Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
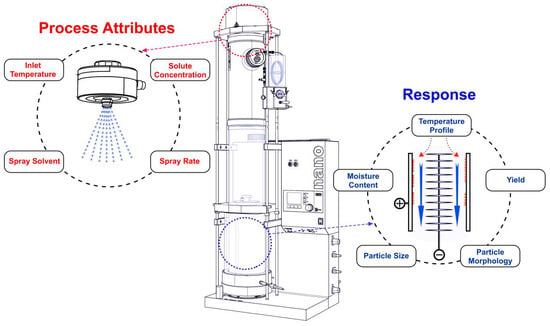
2.2. Experimental Design
2.3. Spray Drying
2.4. Characterization of Spray Solutions
2.5. Characterization of Nano Spray-Dried Powder Materials
2.5.1. Moisture Content
2.5.2. Particle Morphology and Size
2.5.3. Thermal Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Spray Generation
3.2. Temperature Profile
3.2.1. Yield
3.2.2. Moisture Content
3.2.3. Particle Size Distribution

3.3. Spray Solvent
3.3.1. Yield
3.3.2. Moisture Content
3.3.3. Particle Size Distribution

3.4. Solute Concentration
3.4.1. Yield
3.4.2. Moisture Content
3.4.3. Particle Size Distribution
4. Conclusions
- Optimization of spray flow in the Nano Spray Dryer B-90 is one key to a successful process and yield optimization. The addition of hydrochloric acid at a low concentration of 0.02% w/w here improved the spray performance for mannitol solutions. Possibly, hydrochloric acid influenced the crystallization behavior of mannitol in a way that prevented blockage of the spray mesh pores or the spray mesh vibration by mannitol crystals. The observation is worth further investigation. Using additives that modulate the crystallization behavior of the material to be spray dried is suggested as an approach to optimize spray generation by the vibrating-mesh atomizer. An alternative is the use of surface-active additives, e.g., polysorbate 20.
- Powder yields (2.0 g batches) were generally 71% w/w for lactose and 66% w/w for mannitol. Using a hydroethanolic spray solvent increased the yield to 76% w/w for lactose. The increase is attributed to lower turbulence around the spray head. The influence of the spray solvent on the flow of spray droplets can be considered as an approach for yield optimization.
- Powder analysis revealed that lactose and mannitol particles were spherical. Spray-dried lactose materials were amorphous, whereas mannitol materials were crystalline.
- Particle size was influenced by the spray solvent. The spray solvent viscosity and surface tension and the time required for the solute to reach saturation at surfaces of drying droplets contribute to the final particle size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Vehring, R.; Snyder, H.; Lechuga-Ballesteros, D. Spray drying. In Drying Technologies for Biotechnology and Pharmaceutical Applications; Ohtake, S., Izutsu, K.-I., Lechuga-Ballesteros, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 179–216. [Google Scholar]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: London, UK, 2017; pp. 346–401. [Google Scholar]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Arpagaus, C. Pharmaceutical particle engineering via nano spray drying—Process parameters and application examples on the laboratory-scale. Int. J. Med. Nano. Res. 2018, 5, 026. [Google Scholar] [CrossRef]
- Feng, A.L.; Boraey, M.A.; Gwin, M.A.; Finlay, P.R.; Kuehl, P.J.; Vehring, R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef]
- Almansour, K.; Alfagih, I.M.; Ali, R.; Elsayed, M.M.A. Inhalable microparticles containing terbinafine for management of pulmonary fungal infections: Spray drying process engineering using lactose vs. mannitol as excipients. J. Drug Deliv. Sci. Technol. 2020, 60, 101991. [Google Scholar] [CrossRef]
- Almansour, K.; Alfagih, I.M.; Aodah, A.H.; Alheibshy, F.; Ali, R.; Al Hagbani, T.; Elsayed, M.M.A. Inhalable, spray-dried terbinafine microparticles for management of pulmonary fungal infections: Optimization of the excipient composition and selection of an inhalation device. Pharmaceutics 2022, 14, 87. [Google Scholar] [CrossRef]
- Schoubben, A.; Giovagnoli, S.; Tiralti, M.C.; Blasi, P.; Ricci, M. Capreomycin inhalable powders prepared with an innovative spray-drying technique. Int. J. Pharm. 2014, 469, 132–139. [Google Scholar] [CrossRef]
- Littringer, E.M.; Zellnitz, S.; Hammernik, K.; Adamer, V.; Friedl, H.; Urbanetz, N.A. Spray drying of aqueous salbutamol sulfate solutions using the nano spray dryer B-90—The impact of process parameters on particle size. Dry. Technol. 2013, 31, 1346–1353. [Google Scholar] [CrossRef]
- Büchi Labortechnik, A.G. Nano Spray Dryer B-90: Operation Manual, Version B.; BÜCHI Labortechnik AG: Flawil, Switzerland, 2009. [Google Scholar]
- Schmid, K.; Arpagaus, C.; Friess, W. Evaluation of the nano spray dryer B-90 for pharmaceutical applications. Pharm. Dev. Technol. 2011, 16, 287–294. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Strehlow, B.; Kissel, T. Direct fractionation of spray-dried polymeric microparticles by inertial impaction. Powder Technol. 2015, 286, 311–317. [Google Scholar] [CrossRef]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.-K.; Tan, R.B.H. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bürki, K.; Jeon, I.; Arpagaus, C.; Betz, G. New insights into respirable protein powder preparation using a nano spray dryer. Int. J. Pharm. 2011, 408, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Draheim, C.; de Crécy, F.; Hansen, S.; Collnot, E.-M.; Lehr, C.-M. A Design of experiment study of nanoprecipitation and nano spray drying as processes to prepare PLGA nano- and microparticles with defined sizes and size distributions. Pharm. Res. 2015, 32, 2609–2624. [Google Scholar] [CrossRef]
- Longest, P.W.; Farkas, D.; Hassan, A.; Hindle, M. Computational fluid dynamics (CFD) simulations of spray drying: Linking drying parameters with experimental aerosolization performance. Pharm. Res. 2020, 37, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Myrdal, P.B.; Sheth, P.; Stein, S.W. Advances in Metered Dose Inhaler Technology: Formulation Development. AAPS PharmSciTech 2014, 15, 434–455. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.K.; Kawai, K.; Suzuki, T. Glass transition and enthalpy relaxation of amorphous lactose glass. Carbohydr. Res. 2006, 341, 1884–1889. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, D.S.; Rigsbee, D.R. Determination of the glass properties of D-mannitol using sorbitol as an impurity. J. Pharm. Sci. 1998, 87, 774–777. [Google Scholar] [CrossRef]
- Shalash, A.O.; Khalafallah, N.M.; Molokhia, A.M.; Elsayed, M.M.A. The relationship between the air permeability and the performance of carrier-based dry powder inhalation mixtures: New insights and practical guidance. AAPS PharmSciTech 2018, 19, 912–922. [Google Scholar] [CrossRef]
- Shalash, A.O.; Molokhia, A.M.; Elsayed, M.M.A. Insights into the roles of carrier microstructure in adhesive/carrier-based dry powder inhalation mixtures: Carrier porosity and fine particle content. Eur. J. Pharm. Biopharm. 2015, 96, 291–303. [Google Scholar] [CrossRef]
- Burger, A.; Henck, J.-O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stöttner, H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.-J. Properties of sugar, polyol, and polysaccharide water−ethanol solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]
- Gu, B.; Linehan, B.; Tseng, Y.-C. Optimization of the Büchi B-90 spray drying process using central composite design for preparation of solid dispersions. Int. J. Pharm. 2015, 491, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Hou, S.; Wu, J.; Li, X.; Shu, H. Practical, regulatory and clinical considerations for development of inhalation drug products. Asian J. Pharm. Sci. 2015, 10, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Weissenborn, P.K.; Pugh, R.J. Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence. J. Colloid Interface Sci. 1996, 184, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Arpargaus, C.; Rütti, D.; Meuri, M. Enhanced solubility of poorly soluble drugs via spray drying. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Douroumis, D., Fahr, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 551–585. [Google Scholar]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The influence of mannitol on morphology and disintegration of spray-dried nano-embedded microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef]
- Haynes, W.M. Thermophysical properties of water and steam. In CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 6-1–6-4. [Google Scholar]
- Ghazanfari, T.; Elhissi, A.M.A.; Ding, Z.; Taylor, K.M.G. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int. J. Pharm. 2007, 339, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ordoubadi, M.; Gregson, F.K.A.; Wang, H.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. On the particle formation of leucine in spray drying of inhalable microparticles. Int. J. Pharm. 2021, 592, 120102. [Google Scholar] [CrossRef]
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Belotti, S.; Rossi, A.; Colombo, P.; Bettini, R.; Rekkas, D.; Politis, S.; Colombo, G.; Balducci, A.G.; Buttini, F. Spray-dried amikacin sulphate powder for inhalation in cystic fibrosis patients: The role of ethanol in particle formation. Eur. J. Pharm. Biopharm. 2015, 93, 165–172. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A. Particle size and density in spray drying—Effects of carbohydrate properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef] [PubMed]




| Property a | Lactose | Mannitol |
|---|---|---|
| MW [g/mol] | 342.30 | 182.17 |
| [°C] | 102 [22] | 10.7 [23] |
| [°C] | 214–219 (α, peak) [24], 236 (β, peak) [25] | 150–158 (δ, onset) [26], 166 (α/β, onset) [26] |
| [% w/w] | 29 (37 °C) [27] | 23.8 (37 °C) [27] |
| [% w/w] | 3.5 (37 °C) [27] | 6.0 (37 °C) [27] |
| Set of Experiments | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Lactose-AQ | Lactose-HE15 | Lactose-HE50 | Mannitol-AQ | Mannitol-HE50 | |
| Solute | Lactose | Lactose | Lactose | Mannitol | Mannitol |
| Concentration of solute [% w/w] | 1.5, 3.0 | 1.5, 3.0 | 1.5, 3.0 | 1.5, 3.0 | 1.5, 3.0 |
| Solvent | Aqueous | Hydroethanolic | Hydroethanolic | Aqueous | Hydroethanolic |
| Concentration of ethanol [% w/w] | 0.0 | 15.0 | 50.0 | 0.0 a | 50.0 a |
| Solution density, 40 °C [g/cm3] | 0.998–1.002 | 0.970–0.979 | 0.904–0.906 | 0.997–1.001 | 0.901–0.904 |
| Solution viscosity, 40 °C [mPa⋅s] | 0.64–0.69 | 1.19–1.21 | 1.69–1.76 | 0.71–0.82 | 1.65–1.69 |
| Spray intensity [%] | 50–100 | 75–85 | 100 | 75 | 100 |
| Spray rate [g/h] | 14–60 | 14–21 | 14–17 | 24–28 | 9–15 |
| Inlet temperature [°C] | 80, 110 | 80, 110 | 80, 110 | 80, 110 | 80, 110 |
| Air flow rate [L/min] | 135 | 135 | 135 | 135 | 135 |
| Number of experiments () | 18 | 7 | 4 | 4 | 4 |
| Set of Experiments | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Lactose-AQ | Lactose-HE15 | Lactose-HE50 | Mannitol-AQ | Mannitol-HE50 | |
| Yield [% w/w] | 68.2 ± 6.6 | 76.1 ± 2.8 | 75.4 ± 2.9 | 65.7 ± 3.5 | 66.4 ± 4.0 |
| Glass cylinder [% w/w] | 11.1 ± 4.5 | 7.2 ± 3.7 | 7.0 ± 2.4 | 11.1 ± 3.4 | 8.8 ± 2.0 |
| Nozzle deposits [% w/w] | 5.3 ± 6.0 | 4.6 ± 3.6 | 4.3 ± 3.5 | 0.8 ± 1.0 | 0.9 ± 0.6 |
| Loss on drying [% w/w] | 4.7 ± 0.9 | 4.5 ± 0.6 | 4.5 ± 0.5 | 1.0 ± 0.1 | 1.3 ± 0.2 |
| [μm] | 2.2 ± 0.3 a | 1.9 ± 0.1 | 1.9 ± 0.2 | 2.3 ± 0.2 | 2.0 ± 0.3 |
| [μm] | 0.7 ± 0.1 a | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 |
| [μm] | 1.8 ± 0.2 a | 1.5 ± 0.1 | 1.5 ± 0.2 | 1.9 ± 0.1 | 1.5 ± 0.2 |
| [μm] | 4.3 ± 0.8 a | 3.5 ± 0.3 | 3.6 ± 0.4 | 4.6 ± 0.5 | 4.0 ± 1.1 |
| 2.0 ± 0.3 a | 1.9 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.5 | |
| [μm] | 5.3 ± 0.7 a | 4.5 ± 0.5 | 4.7 ± 0.5 | 5.7 ± 0.7 | 5.5 ± 1.9 |
| [μm] | 2.6 ± 0.5 a | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.8 ± 0.2 | 2.6 ± 0.9 |
| [μm] | 5.3 ± 0.9 a | 4.4 ± 0.7 | 4.7 ± 0.3 | 5.6 ± 0.6 | 5.6 ± 1.9 |
| [μm] | 8.0 ± 1.0 a | 6.9 ± 0.7 | 7.1 ± 0.7 | 8.6 ± 1.0 | 8.1 ± 2.7 |
| 1.0 ± 0.1 a | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Enthalpy of water evap. [J/g] | 66.2 ± 8.0 a | 61.8 ± 10.9 | 45.8 ± 13.8 | ND * | ND * |
| [°C] | 112.85 ± 0.38 a | NM * | 106.19 ± 0.82 b | ND * | ND * |
| [°C] | ND * | ND * | ND * | 165.91 ± 0.50 | 166.09 ± 0.90 |
| Enthalpy of melting [J/g] | ND * | ND * | ND * | 261.2 ± 13.4 | 261.7 ± 5.4 |
| Process Parameter | Major Influence |
|---|---|
| Spray throughput | Nozzle deposits and yield loss |
| Inlet temperature | Moisture content of amorphous but not crystalline materials |
| Spray mesh | Particle size and spray throughput |
| Spray solvent | Particle size, density, and morphology mediated mainly by the influence of the solvent on the solubility of the solute and the time it requires to reach saturation at surfaces of drying droplets |
| Solute concentration | Particle size mediated mainly by the influence of the concentration on the time the solute requires to reach saturation at surfaces of drying droplets |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, K.; Ali, R.; Alheibshy, F.; Almutairi, T.J.; Alshammari, R.F.; Alhajj, N.; Arpagaus, C.; Elsayed, M.M.A. Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions. Pharmaceutics 2022, 14, 800. https://doi.org/10.3390/pharmaceutics14040800
Almansour K, Ali R, Alheibshy F, Almutairi TJ, Alshammari RF, Alhajj N, Arpagaus C, Elsayed MMA. Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions. Pharmaceutics. 2022; 14(4):800. https://doi.org/10.3390/pharmaceutics14040800
Chicago/Turabian StyleAlmansour, Khaled, Raisuddin Ali, Fawaz Alheibshy, Tariq J. Almutairi, Rakan F. Alshammari, Nasser Alhajj, Cordin Arpagaus, and Mustafa M.A. Elsayed. 2022. "Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions" Pharmaceutics 14, no. 4: 800. https://doi.org/10.3390/pharmaceutics14040800