Nerve Regeneration with a Scaffold Incorporating an Absorbable Zinc-2% Iron Alloy Filament to Improve Axonal Guidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zn-2%Fe Alloy
2.2. Preparation of Thin Zn Filaments
2.3. Zn Metal Characterization
2.4. Preparation of Scaffolds for Surgery
2.5. Animal Use and Surgery
2.6. Live Animal Functional Monitoring
2.7. Sacrifice and Tissue Preparation
2.8. Micro Computed Tomography (Micro-CT)
2.9. Histology: Tissue Preparation
2.10. Analysis of Axon Density
2.11. Analysis of Nerve Histology
2.12. Statistics
3. Results
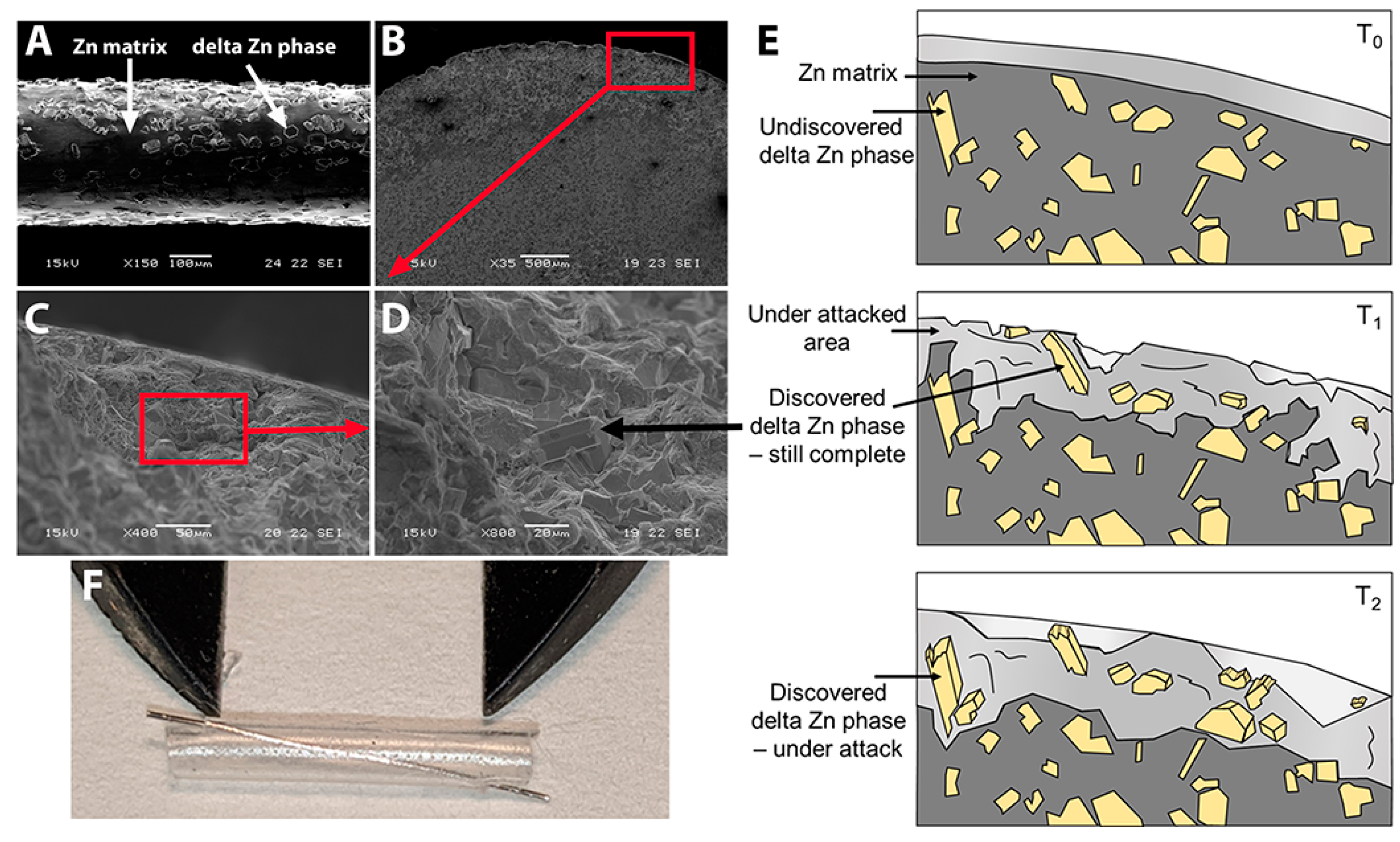
3.1. The Zn-2%Fe Alloy Filaments and Preparation for Surgery
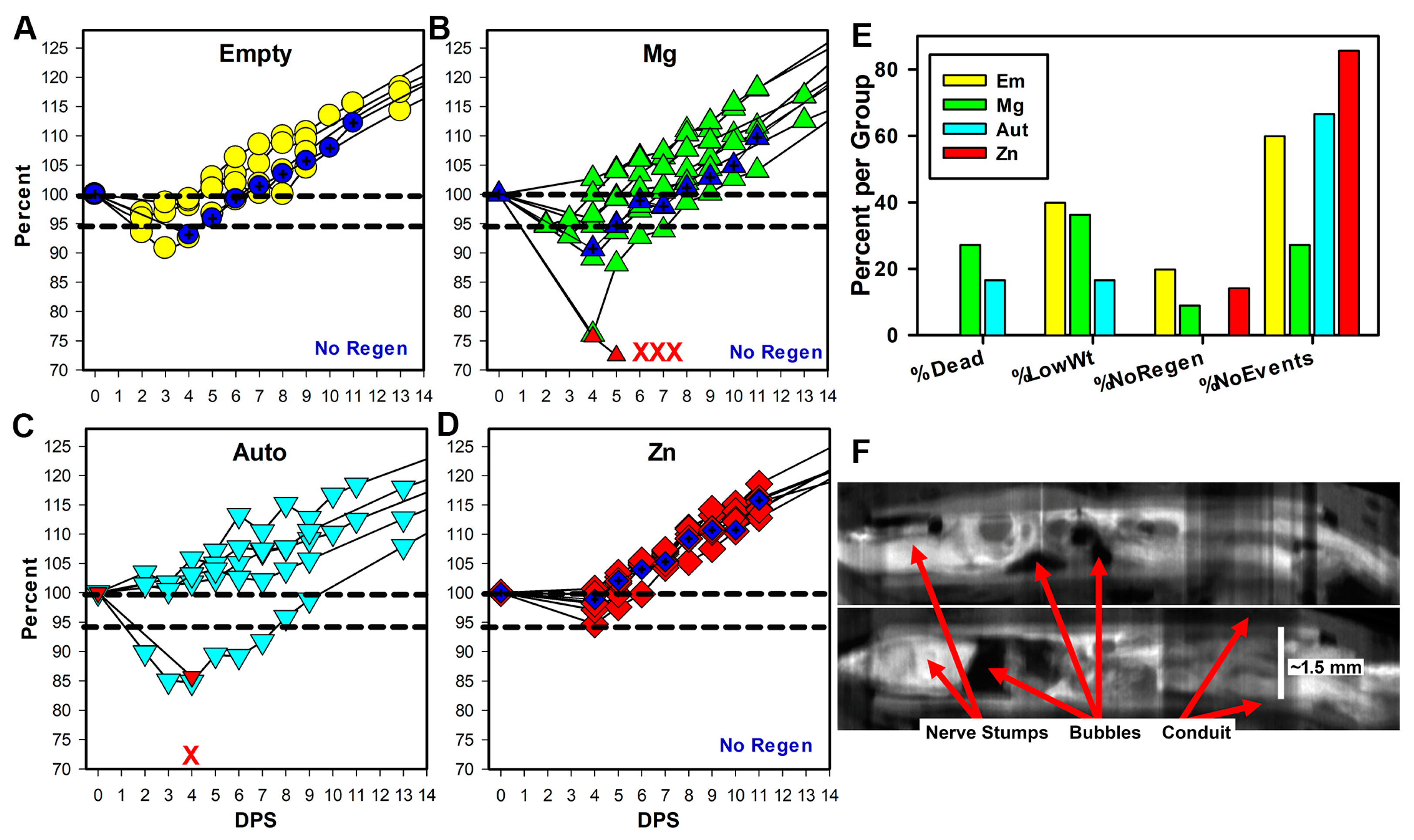
3.2. Early Rat Health
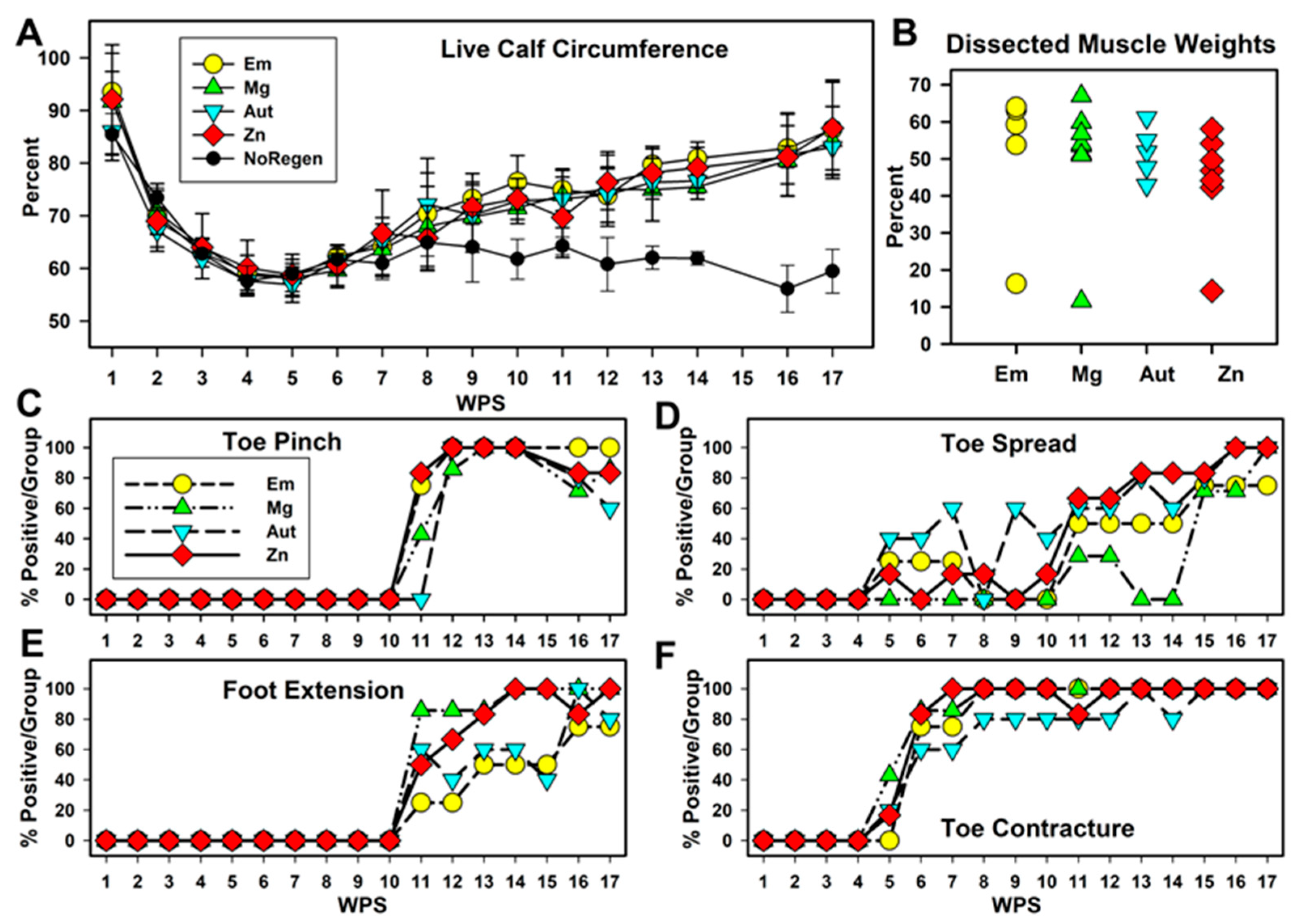
3.3. Functional Changes over Time
3.4. Micro-CT with Iodine Contrast
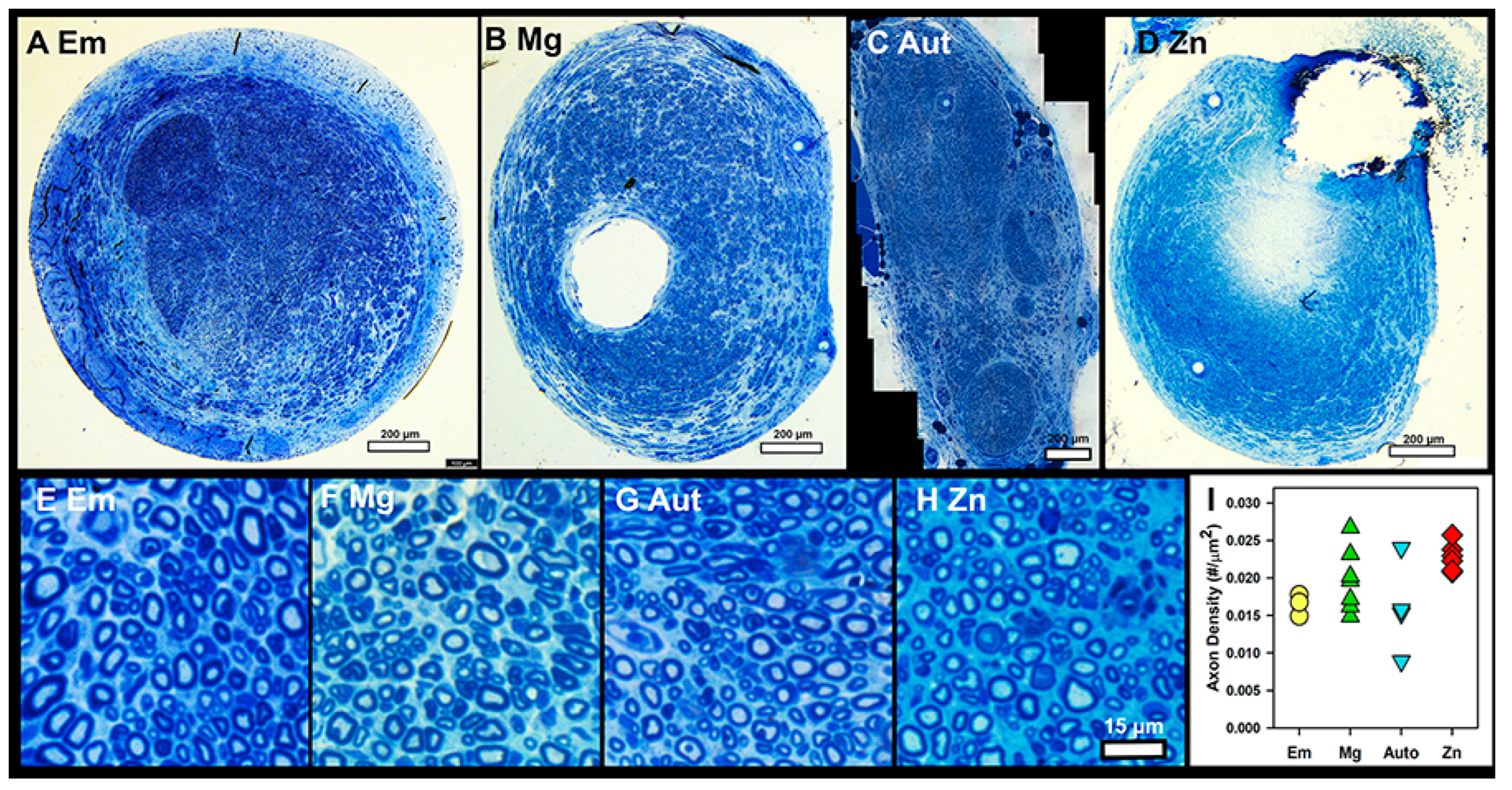
3.5. Histology
4. Discussion
4.1. Zn Alloy and Surgical Placement
4.2. Early Health
4.3. Functional Recovery
4.4. Micro-CT Imaging
4.5. Axon Density
4.6. Histology
5. Conclusions
- Zn-2%Fe and pure Mg filaments placed inside silicone nerve conduits supported cellular attachment to both metals and the regeneration of sciatic nerves across a 6 mm, noncritical injury gap in adult rats.
- Zn-2%Fe filaments were well tolerated and had fewer adverse effects than Mg filaments, empty conduits, or autograft controls during early recovery from surgery.
- All groups supported the regeneration of equal densities of axons distal to the injury site and equal recovery of functions, although the Zn group showed a trend towards an increase in earlier recovery of function.
- The Zn-2%Fe filaments showed a greater local accumulation of degradation products and inflammatory cells than Mg filaments, but no difference in connective tissue capsule thickness and no toxicity or significant inflammation in newly regenerated nerve tissues.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Munro, C.A.; Prasad, V.S.S.V.; Midha, R. Analysis of Upper and Lower Extremity Peripheral Nerve Injuries in a Population of Patients with Multiple Injuries. J. Trauma Inj. Infect. Crit. Care 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2022, 66, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Brattain, K. Analysis the Peripheral Nerve Repair Market in the United States; Magellan Medical Technology Consultants, Inc.: Minneap, MN, USA, 2014; pp. 1–11. [Google Scholar]
- Lee, S.K.; Wolfe, S.W. Peripheral Nerve Injury and Repair. J. Am. Acad. Orthop. Surg. 2000, 8, 243–252. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef]
- Pan, D.; Mackinnon, S.E.; Wood, M.D. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve 2020, 61, 726–739. [Google Scholar] [CrossRef]
- Kasper, M.; Deister, C.; Beck, F.; Schmidt, C.E. Bench-to-Bedside Lessons Learned: Commercialization of an Acellular Nerve Graft. Adv. Healthc. Mater. 2020, 9, e2000174. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2018, 169, 240–251. [Google Scholar] [CrossRef]
- Lin, X.; Saijilafu; Wu, X.; Wu, K.; Chen, J.; Tan, L.; Witte, F.; Yang, H.; Mantovani, D.; Zhou, H.; et al. Biodegradable Mg-based alloys: Biological implications and restorative opportunities. Int. Mater. Rev. 2023, 68, 365–403. [Google Scholar] [CrossRef]
- Vennemeyer, J.; Hopkins, T.; Hershcovitch, M.; Little, K.; Hagen, M.; Minteer, D.; Hom, D.; Marra, K.; Pixley, S. Initial observations on using magnesium metal in peripheral nerve repair. J. Biomater. Appl. 2015, 29, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.M.; Little, K.J.; Vennemeyer, J.J.; Triozzi, J.L.; Turgeon, M.K.; Heilman, A.M.; Minteer, D.; Marra, K.; Hom, D.B.; Pixley, S.K. Short and long gap peripheral nerve repair with magnesium metal filaments. J. Biomed. Mater. Res. Part A 2017, 105, 3148–3158. [Google Scholar] [CrossRef]
- Tatu, R.; White, L.G.; Yun, Y.; Hopkins, T.; An, X.; Ashraf, A.; Little, K.J.; Hershcovitch, M.; Hom, D.B.; Pixley, S. Effects of Altering Magnesium Metal Surfaces on Degradation In Vitro and In Vivo during Peripheral Nerve Regeneration. Materials 2023, 16, 1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Cheng, F.-C.; Chen, C.-J.; Su, H.-L.; Sheu, M.-L.; Sheehan, J.; Pan, H.-C. Down-Regulated Expression of Magnesium Transporter Genes following a High Magnesium Diet Attenuates Sciatic Nerve Crush Injury. Neurosurgery 2019, 84, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-C.; Sheu, M.-L.; Su, H.-L.; Chen, Y.-J.; Chen, C.-J.; Yang, D.-Y.; Chiu, W.-T.; Cheng, F.-C. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes. Res. 2011, 24, 54–70. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Lin, W.; Zhang, S. Magnesium Promotes the Regeneration of the Peripheral Nerve. Front. Cell Dev. Biol. 2021, 9, 717854. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef]
- Ben Tzion-Mottye, L.; Ron, T.; Eliezer, D.; Aghion, E. The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy. Metals 2022, 12, 1291. [Google Scholar] [CrossRef]
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The Galvanization of Biology: A Growing Appreciation for the Roles of Zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Peng, F.; Xie, J.; Liu, H.; Zheng, Y.; Qian, X.; Zhou, R.; Zhong, H.; Zhang, Y.; Li, M. Shifting focus from bacteria to host neutrophil extracellular traps of biodegradable pure Zn to combat implant centered infection. Bioact. Mater. 2023, 21, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Burwinkel, M.; Palissa, C.; Ephraim, E.; Schmidt, M.F. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Veter-Microbiol. 2012, 160, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef]
- Oliver, A.A.; Sikora-Jasinska, M.; Demir, A.G.; Guillory, R.J. Recent advances and directions in the development of bioresorbable metallic cardiovascular stents: Insights from recent human and in vivo studies. Acta Biomater. 2021, 127, 1–23. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn–Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. The Effects of 4%Fe on the Performance of Pure Zinc as Biodegradable Implant Material. Ann. Biomed. Eng. 2019, 47, 1400–1408. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The Suitability of Zn–1.3%Fe Alloy as a Biodegradable Implant Material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Guillory, R.J.; Bowen, P.K.; Hopkins, S.P.; Shearier, E.R.; Earley, E.J.; Gillette, A.A.; Aghion, E.; Bocks, M.L.; Drelich, J.W.; Goldman, J. Corrosion Characteristics Dictate the Long-Term Inflammatory Profile of Degradable Zinc Arterial Implants. ACS Biomater. Sci. Eng. 2016, 2, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.M.; Heilman, A.M.; Liggett, J.A.; LaSance, K.; Little, K.J.; Hom, D.B.; Minteer, D.M.; Marra, K.G.; Pixley, S.K. Combining micro-computed tomography with histology to analyze biomedical implants for peripheral nerve repair. J. Neurosci. Methods 2015, 255, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Pixley, S.K.; Hopkins, T.M.; Little, K.J.; Hom, D.B. Evaluation of peripheral nerve regeneration through biomaterial conduits via micro-CT imaging. Laryngoscope Investig. Otolaryngol. 2016, 1, 185–190. [Google Scholar] [CrossRef]
- Geuna, S.; Tos, P.; Battiston, B.; Guglielmone, R. Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann. Anat.-Anat. Anz. 2000, 182, 23–34. [Google Scholar] [CrossRef]
- Geuna, S.; Raimondo, S.; Ronchi, G.; Di Scipio, F.; Tos, P.; Czaja, K.; Fornaro, M. Chapter 3 Histology of the Peripheral Nerve and Changes Occurring During Nerve Regeneration. Int. Rev. Neurobiol. 2009, 87, 27–46. [Google Scholar] [CrossRef]
- García-Parra, P.; Cavaliere, F.; Maroto, M.; Bilbao, L.; Obieta, I.; de Munain, A.L.; Álava, J.I.; Izeta, A. Modeling neural differentiation on micropatterned substrates coated with neural matrix components. Front. Cell. Neurosci. 2012, 6, 10. [Google Scholar] [CrossRef]
- Guillory, R.J.; Sikora-Jasinska, M.; Drelich, J.W.; Goldman, J. In Vitro Corrosion and in Vivo Response to Zinc Implants with Electropolished and Anodized Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 19884–19893. [Google Scholar] [CrossRef]
- Tubbs, J.T.; Kissling, G.E.; Travlos, G.S.; Goulding, D.R.; Clark, J.A.; King-Herbert, A.P.; Blankenship-Paris, T.L. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 185–191. [Google Scholar]
- Flecknell, P. Rodent analgesia: Assessment and therapeutics. Veter. J. 2018, 232, 70–77. [Google Scholar] [CrossRef]
- Jablonski, P.; Howden, B.O.; Baxter, K. Influence of buprenorphine analgesia on post-operative recovery in two strains of rats. Lab. Anim. 2001, 35, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hestehave, S.; Munro, G.; Christensen, R.; Pedersen, T.B.; Arvastson, L.; Hougaard, P.; Abelson, K.S.P. Is there a reasonable excuse for not providing post-operative analgesia when using animal models of peripheral neuropathic pain for research purposes? PLoS ONE 2017, 12, e0188113. [Google Scholar] [CrossRef]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C. Adverse effects on growth rates in rats caused by buprenorphine administration. Lab. Anim. 2000, 34, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Ullah, S.; Mueller, P.P. Advances and Challenges of Biodegradable Implant Materials with a Focus on Magnesium-Alloys and Bacterial Infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Yannas, I.V.; Zhang, M.; Spilker, M.H. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2007, 18, 943–966. [Google Scholar] [CrossRef]
- Cobianchi, S.; de Cruz, J.; Navarro, X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef]
- de Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Bozkurt, A.; Tholl, S.; Wehner, S.; Tank, J.; Cortese, M.; O’Dey, D.M.; Deumens, R.; Lassner, F.; Schügner, F.; Gröger, A.; et al. Evaluation of functional nerve recovery with Visual-SSI—A novel computerized approach for the assessment of the static sciatic index (SSI). J. Neurosci. Methods 2008, 170, 117–122. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Buxbaum, R.E.; Rajachar, R.M.; Goldman, J. New approaches in evaluating metallic candidates for bioabsorbable stents. Emerg. Mater. Res. 2012, 1, 237–255. [Google Scholar] [CrossRef]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. A new in vitro–in vivo correlation for bioabsorbable magnesium stents from mechanical behavior. Mater. Sci. Eng. C 2013, 33, 5064–5070. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Magnesium in the murine artery: Probing the products of corrosion. Acta Biomater. 2014, 10, 1475–1483. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Huang, G.; Zhang, J.; Pan, S. In Vitro and In Vivo Applications of Magnesium-Enriched Biomaterials for Vascularized Osteogenesis in Bone Tissue Engineering: A Review of Literature. J. Funct. Biomater. 2023, 14, 326. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Russell, T.; Sankar, J.; Yun, Y. The Biological Responses to Magnesium-Based Biodegradable Medical Devices. Metals 2017, 7, 514. [Google Scholar] [CrossRef]
- Bowen, P.K.; Gelbaugh, J.A.; Mercier, P.J.; Goldman, J.; Drelich, J. Tensile testing as a novel method for quantitatively evaluating bioabsorbable material degradation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2101–2113. [Google Scholar] [CrossRef]
- Fu, J.; Su, Y.; Qin, Y.-X.; Zheng, Y.; Wang, Y.; Zhu, D. Evolution of metallic cardiovascular stent materials: A comparative study among stainless steel, magnesium and zinc. Biomaterials 2020, 230, 119641. [Google Scholar] [CrossRef]
- Bowen, P.K.; Roger, J.G.; Shearier, E.R.; Seitz, J.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Zhao, S.; Seitz, J.-M.; Eifler, R.; Maier, H.J.; Guillory, R.J.; Earley, E.J.; Drelich, A.; Goldman, J.; Drelich, J.W. Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C 2017, 76, 301–312. [Google Scholar] [CrossRef]
- Bowen, P.K.; Seitz, J.; Roger, J.G.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of wrought Zn-Al alloys (1, 3, and 5 wt% Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Guillory, R.J.; Kolesar, T.M.; Oliver, A.A.; Stuart, J.A.; Bocks, M.L.; Drelich, J.W.; Goldman, J. Zn2+-dependent suppression of vascular smooth muscle intimal hyperplasia from biodegradable zinc implants. Mater. Sci. Eng. C 2020, 111, 110826. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (< 0.1wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C 2018, 84, 67–79. [Google Scholar]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef]
- Wurth, S.; Capogrosso, M.; Raspopovic, S.; Gandar, J.; Federici, G.; Kinany, N.; Cutrone, A.; Piersigilli, A.; Pavlova, N.; Guiet, R.; et al. Long-term usability and bio-integration of polyimide-based intra-neural stimulating electrodes. Biomaterials 2017, 122, 114–129. [Google Scholar] [CrossRef]
- Shi, J.; Fang, Y. Flexible and Implantable Microelectrodes for Chronically Stable Neural Interfaces. Adv. Mater. 2018, 31, e1804895. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ron, T.; Leon, A.; Kafri, A.; Ashraf, A.; Na, J.; Babu, A.; Banerjee, R.; Brookbank, H.; Muddaluri, S.R.; Little, K.J.; et al. Nerve Regeneration with a Scaffold Incorporating an Absorbable Zinc-2% Iron Alloy Filament to Improve Axonal Guidance. Pharmaceutics 2023, 15, 2595. https://doi.org/10.3390/pharmaceutics15112595
Ron T, Leon A, Kafri A, Ashraf A, Na J, Babu A, Banerjee R, Brookbank H, Muddaluri SR, Little KJ, et al. Nerve Regeneration with a Scaffold Incorporating an Absorbable Zinc-2% Iron Alloy Filament to Improve Axonal Guidance. Pharmaceutics. 2023; 15(11):2595. https://doi.org/10.3390/pharmaceutics15112595
Chicago/Turabian StyleRon, Tomer, Avi Leon, Alon Kafri, Ahmed Ashraf, John Na, Ashvin Babu, Runima Banerjee, Hunter Brookbank, Saimahesh Raju Muddaluri, Kevin J. Little, and et al. 2023. "Nerve Regeneration with a Scaffold Incorporating an Absorbable Zinc-2% Iron Alloy Filament to Improve Axonal Guidance" Pharmaceutics 15, no. 11: 2595. https://doi.org/10.3390/pharmaceutics15112595





