Novel Gold Nanorods@Thiolated Pectin on the Killing of HeLa Cells by Photothermal Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Potentiometric Titration
2.3. Thiolated Pectin (SH-PEC) Synthesis
2.4. Infrared Spectroscopy of SH-PEC
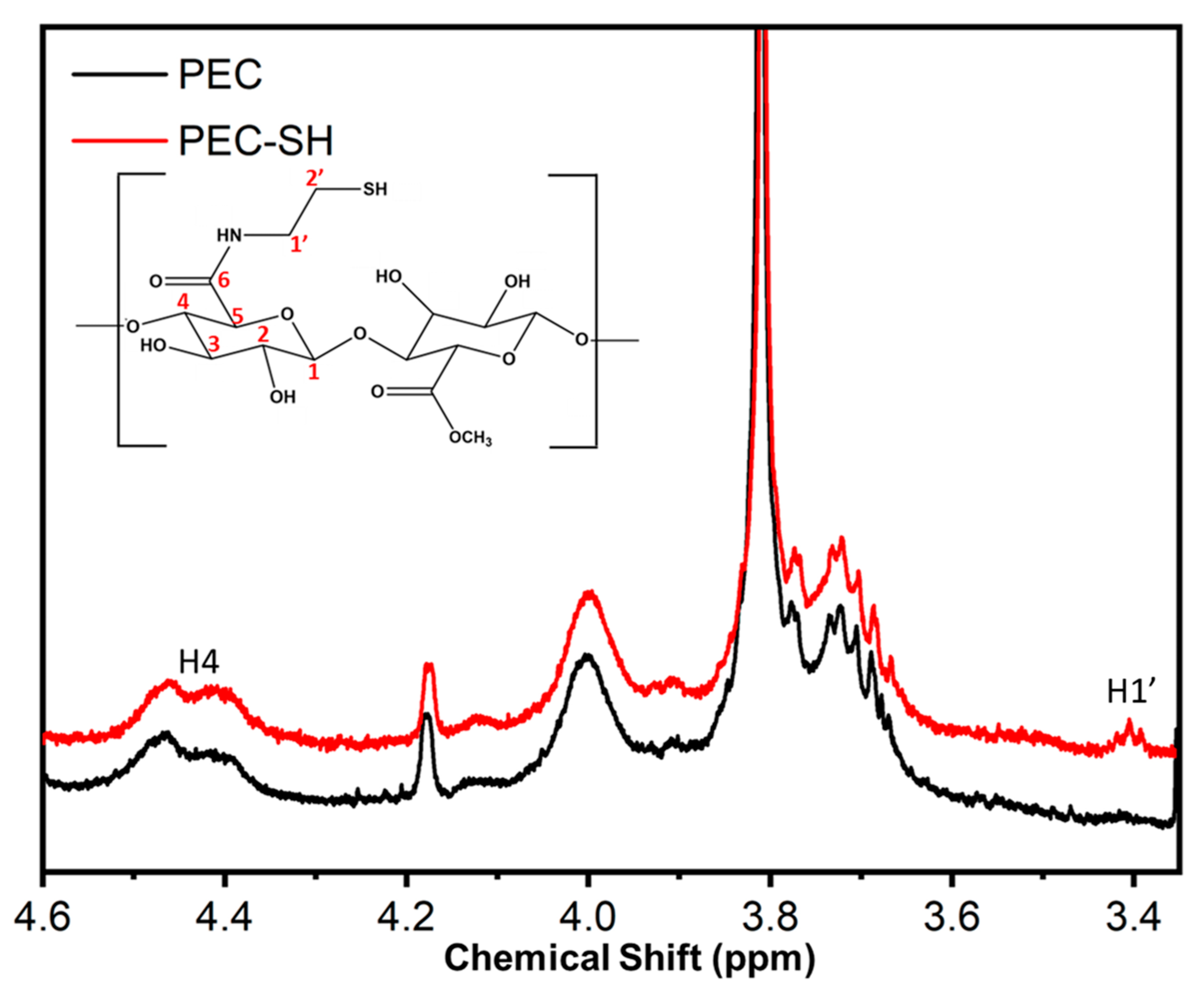
2.5. Nuclear Magnetic Resonance (1H NMR)
2.6. Gold Nanorods Synthesis
2.7. SH-PEC Coated AuNRs
2.8. UV-Vis Absorption Spectroscopy, STEM, AFM, DLS and Zeta Potential Measurements of AuNRs and AuNR@SH-PECs
2.9. Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS)
2.10. Photothermal Properties of AuNR@SH-PEC
2.11. In Vitro Cell Cytotoxicity and Photothermal Effect
2.12. ROS Generation
2.13. Statistical Analysis
3. Results and Discussion
3.1. Number of Ionizable Groups on PEC
3.2. SH-PEC Synthesis
3.3. Determination of AuNRs Concentration
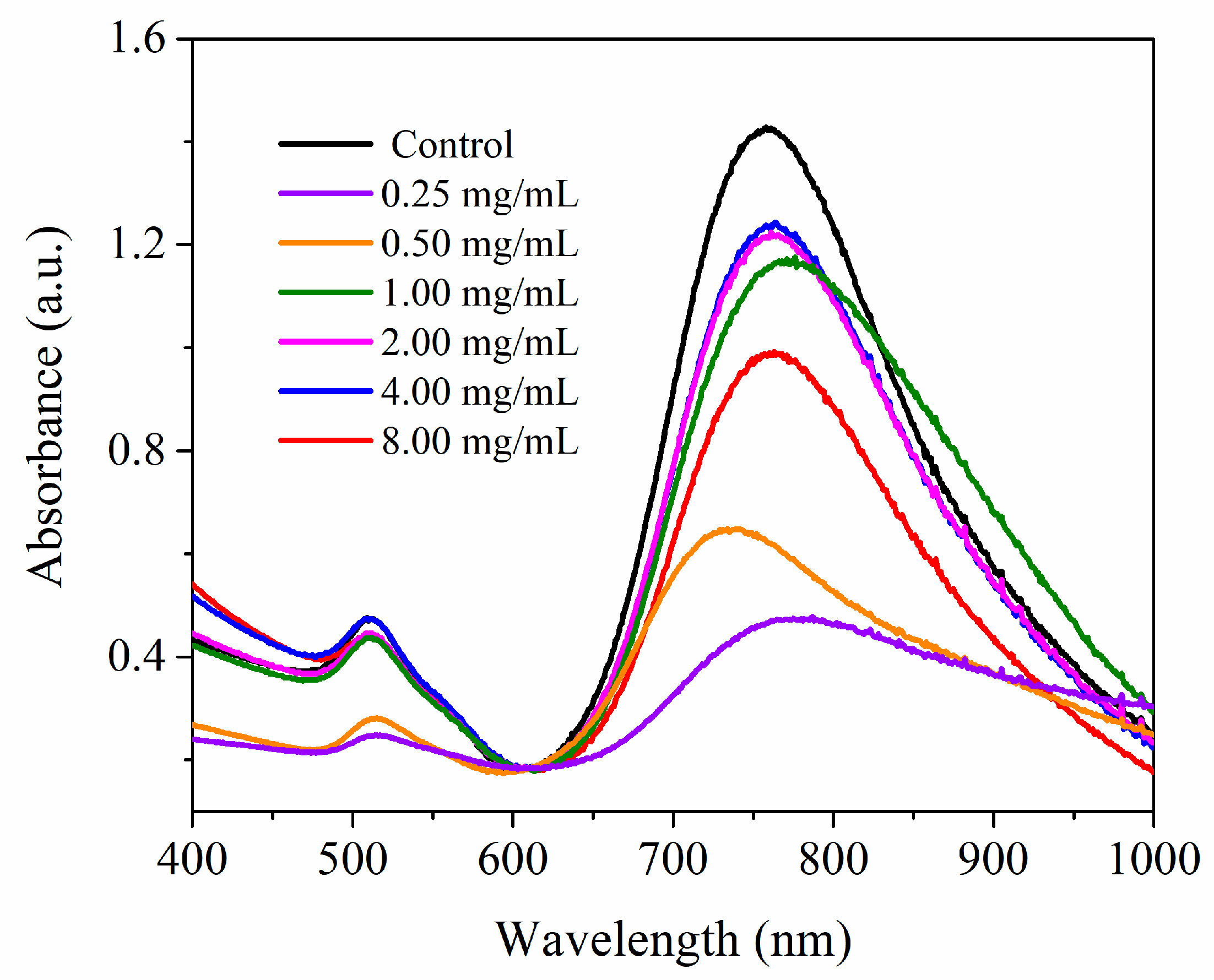
3.4. AuNRs Coated with SH-PEC (AuNR@SH-PEC)
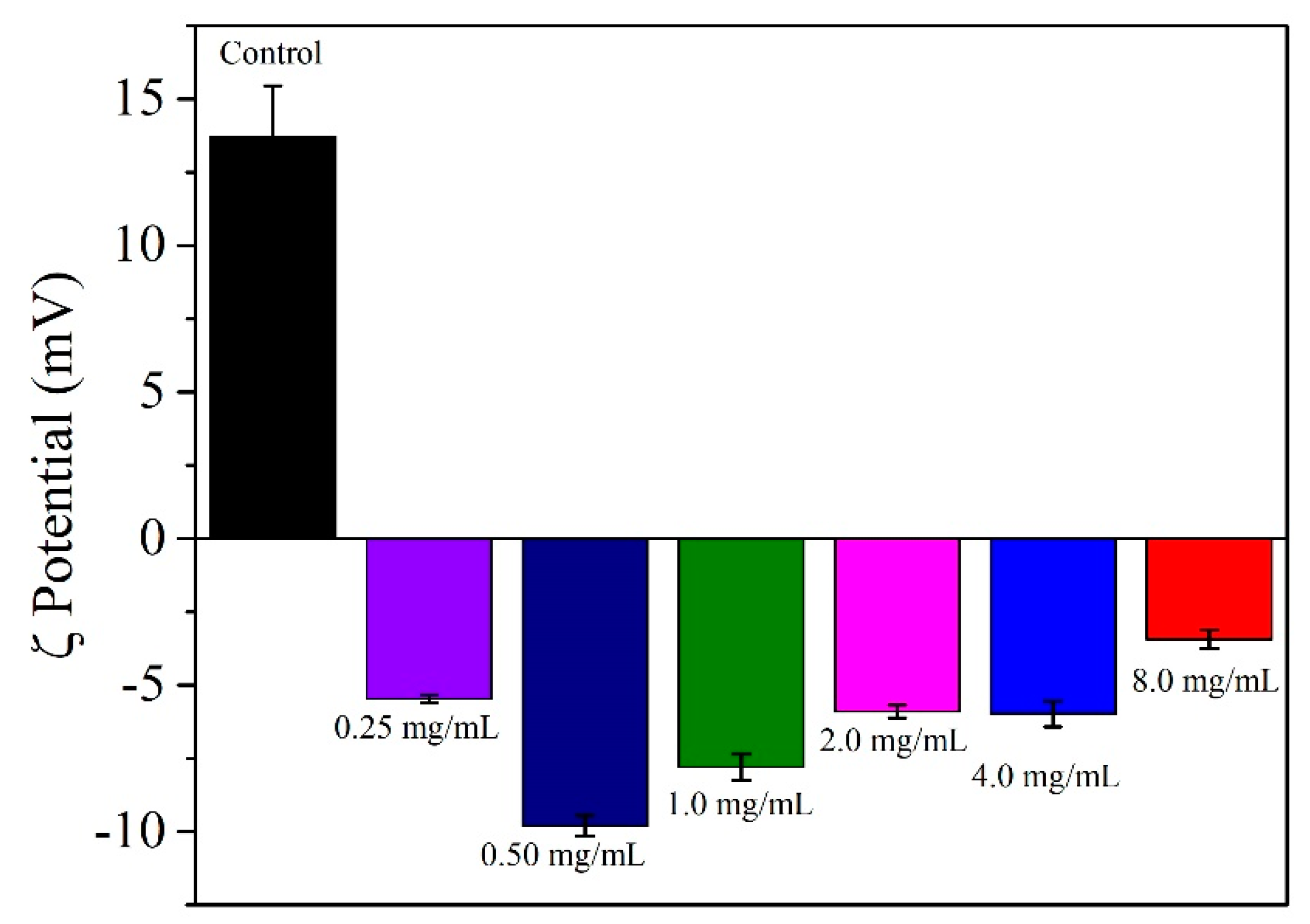
3.5. Dynamic Light Scattering (DLS) and Zeta Potential (ζ) Measurements
3.6. Scanning Transmission Electron Microscopy (STEM) and Atomic Force Microscopy (AFM)
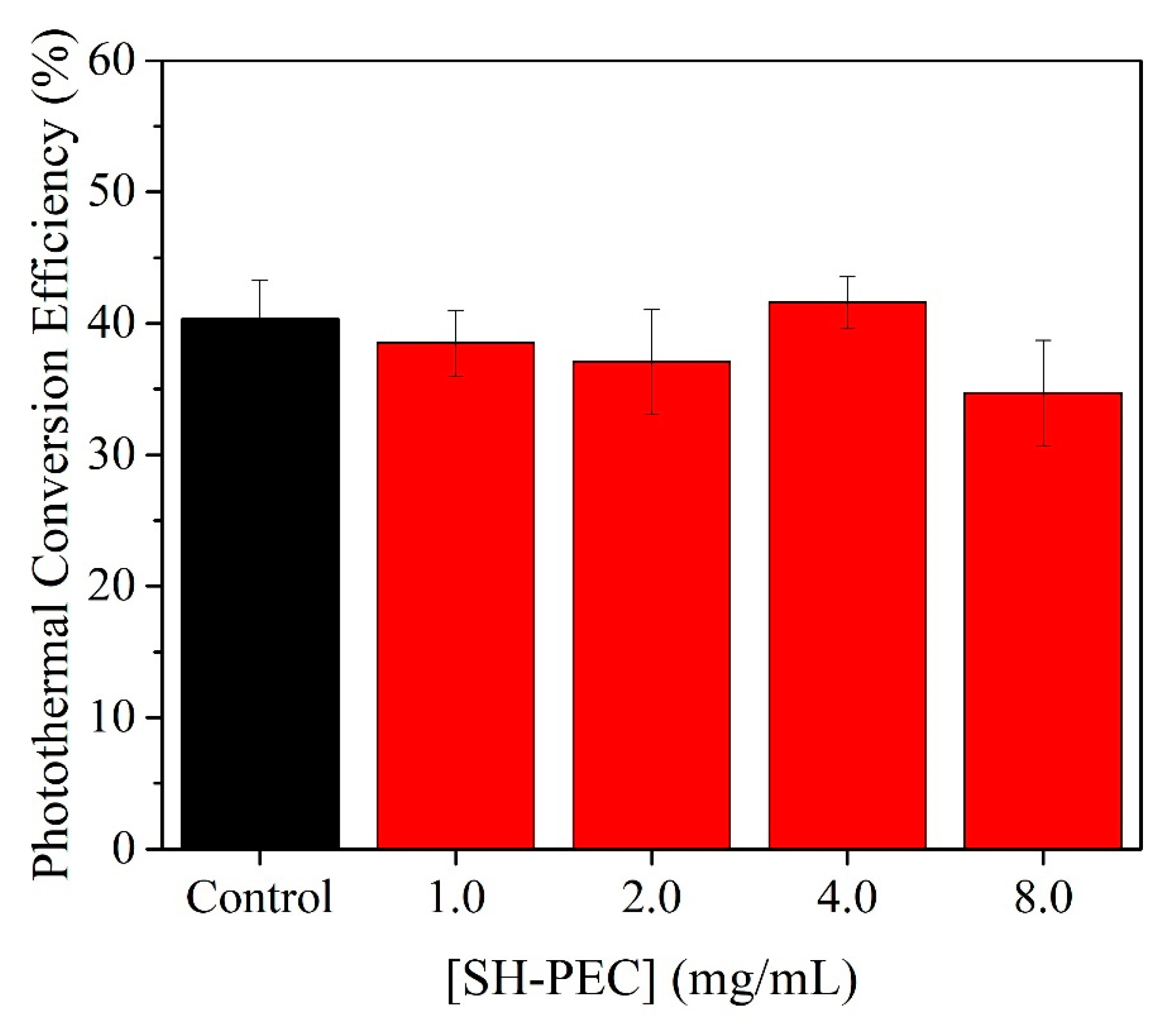
3.7. Photothermal Assays
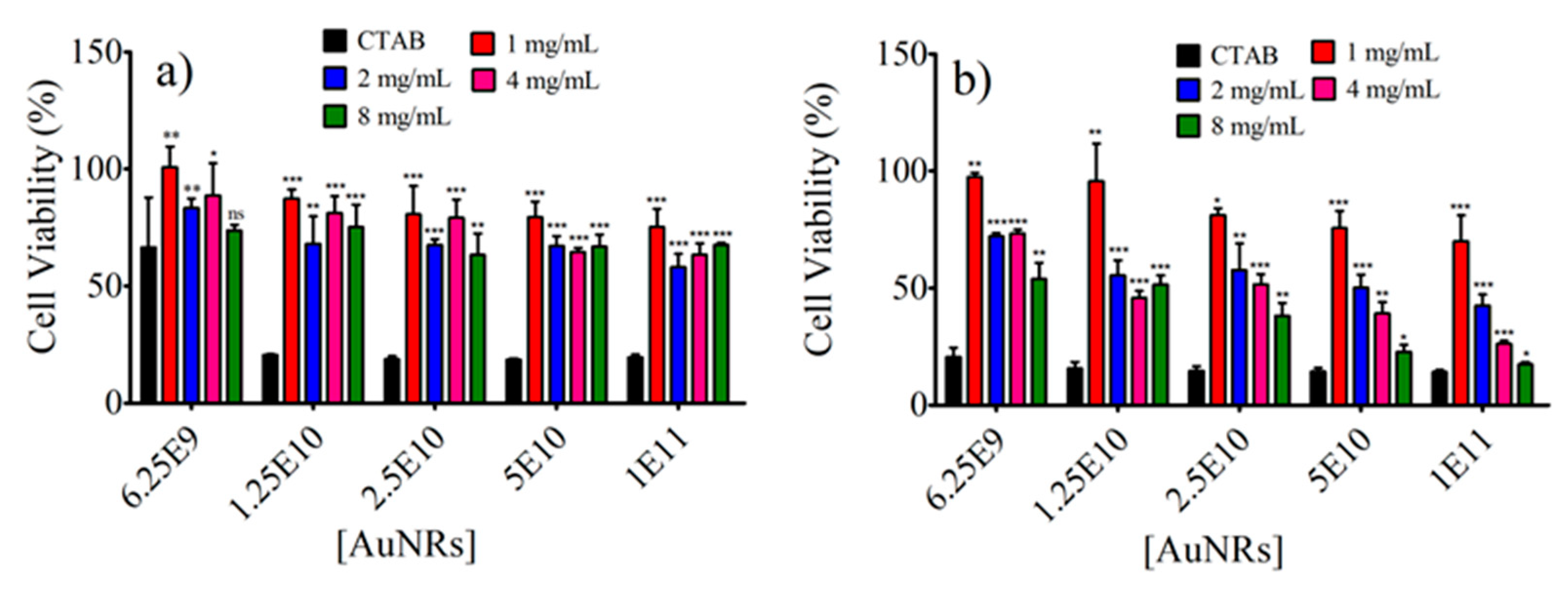
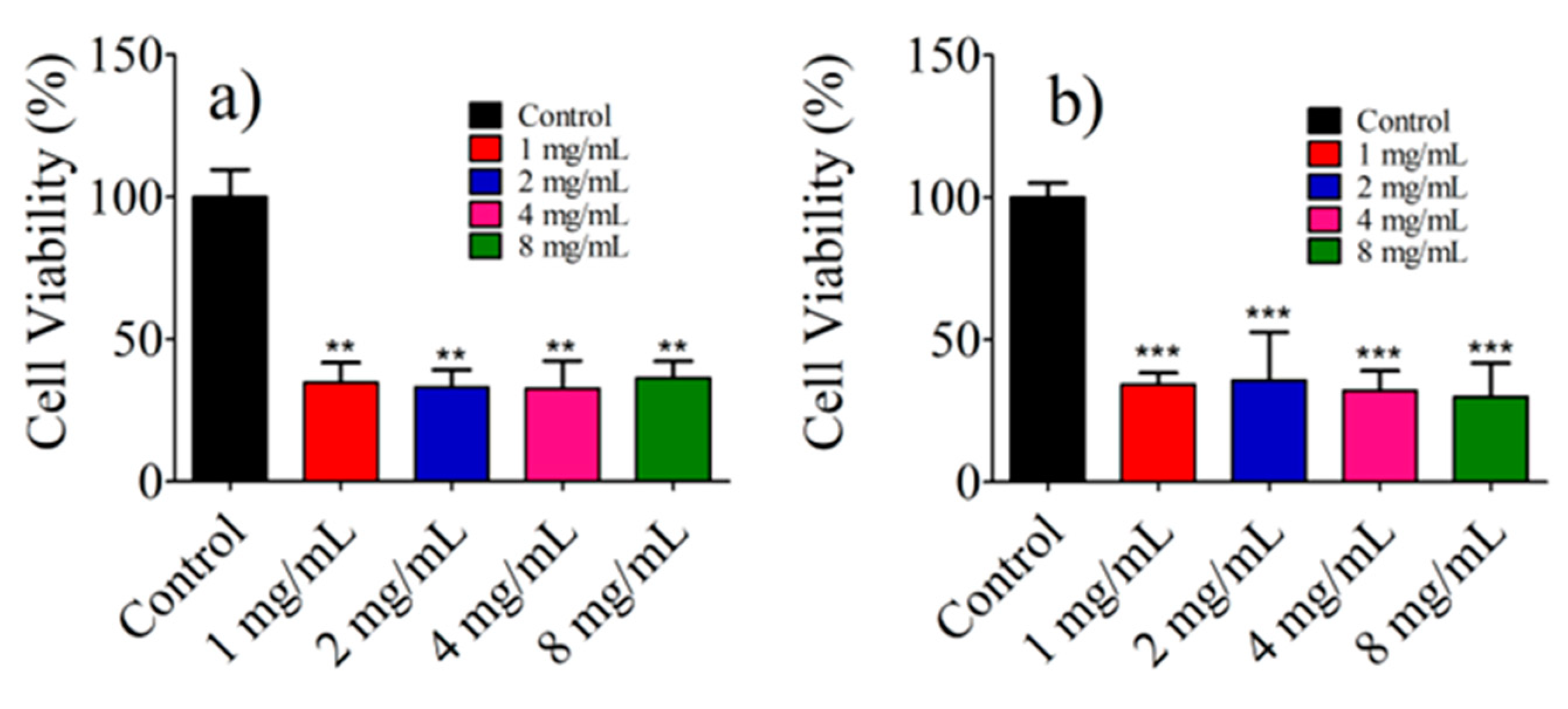
3.8. Photothermal Effect of AuNR@SH-PEC on the Viability of Balb/c 3t3 and HeLa Cell Lines In Vitro Biological Evaluation
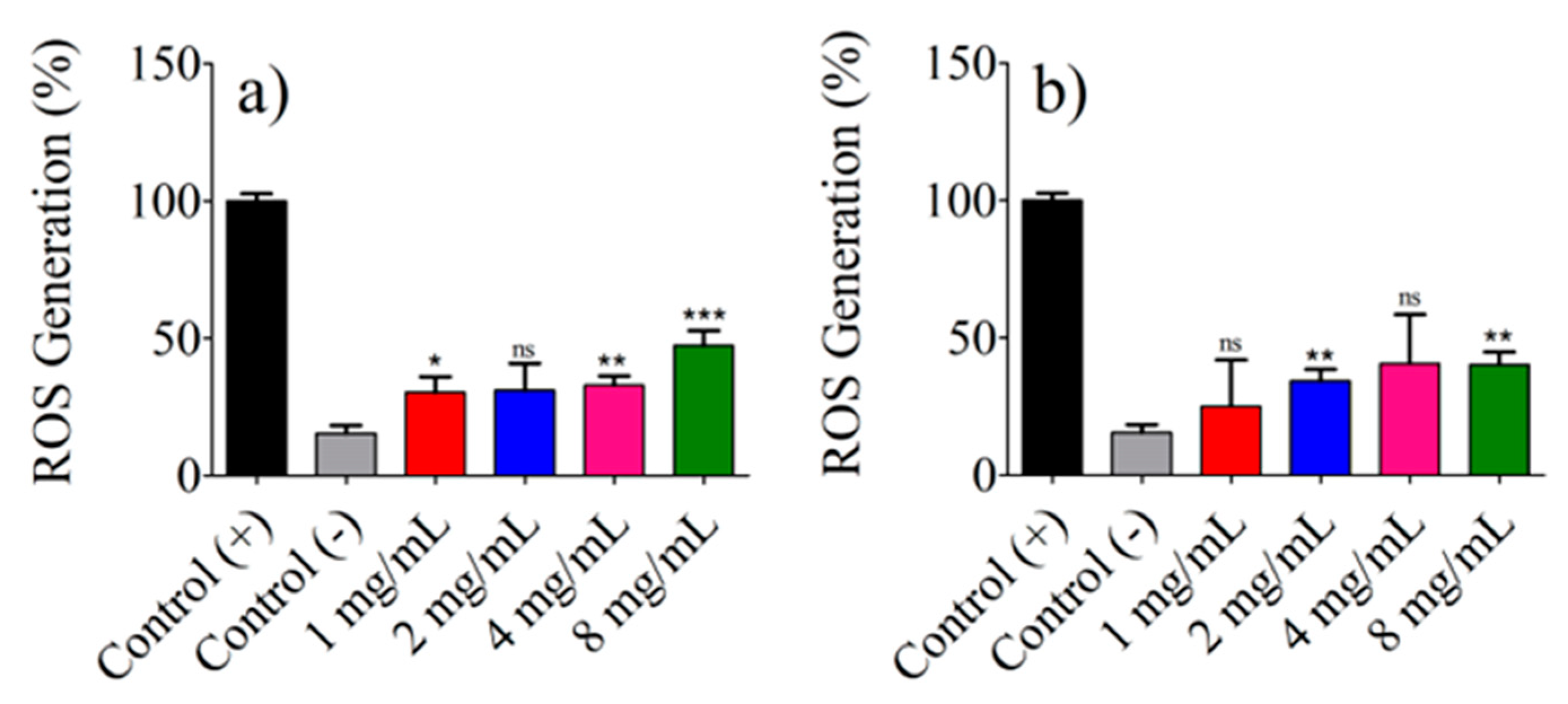
3.9. ROS Generation Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in lipid-based nanoparticles for cancer chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, A.A.; Aguirre-Álvarez, G.; Cariño-Cortés, R.; Mendoza-Huizar, L.H.; Jiménez-Alvarado, R. Iron oxide nanoparticles: Synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem. Pap. 2020, 74, 3809–3824. [Google Scholar] [CrossRef]
- Naief, M.F.; Mohammed, S.N.; Mayouf, H.J.; Mohammed, A.M. A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J. Organomet. Chem. 2023, 999, 122819. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Koohi, M.; Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Pontremoli, C.; Fiorilli, S.; Etrych, T.; Koohi, M.; Esfahani, M.; et al. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Zhang, C.; Liu, H.; Hao, M.; Kan, S.; Liu, D.; Liu, W. The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front. Oncol. 2021, 11, 819329. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis *. In Colloidal Synthesis of Plasmonic Nanometals; Jenny Stanford Publishing: New Delhi, India, 2020; pp. 197–220. [Google Scholar] [CrossRef]
- Hosseinniya, S.; Rezayan, A.H.; Ghasemi, F.; Malekmohamadi, M.; Taheri, R.A.; Hosseini, M.; Alvandi, H. Fabrication and evaluation of optical nanobiosensor based on localized surface plasmon resonance (LSPR) of gold nanorod for detection of CRP. Anal. Chim. Acta 2023, 1237, 340580. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.Z.; Li, R.S.; Huang, C.Z. Localized surface plasmon resonance of gold nanorods and assemblies in the view of biomedical analysis. TrAC Trends Anal. Chem. 2016, 80, 429–443. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Li, W.T.; Peng, J.R.; Yang, Q.; Li, H.; Wei, Y.Q.; Zhang, X.N.; Qian, Z.Y. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics 2015, 5, 345–356. [Google Scholar] [CrossRef]
- Yasun, E.; Li, C.; Barut, I.; Janvier, D.; Qiu, L.; Cui, C.; Tan, W. BSA modification to reduce CTAB induced nonspecificity and cytotoxicity of aptamer-conjugated gold nanorods. Nanoscale 2015, 7, 10240–10248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, X.; Yang, N.; Huang, Z.; Huang, L.; Fang, Z.; Zhang, C.; Li, L.; Yu, C. Surface engineering strategies of gold nanomaterials and their applications in biomedicine and detection. J. Mater. Chem. B 2021, 9, 5583–5598. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, K.; Patel, P.; Mehta, T. Surface modifications of gold nanoparticles: Stabilization and recent applications in cancer therapy. Pharm. Dev. Technol. 2022, 27, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef]
- Goodwin, A. Lipid-stabilized hydrophobically modified silica-coated gold nanorods generate enhanced photoacoustic signal from cavitation with nonlinear dependence on laser fluence. J. Acoust. Soc. Am. 2021, 150, A27. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Mujokoro, B.; Dorkoosh, F.; Khosravani, M.; Adabi, M. Investigation of effective parameters on size of paclitaxel loaded PLGA nanoparticles. Adv. Pharm. Bull. 2018, 8, 77–84. [Google Scholar] [CrossRef]
- Hakimian, F.; Ghourchian, H. Simple and rapid method for synthesis of porous gold nanoparticles and its application in improving DNA loading capacity. Mater. Sci. Eng. C 2019, 103, 109795. [Google Scholar] [CrossRef]
- Figueiredo, A.Q.; Rodrigues, C.F.; Fernandes, N.; Correia, I.J.; Moreira, A.F. In situ formation of alginic acid-gold nanohybrids for application in cancer photothermal therapy. Biotechnol. J. 2023, e2300019. [Google Scholar] [CrossRef]
- Li, L.S.; Ren, B.; Yang, X.; Cai, Z.C.; Zhao, X.J.; Zhao, M.X. Hyaluronic acid-modified and doxorubicin-loaded gold nanoparticles and evaluation of their bioactivity. Pharmaceuticals 2021, 14, 101. [Google Scholar] [CrossRef]
- de Barros, H.R.; Piovan, L.; Sassaki, G.L.; de Araujo Sabry, D.; Mattoso, N.; Nunes, Á.M.; Meneghetti, M.R.; Riegel-Vidotti, I.C. Surface interactions of gold nanorods and polysaccharides: From clusters to individual nanoparticles. Carbohydr. Polym. 2016, 152, 479–486. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; da Cruz, A.F.; Nunes, Á.M.; Meneghetti, M.R.; de Barros, H.R.; Borges, B.S.; de Medeiros, L.C.A.S.; Soares, M.J.; dos Santos, M.P.; Grassi, M.T.; et al. Biocompatible gum arabic-gold nanorod composite as an effective therapy for mistreated melanomas. Int. J. Biol. Macromol. 2021, 185, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Doghish, A.S.; Hashem, A.H.; Shehabeldine, A.M.; Sallam, A.A.M.; El-Sayyad, G.S.; Salem, S.S. Nanocomposite based on gold nanoparticles and carboxymethyl cellulose: Synthesis, characterization, antimicrobial, and anticancer activities. J. Drug Deliv. Sci. Technol. 2022, 77, 103874. [Google Scholar] [CrossRef]
- You, S.-M.; Luo, K.; Jung, J.-Y.; Jeong, K.-B.; Lee, E.-S.; Oh, M.-H.; Kim, Y.-R. Gold Nanoparticle-Coated Starch Magnetic Beads for the Separation, Concentration, and SERS-Based Detection of E. coli O157:H7. ACS Appl. Mater. Interfaces 2020, 12, 18292–18300. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Yang, J.; Choi, J.; Lim, E.-K.; Kim, E.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Thiolated Dextran-Coated Gold Nanorods for Photothermal Ablation of Inflammatory Macrophages. Langmuir 2010, 26, 17520–17527. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Pectin modified metal nanoparticles and their application in property modification of biosensors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100164. [Google Scholar] [CrossRef]
- Cisneros, J.S.; Chain, C.Y.; Rivas Aiello, M.B.; Parisi, J.; Castrogiovanni, D.C.; Bosio, G.N.; Mártire, D.O.; Vela, M.E. Pectin-Coated Plasmonic Nanoparticles for Photodynamic Therapy: Inspecting the Role of Serum Proteins. ACS Omega 2021, 6, 12567–12576. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O.A.; Al-Ghobashy, M.A.; Nebsen, M.; El-Kholy, R.; Salem, M.Y. Assessment of pectin-coated magnetite nanoparticles in low-energy water desalination applications. Environ. Sci. Pollut. Res. 2018, 25, 18476–18483. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Suo, A.; Wang, Y.; Wang, J.; Sun, T.; Yang, M.; Wan, X.; Yao, Y. Hyaluronic Acid-Functionalized Gold Nanorods with pH/NIR Dual-Responsive Drug Release for Synergetic Targeted Photothermal Chemotherapy of Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 36533–36547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, M. Fast loading of PEG–SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760. [Google Scholar] [CrossRef]
- Yoon, S.; Chang, J.; Kwon, N.; Moon, S.; Park, Y.; Han, K.H.; Lim, B.; Lee, J.H. Multifunctional Nanomaterial-alginate Drug Delivery and Imaging System for Cancer Therapy. Biochip J. 2019, 13, 236–242. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Liu, C.-H.; Chin, Y.; Chen, S.-Y.; Liu, S.H.; Hsu, Y.-C.; Wu, K.C.-W. Biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B 2019, 7, 4451–4460. [Google Scholar] [CrossRef]
- de Almeida, D.A.; Sabino, R.M.; Souza, P.R.; Bonafé, E.G.; Venter, S.A.S.; Popat, K.C.; Martins, A.F.; Monteiro, J.P. Pectin-capped gold nanoparticles synthesis in-situ for producing durable, cytocompatible, and superabsorbent hydrogel composites with chitosan. Int. J. Biol. Macromol. 2020, 147, 138–149. [Google Scholar] [CrossRef]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Beltrán, O.; Luna, M.; Valbuena-Gregorio, E.; Valdez-Melchor, R.G.; Burruel-Ibarra, S.E.; Quihui-Cota, L.; Ruiz-Cruz, S.; Juárez, J.; López-Mata, M.A. Optimization of Carvacrol Nanoemulsion for the Incorporation in Pectin Membranes: Influence on Their Load Capacity, Microstructure and Antibacterial Properties. Mater. Res. 2022, 25, e20210534. [Google Scholar] [CrossRef]
- Borker, S.; Pokharkar, V. Engineering of pectin-capped gold nanoparticles for delivery of doxorubicin to hepatocarcinoma cells: An insight into mechanism of cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46, 826–835. [Google Scholar] [CrossRef]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge density quantification of polyelectrolyte polysaccharides by conductometric titration: An analytical chemistry experiment. J. Chem. Educ. 2012, 89, 121–124. [Google Scholar] [CrossRef]
- Eliyahu, S.; Galitsky, A.; Ritov, E.; Bianco-Peled, H. Hybrid Acrylated Chitosan and Thiolated Pectin Cross-Linked Hydrogels with Tunable Properties. Polymers 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.A.M.S.; Facchi, S.P.; Martins, A.F.; Nocchi, S.; Schuquel, I.T.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr. Polym. 2015, 115, 139–145. [Google Scholar] [CrossRef]
- Almada, M.; Ruiz, E.D.; Ibarra-Hurtado, J.; Hassan, N.; Kogan, M.J.; Cadena-Nava, R.D.; Valdés, M.A.; Juárez, J. Growth kinetics of gold nanorods synthesized by a seed-mediated method under pH acidic conditions. J. Nanosci. Nanotechnol. 2016, 16, 7707–7714. [Google Scholar] [CrossRef]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected standard protocols for the synthesis, phase transfer, and characterization of inorganic colloidal nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Z.; Panwar, N.; Hu, R.; Wang, X.; Qu, J.; Tjin, S.C.; Xu, G.; Yong, K.T. Functionalized gold nanorods for nanomedicine: Past, present and future. Coord. Chem. Rev. 2017, 352, 15–66. [Google Scholar] [CrossRef]
- Knoll, P.; Le, N.M.N.; Wibel, R.; Baus, R.A.; Kali, G.; Asim, M.H.; Bernkop-Schnürch, A. Thiolated pectins: In vitro and ex vivo evaluation of three generations of thiomers. Acta Biomater. 2021, 135, 139–149. [Google Scholar] [CrossRef]
- Hong, T.; Zhao, J.; Yin, J.; Nie, S.; Xie, M. Structural Characterization of a Low Molecular Weight HG-Type Pectin From Gougunao Green Tea. Front. Nutr. 2022, 9, 878249. [Google Scholar] [CrossRef]
- Bag, N.; Mathur, R.; Hussain, F.; Indracanti, N.; Singh, S.; Singh, S.; Chauhan, R.P.; Chuttani, K.; Mishra, A.K. Synthesis and in vivo toxicity assessment of CdSe:ZnS quantum dots functionalized with EDTA-Bis-Cysteamine. Toxicol. Res. 2015, 4, 1416–1425. [Google Scholar] [CrossRef]
- Del Caño, R.; Gisbert-González, J.M.; González-Rodríguez, J.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Effective replacement of cetyltrimethylammonium bromide (CTAB) by mercaptoalkanoic acids on gold nanorod (AuNR) surfaces in aqueous solutions. Nanoscale 2020, 12, 658–668. [Google Scholar] [CrossRef]
- Narayanan, J.; Deotare, V.W.; Bandyopadhyay, R.; Sood, A.K. Gelation of aqueous pectin solutions: A dynamic light scattering study. J. Colloid Interface Sci. 2002, 245, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Peng Jia, Y.; Shi, K.; Feng Liao, J.; Rong Peng, J.; Hao, Y.; Qu, Y.; Juan Chen, L.; Liu, L.; Yuan, X.; Yong Qian, Z.; et al. Effects of Cetyltrimethylammonium Bromide on the Toxicity of Gold Nanorods Both In Vitro and In Vivo: Molecular Origin of Cytotoxicity and Inflammation. Small Methods 2020, 4, 1900799. [Google Scholar] [CrossRef]
- Arellano-Galindo, L.; Villar-Alvarez, E.; Varela, A.; Figueroa, V.; Fernandez-Vega, J.; Cambón, A.; Prieto, G.; Barbosa, S.; Taboada, P. Hybrid Gold Nanorod-Based Nanoplatform with Chemo and Photothermal Activities for Bimodal Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 13109. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef] [PubMed]




| SH-PEC Concentration (mg/mL) | Hydrodynamic Diameter (nm) |
|---|---|
| 0.25 | 177.0 ± 8.0 |
| 0.50 | 104.6 ± 19.0 |
| 1.00 | 50.8 ± 8.8 |
| 2.00 | 68.4 ± 6.4 |
| 4.00 | 114.2 ± 33.5 |
| 8.00 | 191.0 ± 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran, O.; Luna, M.; Gastelum, M.; Costa-Santos, A.; Cambón, A.; Taboada, P.; López-Mata, M.A.; Topete, A.; Juarez, J. Novel Gold Nanorods@Thiolated Pectin on the Killing of HeLa Cells by Photothermal Ablation. Pharmaceutics 2023, 15, 2571. https://doi.org/10.3390/pharmaceutics15112571
Beltran O, Luna M, Gastelum M, Costa-Santos A, Cambón A, Taboada P, López-Mata MA, Topete A, Juarez J. Novel Gold Nanorods@Thiolated Pectin on the Killing of HeLa Cells by Photothermal Ablation. Pharmaceutics. 2023; 15(11):2571. https://doi.org/10.3390/pharmaceutics15112571
Chicago/Turabian StyleBeltran, Osvaldo, Mariangel Luna, Marisol Gastelum, Alba Costa-Santos, Adriana Cambón, Pablo Taboada, Marco A. López-Mata, Antonio Topete, and Josue Juarez. 2023. "Novel Gold Nanorods@Thiolated Pectin on the Killing of HeLa Cells by Photothermal Ablation" Pharmaceutics 15, no. 11: 2571. https://doi.org/10.3390/pharmaceutics15112571





