Current Research Trends in the Application of In Vitro Three-Dimensional Models of Liver Cells
Abstract
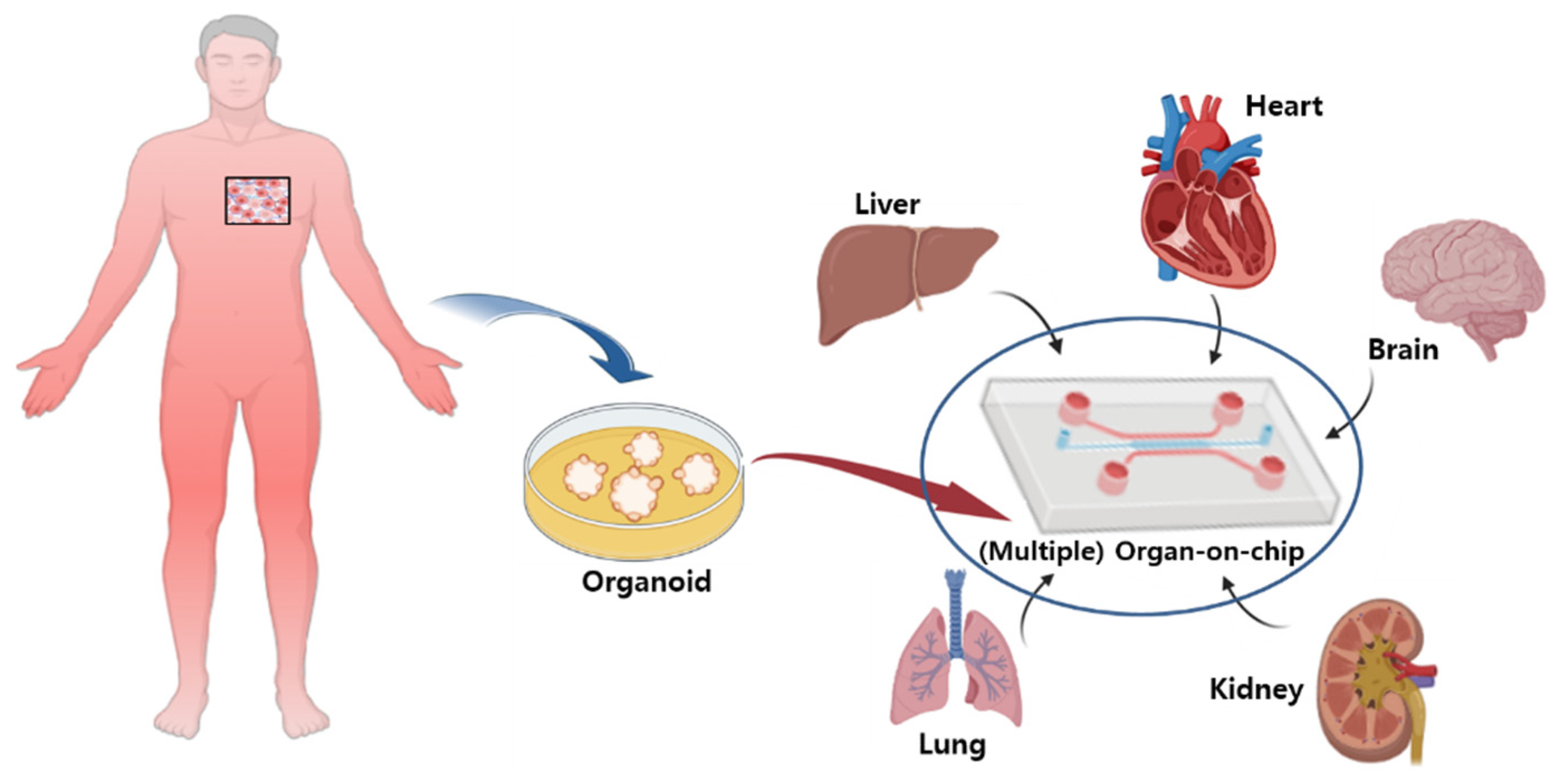
:1. Introduction
2. Three-Dimensional Cell Culture Technology
2.1. Hydrogel Scaffolds
2.2. Organ-On-Chips (Microchips)
2.3. Organoids and Spheroids
2.3.1. Organoids
2.3.2. Spheroids
3. Advantages of 3D Cultures in Studies on the Liver Metabolism
3.1. Liver Study with Scaffolds
3.2. Liver Organ-On-Chip Studies
3.3. Liver Studies Using Organoid or Spheroid Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauschke, V.M.; Vorrink, S.U.; Moro, S.M.; Rezayee, F.; Nordling, Å.; Hendriks, D.F.; Bell, C.C.; Sison-Young, R.; Park, B.K.; Goldring, C.E.; et al. Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology 2016, 64, 1743–1756. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.C.; Lauschke, V.M.; Vorrink, S.U.; Palmgren, H.; Duffin, R.; Andersson, T.B.; Ingelman-Sundberg, M. Transcriptional, Functional, and Mechanistic Comparisons of Stem Cell-Derived Hepatocytes, HepaRG Cells, and Three-Dimensional Human Hepatocyte Spheroids as Predictive In Vitro Systems for Drug-Induced Liver Injury. Drug Metab. Dispos. 2017, 45, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Yamazaki, M.; Shibuya, K.; Yamazaki, M.; Fujii, E.; Nakano, K.; Suzuki, M. Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Sci. Rep. 2020, 10, 3156. [Google Scholar] [CrossRef] [Green Version]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.C.; Dankers, A.C.A.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zheng, W.; Cheng, S.; Zhang, W.; Liu, S.; Jiang, X. In Vitro Evaluation of Essential Mechanical Properties and Cell Behaviors of a Novel Polylactic-co-Glycolic Acid (PLGA)-Based Tubular Scaffold for Small-Diameter Vascular Tissue Engineering. Polymers 2017, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cheng, Y.; Razzaque, S.; Cao, Z.; Ren, S.; Tan, B. Smart Synthesis of Hollow Microporous Organic Capsules with a Polyaniline Modified Shell. Macromol. Rapid Commun. 2022, 43, e2100836. [Google Scholar] [CrossRef]
- Rodriguez-Cabello, J.C.; Gonzalez De Torre, I.; González-Pérez, M.; González-Pérez, F.; Montequi, I. Fibrous Scaffolds From Elastin-Based Materials. Front. Bioeng. Biotechnol. 2021, 9, 652384. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Won, S.Y.; Sood, A.; Choi, S.Y.; Singhmar, R.; Bhaskar, R.; Kumar, V.; Zo, S.M.; Han, S.S. Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 14158. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Lim, C.; Lim, M. Characterization of leukemic cell behaviors in a soft marrow mimetic alginate hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1980–1988. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Xie, H.G.; Zhao, S.; Xu, X.X.; Fan, L.X.; Guo, X.; Lu, T.; Sun, G.W.; Ma, X.J. Role of three-dimensional matrix stiffness in regulating the chemoresistance of hepatocellular carcinoma cells. Biotechnol. Appl. Biochem. 2015, 62, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular weight dependence of structure and properties of chitosan oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- da Silva Morais, A.; Vieira, S.; Zhao, X.; Mao, Z.; Gao, C.; Oliveira, J.M.; Reis, R.L. Advanced Biomaterials and Processing Methods for Liver Regeneration: State-of-the-Art and Future Trends. Adv. Healthc. Mater. 2020, 9, e1901435. [Google Scholar] [CrossRef]
- Leung, M.; Kievit, F.M.; Florczyk, S.J.; Veiseh, O.; Wu, J.; Park, J.O.; Zhang, M. Chitosan-alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance. Pharm. Res. 2010, 27, 1939–1948. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T. The effect of amines added to an alkali-pretreatment on the solubilisation of collagen and on the properties of gelatin. Hoppe Seylers Z. Physiol. Chem. 1969, 350, 1257–1265. [Google Scholar] [CrossRef]
- Brancato, V.; Gioiella, F.; Profeta, M.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Melone, P.; Netti, P.A. 3D tumor microtissues as an in vitro testing platform for microenvironmentally-triggered drug delivery systems. Acta Biomater. 2017, 57, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering--a review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Turner, R.A.; Wauthier, E.; Lozoya, O.; McClelland, R.; Bowsher, J.E.; Barbier, C.; Prestwich, G.; Hsu, E.; Gerber, D.A.; Reid, L.M. Successful transplantation of human hepatic stem cells with restricted localization to liver using hyaluronan grafts. Hepatology 2013, 57, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, R.B.; Shahdadfar, A.; Reinholt, F.P.; Brinchmann, J.E. Chondrogenesis in a hyaluronic acid scaffold: Comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Payne, S.L. Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater. Res. 2021, 25, 5. [Google Scholar] [CrossRef]
- Vasanthan, K.S.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Role of biomaterials, therapeutic molecules and cells for hepatic tissue engineering. Biotechnol. Adv. 2012, 30, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Balali, S.; Davachi, S.M.; Sahraeian, R.; Shiroud Heidari, B.; Seyfi, J.; Hejazi, I. Preparation and Characterization of Composite Blends Based on Polylactic Acid/Polycaprolactone and Silk. Biomacromolecules 2018, 19, 4358–4369. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.H.; Kuo, C.Y.; Tagadur Govindaraju, D.; Chen, K.S.; Chen, J.P. Polycaprolactone/Chitosan Composite Nanofiber Membrane as a Preferred Scaffold for the Culture of Mesothelial Cells and the Repair of Damaged Mesothelium. Int. J. Mol. Sci. 2022, 23, 9517. [Google Scholar] [CrossRef]
- Yoon, S.K.; Chung, D.J. In Vivo Degradation Studies of PGA-PLA Block Copolymer and Their Histochemical Analysis for Spinal-Fixing Application. Polymers 2022, 14, 3322. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2018, 24, 5471–5486. [Google Scholar] [CrossRef]
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines 2020, 11, 381. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.Y.; Weber, E.J.; Sidorenko, V.S.; Chapron, A.; Yeung, C.K.; Gao, C.; Mao, Q.; Shen, D.; Wang, J.; Rosenquist, T.A.; et al. Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2017, 2, e95978. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach To Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef]
- Filipiak-Duliban, A.; Brodaczewska, K.; Kajdasz, A.; Kieda, C. Spheroid Culture Differentially Affects Cancer Cell Sensitivity to Drugs in Melanoma and RCC Models. Int. J. Mol. Sci. 2022, 23, 1166. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Rabbani, Z.; Soveyzi, F.; Tayanloo-Beik, A.; Rezaei-Tavirani, M.; Biglar, M.; Adibi, H.; Larijani, B. Advancement of Organoid Technology in Regenerative Medicine. Regen. Eng. Transl. Med. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, S.; Win Naing, M. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol. Bioeng. 2021, 118, 542–554. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Shen, H. Core Concept: Organoids have opened avenues into investigating numerous diseases. But how well do they mimic the real thing? Proc. Natl. Acad. Sci. USA 2018, 115, 3507–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Vidyasekar, P.; Shyamsunder, P.; Sahoo, S.K.; Verma, R.S. Scaffold-free and scaffold-assisted 3D culture enhances differentiation of bone marrow stromal cells. In Vitro Cell Dev. Biol. Anim. 2016, 52, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; Ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1653. [Google Scholar] [CrossRef]
- Riffle, S.; Pandey, R.N.; Albert, M.; Hegde, R.S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer 2017, 17, 338. [Google Scholar] [CrossRef] [Green Version]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.A.; Rink, A.; Beattie, C.W.; Hu, W.S. Differential gene expression analysis during porcine hepatocyte spheroid formation. Mamm. Genome 2002, 13, 515–523. [Google Scholar] [CrossRef]
- Yang, J.; He, M.M.; Niu, W.; Wrighton, S.A.; Li, L.; Liu, Y.; Li, C. Metabolic capabilities of cytochrome P450 enzymes in Chinese liver microsomes compared with those in Caucasian liver microsomes. Br. J. Clin. Pharmacol. 2012, 73, 268–284. [Google Scholar] [CrossRef] [Green Version]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef]
- Cox, C.R.; Lynch, S.; Goldring, C.; Sharma, P. Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury. Front. Med. Technol. 2020, 2, 611913. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yang, Z.; Dong, D.L.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Jin, G.Z.; Xuan, Y. 3D culture technologies of cancer stem cells: Promising ex vivo tumor models. J. Tissue Eng. 2020, 11, 2041731420933407. [Google Scholar] [CrossRef]
- Uchida, Y.; Tanaka, S.; Aihara, A.; Adikrisna, R.; Yoshitake, K.; Matsumura, S.; Mitsunori, Y.; Murakata, A.; Noguchi, N.; Irie, T.; et al. Analogy between sphere forming ability and stemness of human hepatoma cells. Oncol. Rep. 2010, 24, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Eales, K.L.; Hollinshead, K.E.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Burdett, E.; Kasper, F.K.; Mikos, A.G.; Ludwig, J.A. Engineering tumors: A tissue engineering perspective in cancer biology. Tissue Eng. Part B Rev. 2010, 16, 351–359. [Google Scholar] [CrossRef]
- Mehta, M.; Khan, A.; Danish, S.; Haffty, B.G.; Sabaawy, H.E. Radiosensitization of Primary Human Glioblastoma Stem-like Cells with Low-Dose AKT Inhibition. Mol. Cancer Ther. 2015, 14, 1171–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Boeter, J.W.B.; Penning, L.C.; Spee, B.; Schneeberger, K. Hydrogels for Liver Tissue Engineering. Bioengineering 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Angelo, M.; Benedetti, E.; Tupone, M.G.; Catanesi, M.; Castelli, V.; Antonosante, A.; Cimini, A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells 2019, 8, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, C.; Li, H.; Ding, Y. Decreased liver stiffness by transient elastography indicates lower incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Medicine 2019, 98, e13929. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.C.; Du, C.; Gao, S.; Wan, A.C.; Ying, J.Y. The use of a polyelectrolyte fibrous scaffold to deliver differentiated hMSCs to the liver. Biomaterials 2010, 31, 48–57. [Google Scholar] [CrossRef]
- Poorna, M.R.; Sudhindran, S.; Thampi, M.V.; Mony, U. Differentiation of induced pluripotent stem cells to hepatocyte-like cells on cellulose nanofibril substrate. Colloids Surf. B Biointerfaces 2021, 198, 111466. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Chu, X.H.; Huang, N.P.; Leach, M.K.; Wang, G.; Wang, Y.C.; Ding, Y.T.; Gu, Z.Z. Rat hepatocyte aggregate formation on discrete aligned nanofibers of type-I collagen-coated poly(L-lactic acid). Biomaterials 2010, 31, 3604–3612. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Preparation and evaluation of ammonia-treated collagen/chitosan matrices for liver tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 91–98. [Google Scholar] [CrossRef]
- Denuzière, A.; Ferrier, D.; Domard, A. Interactions between chitosan and glycosaminoglycans (chondroitin sulfate and hyaluronic acid): Physicochemical and biological studies. Ann. Pharm. Fr. 2000, 58, 47–53. [Google Scholar]
- Hou, Y.T.; Hsu, C.C. Development of a 3D porous chitosan/gelatin liver scaffold for a bioartificial liver device. J. Biosci. Bioeng. 2020, 129, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, S.J.; Kim, C.B.; Kim, J.K.; Cho, Y.W.; Chung, B.G.; Lee, S.H. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010, 10, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Ruoß, M.; Häussling, V.; Schügner, F.; Olde Damink, L.H.H.; Lee, S.M.L.; Ge, L.; Ehnert, S.; Nussler, A.K. A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering 2018, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.R.; Lee, Y.M.; Akaike, T. Evaluation of a galactose-carrying gelatin sponge for hepatocytes culture and transplantation. J. Biomed. Mater. Res. A 2003, 67, 733–741. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.W.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Healthc. Mater. 2020, 9, e1901658. [Google Scholar] [CrossRef] [Green Version]
- Bot, P.T.; Hoefer, I.E.; Piek, J.J.; Pasterkamp, G. Hyaluronic acid: Targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr. Med. Chem. 2008, 15, 786–791. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kurbatov, L.K.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells 2019, 8, 1127. [Google Scholar] [CrossRef] [Green Version]
- Nevi, L.; Carpino, G.; Costantini, D.; Cardinale, V.; Riccioni, O.; Di Matteo, S.; Melandro, F.; Berloco, P.B.; Reid, L.; Gaudio, E.; et al. Hyaluronan coating improves liver engraftment of transplanted human biliary tree stem/progenitor cells. Stem Cell Res. Ther. 2017, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Christoffersson, J.; Aronsson, C.; Jury, M.; Selegård, R.; Aili, D.; Mandenius, C.F. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication 2018, 11, 015013. [Google Scholar] [CrossRef]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. A 2012, 100, 406–423. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.W. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 2011, 3, 034102. [Google Scholar] [CrossRef]
- Das, P.; DiVito, M.D.; Wertheim, J.A.; Tan, L.P. Collagen-I and fibronectin modified three-dimensional electrospun PLGA scaffolds for long-term in vitro maintenance of functional hepatocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110723. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Tripathi, D.M.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S.; Kaur, S. Evolution of Electrospinning in Liver Tissue Engineering. Biomimetics 2022, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, C.; Duan, L.; Hou, X.; Huang, Y.; Gao, L.; Zong, C.; Liu, W.; Jiang, J.; Ye, F.; et al. Oncostatin M promotes hepatic progenitor cell activation and hepatocarcinogenesis via macrophage-derived tumor necrosis factor-α. Cancer Lett. 2021, 517, 46–54. [Google Scholar] [CrossRef]
- Davidson, I.W.; Beliles, R.P. Consideration of the target organ toxicity of trichloroethylene in terms of metabolite toxicity and pharmacokinetics. Drug Metab. Rev. 1991, 23, 493–599. [Google Scholar] [CrossRef] [PubMed]
- Horner, S.; Ryan, D.; Robinson, S.; Callander, R.; Stamp, K.; Roberts, R.A. Target organ toxicities in studies conducted to support first time in man dosing: An analysis across species and therapy areas. Regul. Toxicol. Pharmacol. 2013, 65, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Meunier, L.; Larrey, D. Drug-Induced Liver Injury: Biomarkers, Requirements, Candidates, and Validation. Front. Pharmacol. 2019, 10, 1482. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Messelmani, T.; Le Goff, A.; Souguir, Z.; Maes, V.; Roudaut, M.; Vandenhaute, E.; Maubon, N.; Legallais, C.; Leclerc, E.; Jellali, R. Development of Liver-on-Chip Integrating a Hydroscaffold Mimicking the Liver’s Extracellular Matrix. Bioengineering 2022, 9, 443. [Google Scholar] [CrossRef]
- Docci, L.; Milani, N.; Ramp, T.; Romeo, A.A.; Godoy, P.; Franyuti, D.O.; Krähenbühl, S.; Gertz, M.; Galetin, A.; Parrott, N.; et al. Exploration and application of a liver-on-a-chip device in combination with modelling and simulation for quantitative drug metabolism studies. Lab Chip 2022, 22, 1187–1205. [Google Scholar] [CrossRef]
- Shri, M.; Agrawal, H.; Rani, P.; Singh, D.; Onteru, S.K. Hanging Drop, A Best Three-Dimensional (3D) Culture Method for Primary Buffalo and Sheep Hepatocytes. Sci. Rep. 2017, 7, 1203. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, A.; Murekatete, B.; Moedder, D.; Meinert, C.; Bray, L.J. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci. Rep. 2021, 11, 15566. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Ozawa, F.; Nie, M.; Takeuchi, S. 3D culture of functional human iPSC-derived hepatocytes using a core-shell microfiber. PLoS ONE 2020, 15, e0234441. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cong, Y.; Han, X.; Wei, W.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B.; Luo, Y.; Zhang, X. A liver-on-a-chip for hepatoprotective activity assessment. Biomicrofluidics 2020, 14, 064107. [Google Scholar] [CrossRef]
- Maher, S.P.; Crouse, R.B.; Conway, A.J.; Bannister, E.C.; Achyuta, A.K.; Clark, A.Y.; Sinatra, F.L.; Cuiffi, J.D.; Adams, J.H.; Kyle, D.E.; et al. Microphysical space of a liver sinusoid device enables simplified long-term maintenance of chimeric mouse-expanded human hepatocytes. Biomed. Microdevices 2014, 16, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, L.J.Y.; Chong, L.H.; Jin, L.; Singh, P.K.; Lee, P.S.; Yu, H.; Ananthanarayanan, A.; Leo, H.L.; Toh, Y.C. A pump-free microfluidic 3D perfusion platform for the efficient differentiation of human hepatocyte-like cells. Biotechnol. Bioeng. 2017, 114, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef]
- Materne, E.M.; Tonevitsky, A.G.; Marx, U. Chip-based liver equivalents for toxicity testing--organotypicalness versus cost-efficient high throughput. Lab Chip 2013, 13, 3481–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions—A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Yamada, D.; Takarada, T. A protocol to induce expandable limb-bud mesenchymal cells from human pluripotent stem cells. STAR Protoc. 2022, 3, 101786. [Google Scholar] [CrossRef]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B.t. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalopoulos, G.K.; Bowen, W.C.; Mulè, K.; Luo, J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003, 11, 55–75. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, Z.P.; Liu, H.J.; Wang, L.J.; Cheng, Z.; Tang, N.; Li, T.; Liu, T.; Han, H.X.; Cao, G.; et al. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5(+) liver stem cells. Nat. Commun. 2017, 8, 1175. [Google Scholar] [CrossRef] [Green Version]
- Asai, A.; Aihara, E.; Watson, C.; Mourya, R.; Mizuochi, T.; Shivakumar, P.; Phelan, K.; Mayhew, C.; Helmrath, M.; Takebe, T.; et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 2017, 144, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Xu, D.; Garfin, P.M.; Ehmer, U.; Hurwitz, M.; Enns, G.; Michie, S.; Wu, M.; Zheng, M.; Nishimura, T.; et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2017, 2, e94954. [Google Scholar] [CrossRef] [Green Version]
- Jalan-Sakrikar, N.; Brevini, T.; Huebert, R.C.; Sampaziotis, F. Organoids and regenerative hepatology. Hepatology 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Calvisi, D.F.; Chen, X. Organoids for the Study of Liver Cancer. Semin. Liver Dis. 2021, 41, 19–27. [Google Scholar] [CrossRef]
- Görlach, A.; Holtermann, G.; Jelkmann, W.; Hancock, J.T.; Jones, S.A.; Jones, O.T.; Acker, H. Photometric characteristics of haem proteins in erythropoietin-producing hepatoma cells (HepG2). Biochem. J. 1993, 290 Pt 3, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Ma, M.; Purcell, W.M. Characterisation of some cytotoxic endpoints using rat liver and HepG2 spheroids as in vitro models and their application in hepatotoxicity studies. II. Spheroid cell spreading inhibition as a new cytotoxic marker. Toxicol. Appl. Pharmacol. 2003, 189, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Hendriks, D.F.; Moro, S.M.; Ellis, E.; Walsh, J.; Renblom, A.; Fredriksson Puigvert, L.; Dankers, A.C.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingelman-Sundberg, M.; Lauschke, V.M. 3D human liver spheroids for translational pharmacology and toxicology. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. 1), 5–15. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research progress in toxicological effects and mechanism of aflatoxin B(1) toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Dalsbecker, P.; Beck Adiels, C.; Goksör, M. Liver-on-a-chip devices: The pros and cons of complexity. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G188–G204. [Google Scholar] [CrossRef] [PubMed]
- Messelmani, T.; Morisseau, L.; Sakai, Y.; Legallais, C.; Le Goff, A.; Leclerc, E.; Jellali, R. Liver organ-on-chip models for toxicity studies and risk assessment. Lab Chip 2022, 22, 2423–2450. [Google Scholar] [CrossRef] [PubMed]


| 2D | 3D | Reference | |
|---|---|---|---|
| Cell morphology | Growing in a flat form on the floor. | Growing up in a 3D shape that can be found in tissues. | [5] |
| Cell–cell interaction | Cell-to-cell interactions are limited owing to the large proportion of interactions between the floor and the cell. | Cell-to-cell interactions are all connected and can form physiologically active conditions which are close to those of living organisms [6]. | [6,7] |
| Cell differentiation and proliferation | Differentiation rate is not significantly improved, and proliferation shows a high growth rate. | Shows a very high cell differentiation rate compared to that of 2D cell cultures, and cell proliferation rates are achieved under conditions similar to the in vivo environment. Easy to control for rate. | [7] |
| Drug metabolism | Poor observation of drug metabolism. | The expression of various enzymes involved in drug metabolism has increased significantly, leading to a good drug metabolism process. | [8,9] |
| Drug sensitivity | Differences depending on the concentration of the drug; drug effects occur well. | High resistance to drugs compared to 2D cell cultures. | [9] |
| Spheroids | Organoids | Reference | |
|---|---|---|---|
| Sources | Can be formed through the tendency of aggregation of adhesive cells. It can be composed of several types of cells and can be formed by the cell’s own tissues. | Generated from both iPSCs and adult stem cells by mimicking the biochemical and physical cues of tissue development and homeostasis. | [49,50] |
| Applications | Spheroids can be useful for the discovery of disease-related cancers or drugs. | Organoids are useful for regenerative medicine, drug discovery, and disease modeling. | [51,52] |
| Considerations | It is economical and easy to manufacture, but it is difficult to cultivate for a long time. | Multiple cells can be combined into a single component and can closely imitate the actual organ, but a well-organized surrounding environment is needed. | [53] |
| 3D culture conditions | Cultured with or without extracellular matrix and growth factors. | Needs extracellular substrates and growth factors | [49] |
| In Vitro Application | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Hydrogel 3D scaffold | It can be designed by calculating the 3D microenvironment and ECM characteristics. The expression of proteins and genes expressed in hydrogels is high, and the function and life of cells are improving with the development of technology. | Cell cultures are complicated due to the difference in materials, and the necrosis rate of cells occurring in the 3D cell culture model is a task to be overcome. | [128,129,130] |
| Liver-on-a-chip | 3D cultures of liver tissues and cocultivation with other tissues are possible, and a fine environment suitable for spheroids can be manipulated in the laboratory. As a result, the expression rate of the CYP protein expressed in the reinforced liver is high, and a secondary structure can be formed. In addition, owing to the pattern created in the 3D space, the throughput is high, and the cost is relatively low. | The method of manipulating the chip is still complicated, and it is necessary to develop the supply system necessary for cell cultures on the chip. In addition, the growth of liver cells varies depending on the material of the chip. This is because standardized methods have not yet been established. | [131,132] |
| Liver Spheroid/ Liver Organoid | It can provide a microenvironment similar to a complex environment in vivo, enabling cell interactions, long incubation periods, and high expression of CYP 450 and transporters in the liver, and liver-specific functions are well maintained. | Owing to the limited size of the spheroids, it is still difficult to form large-sized spheroids. Additionally, cell properties may deform, or necrosis may occur in long-term cultures. | [101,132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.; Kim, S.H.; Jung, Y.-S. Current Research Trends in the Application of In Vitro Three-Dimensional Models of Liver Cells. Pharmaceutics 2023, 15, 54. https://doi.org/10.3390/pharmaceutics15010054
Yun C, Kim SH, Jung Y-S. Current Research Trends in the Application of In Vitro Three-Dimensional Models of Liver Cells. Pharmaceutics. 2023; 15(1):54. https://doi.org/10.3390/pharmaceutics15010054
Chicago/Turabian StyleYun, Chawon, Sou Hyun Kim, and Young-Suk Jung. 2023. "Current Research Trends in the Application of In Vitro Three-Dimensional Models of Liver Cells" Pharmaceutics 15, no. 1: 54. https://doi.org/10.3390/pharmaceutics15010054





