine, Mannitol | ~4–5 µm | [54] | |
| Levofloxacin | Soybean lecithin, D-mannitol, L-leucine | 5.6 µm | [54] | |
| Small interfering RNA | Mannitol | 10–14.9 µm, an aerosol performance study using NGI showed an emitted fraction (EF) and FPF of 91% and 28%, respectively | [55] | |
| Voriconazole | Mannitol | 3.8 µm, FPF 40% in NGI | [56] | |
| Octreotide acetate | Mannitol, ammonium carbonate | 2.6 µm, FPF 40%, 88% bioavailability relative to commercial products | [57] | |
| Human IgG | Hydroxypropyl β-cyclodextrin, trehalose | ~5.32 µm, FPF 51.29%, and particle behavior studied by the Anderson cascade impactor | [58] | |
| PlasmidDNA-Luc | Β-benzyl-L-aspartate N-carboxy-anhydride | 7.6 µm, FPF 54%, in vitro inhalation study performed on ACI deposited in stage 3 and lower parts | [59] | |
| SCF | Fluticasone-17-propionte | Poloxamer 188 | ~1.69 µm, FPF 61.9%, aerosol performance studied by NGI | [60] |
| Salmon calcitonin | Inulin, Trehalose, Chitosan, Sodium taurocholate, β-cyclodextrin | 2.2–2.9 µm, studies performed on Sprague-Dawley rats | [61] | |
| Ibuprofen | Chitosan | 2.1–2.7 µm | [62] | |
| Plasmid DNA | Poly(D,L-lactic-co-glycolic) acid | Not mentioned | [63] | |
| siRNA | Chitosan | <10 µm | [64] | |
| 5-fluorouracil | α-lactose monohydrate | Not mentioned | [65] | |
| Curcumin | Hydroxypropyl-β-cyclodextrin | ~5.8 µm | [66] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, A.; Nimbkar, S.; Moses, J.A. Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches. Pharmaceutics 2023, 15, 2706. https://doi.org/10.3390/pharmaceutics15122706
Negi A, Nimbkar S, Moses JA. Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches. Pharmaceutics. 2023; 15(12):2706. https://doi.org/10.3390/pharmaceutics15122706
Chicago/Turabian StyleNegi, Aditi, Shubham Nimbkar, and Jeyan Arthur Moses. 2023. "Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches" Pharmaceutics 15, no. 12: 2706. https://doi.org/10.3390/pharmaceutics15122706





