Crystal Engineering of Ionic Cocrystals Sustained by Azolium···Azole Heterosynthons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder X-ray Diffraction (PXRD)
2.2. Single-Crystal X-ray Diffraction
2.3. ICC Design (Coformer Selection)
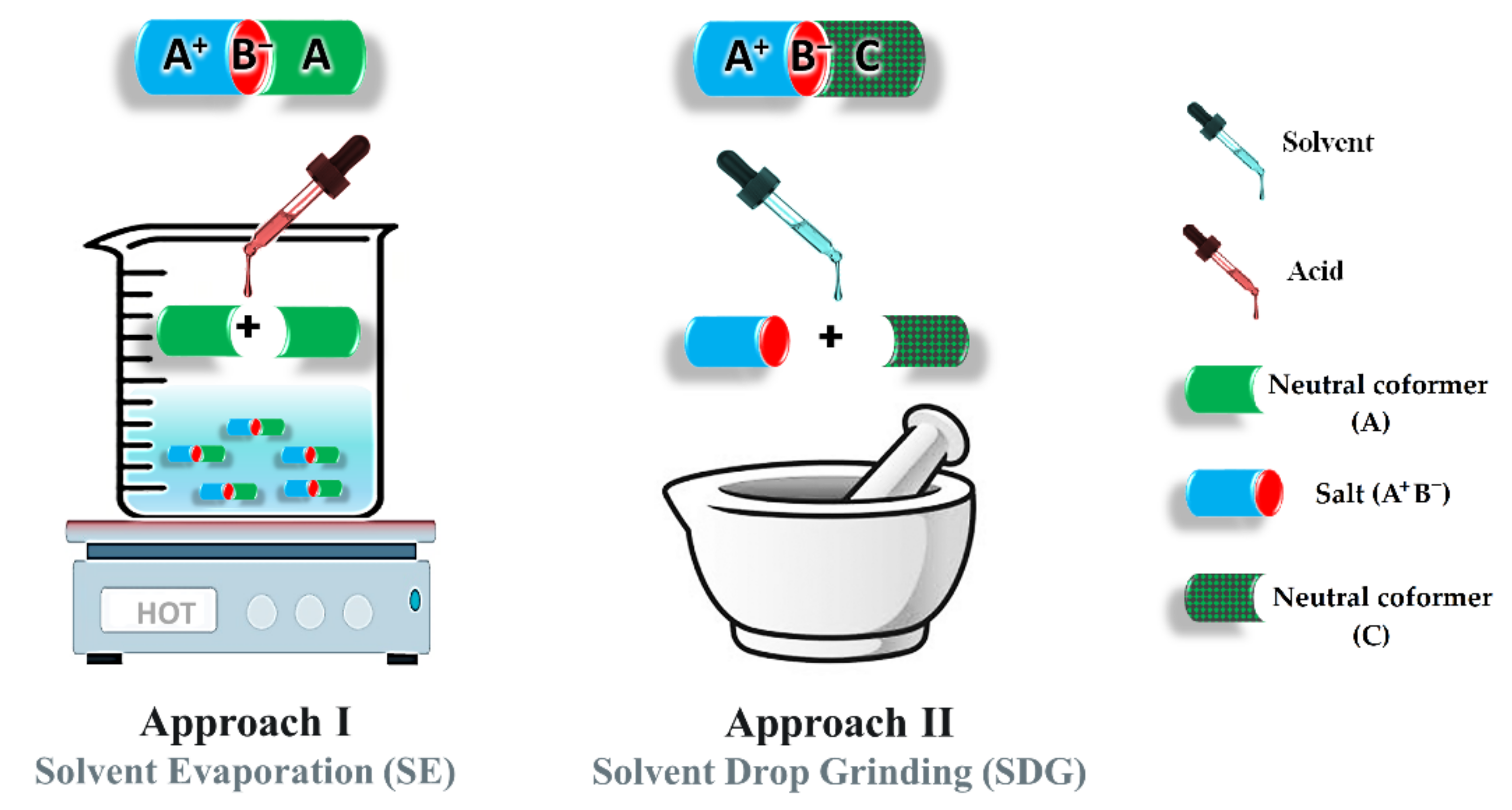
2.4. Synthesis of Ionic Cocrystals
2.4.1. Approach I (Solvent Evaporation)
2.4.2. Approach II (Solvent-Drop Grinding)
2.5. Computational Methods
2.6. Cambridge Structural Database (CSD) Analysis
3. Results and Discussion
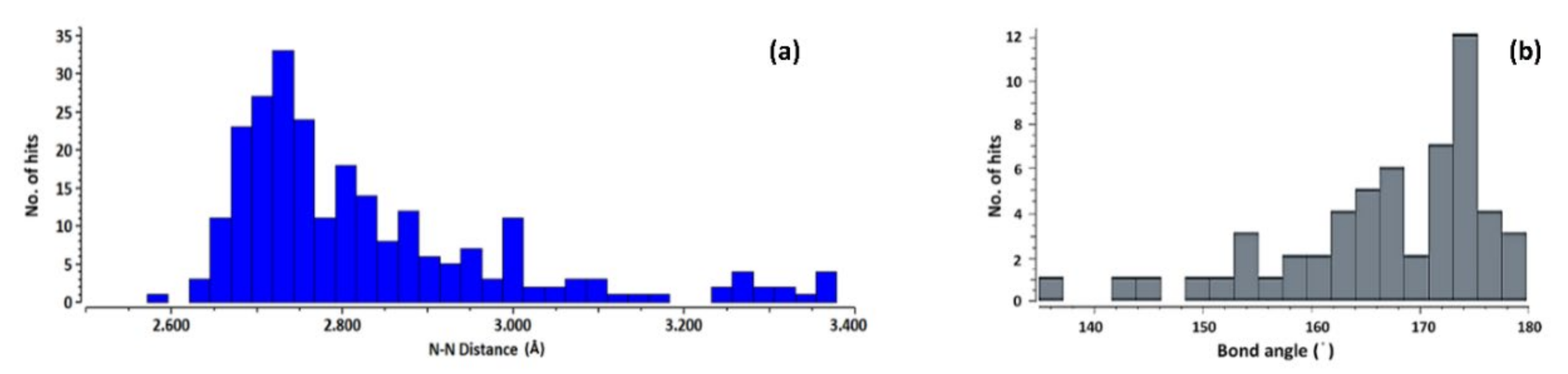
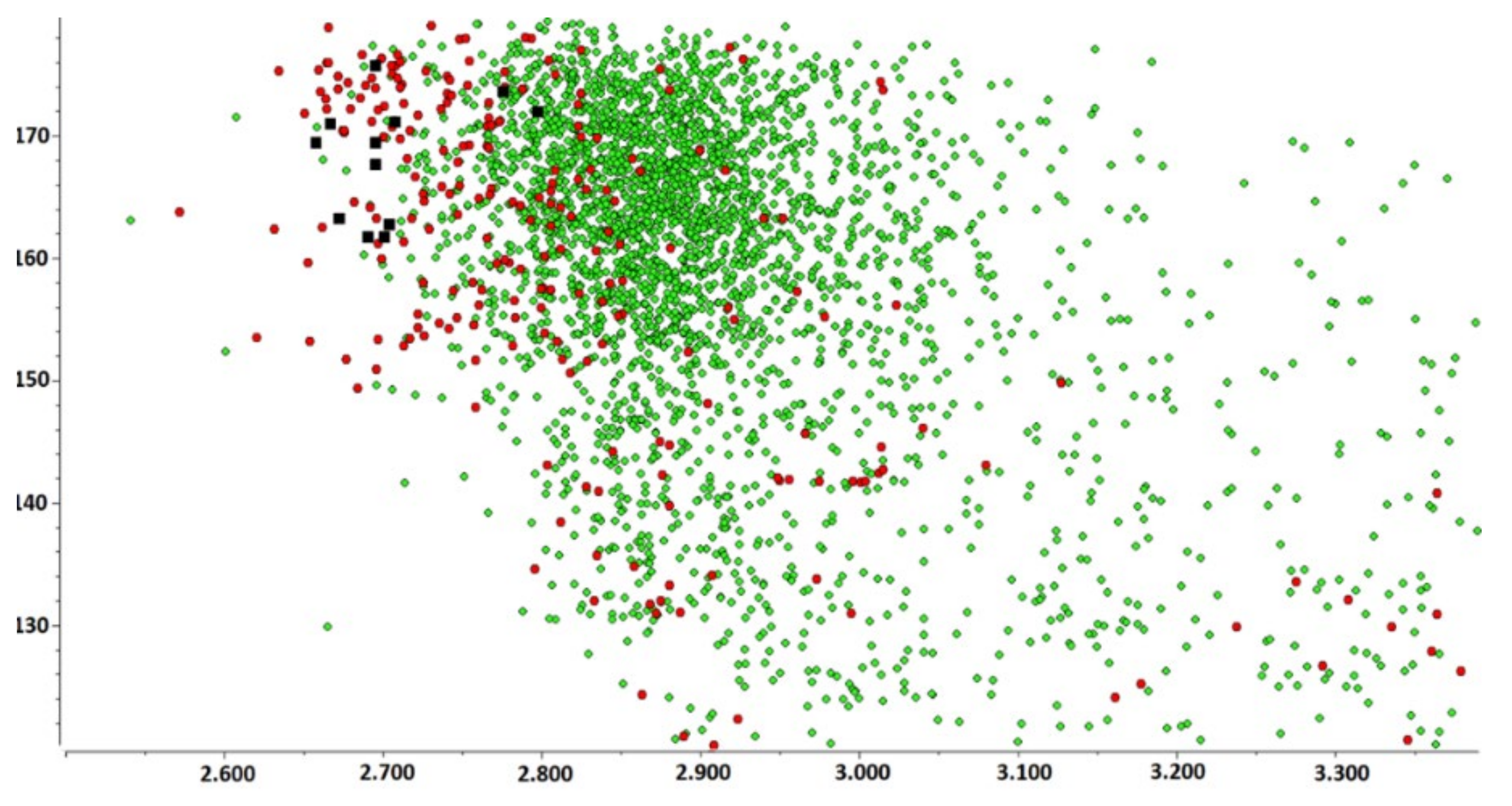
3.1. CSD Analysis of Azolium-Azole Supramolecular Heterosynthons
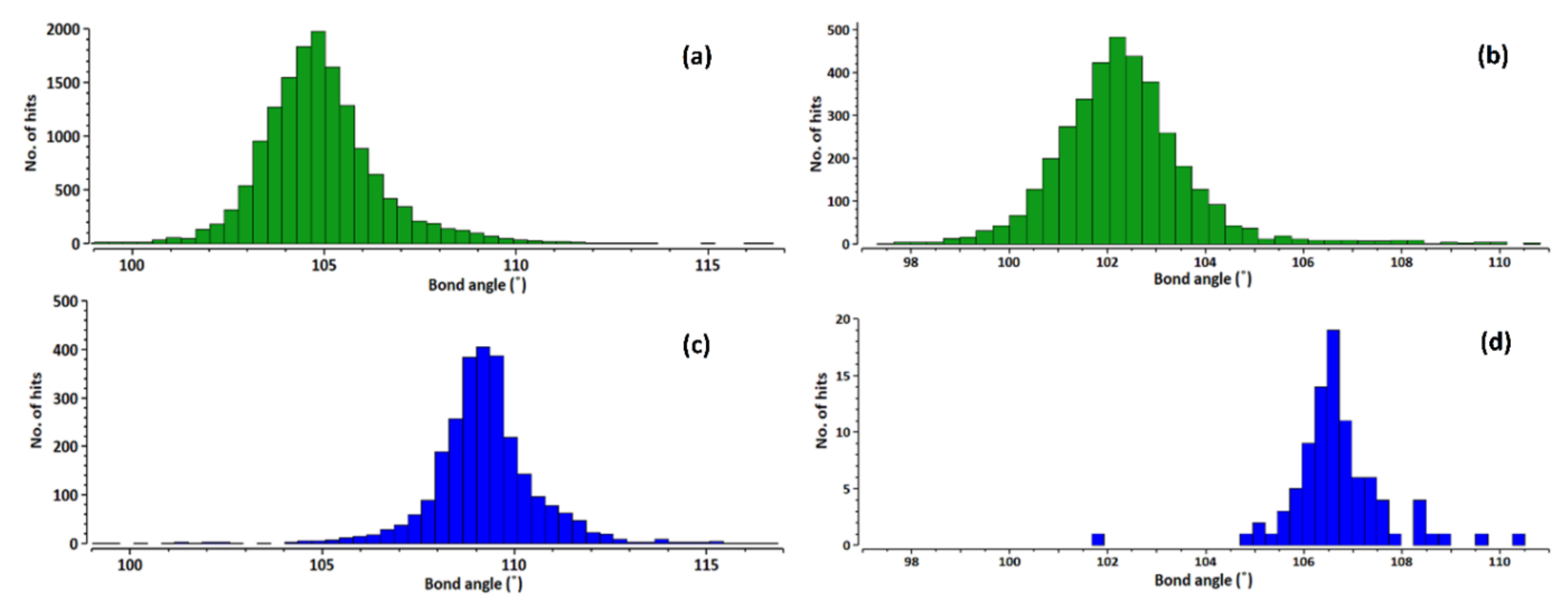
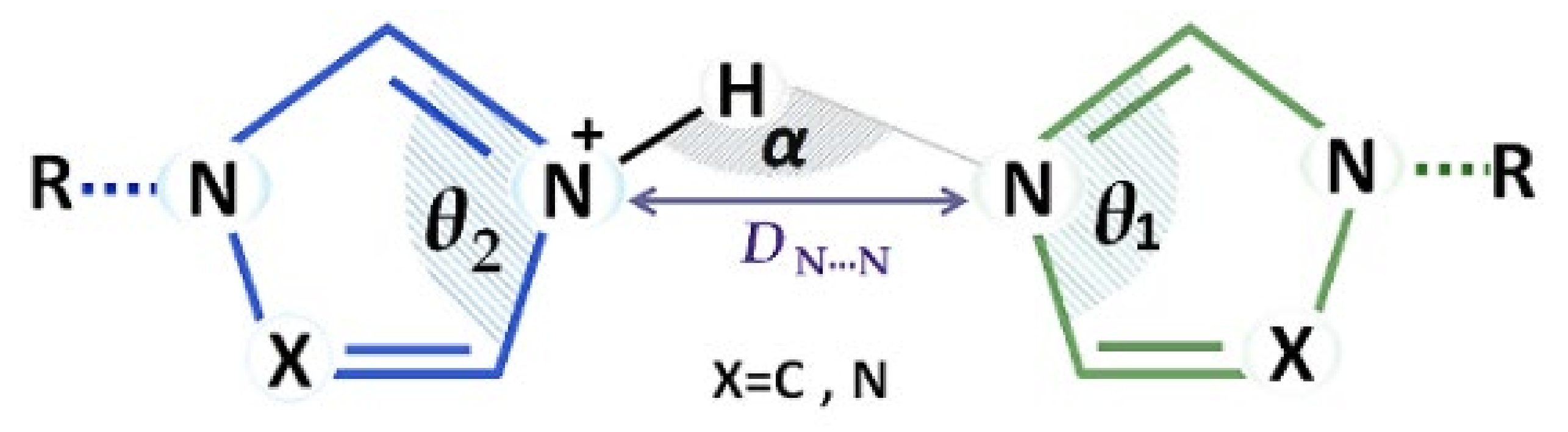
3.2. Structural Parameters That Distinguish between Azole and Azolium Rings
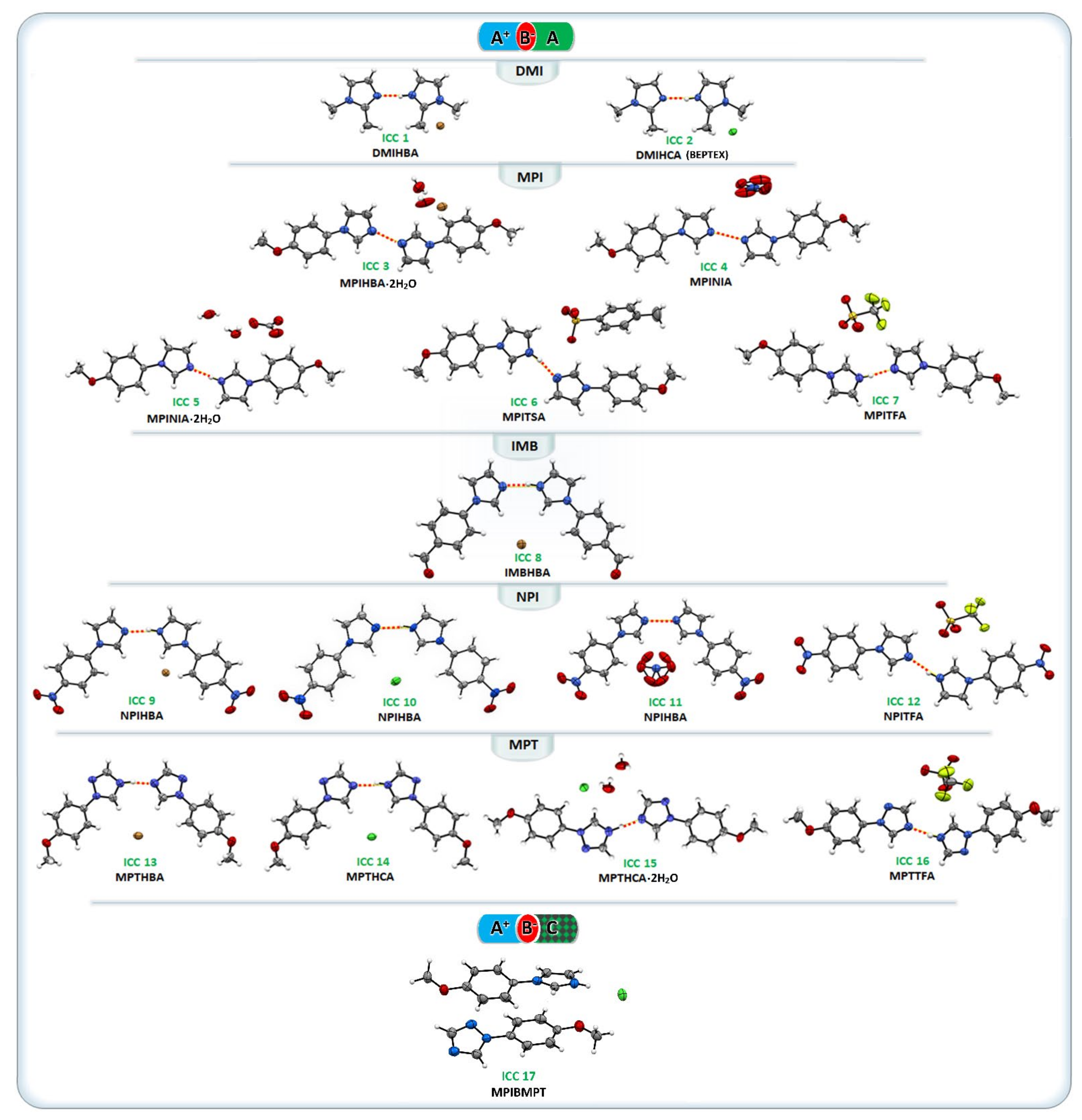
3.3. Crystal Structure Descriptions
3.3.1. ICCs Containing 1,2-Dimethylimidazole, DMI (1 and 2)
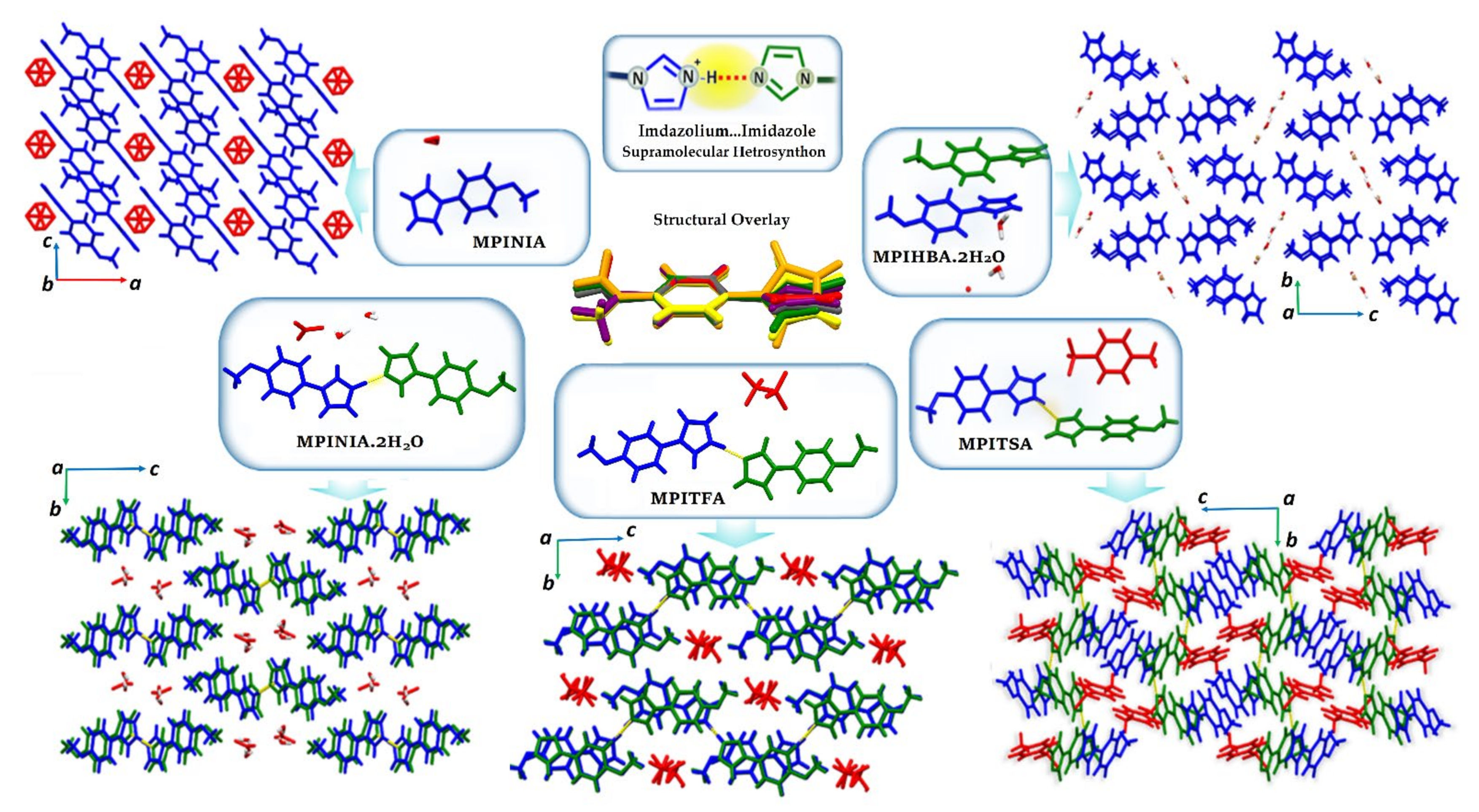
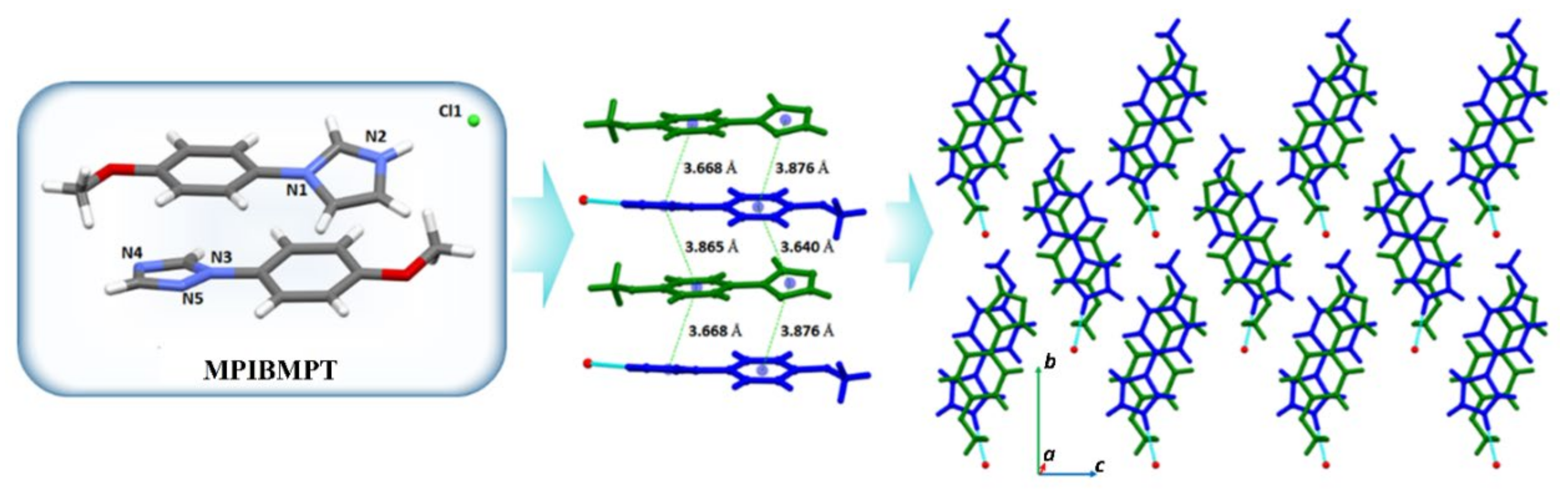
3.3.2. ICCs Containing 1-(4-Methoxyphenyl)-1H-Imidazole, MPI, 3–7
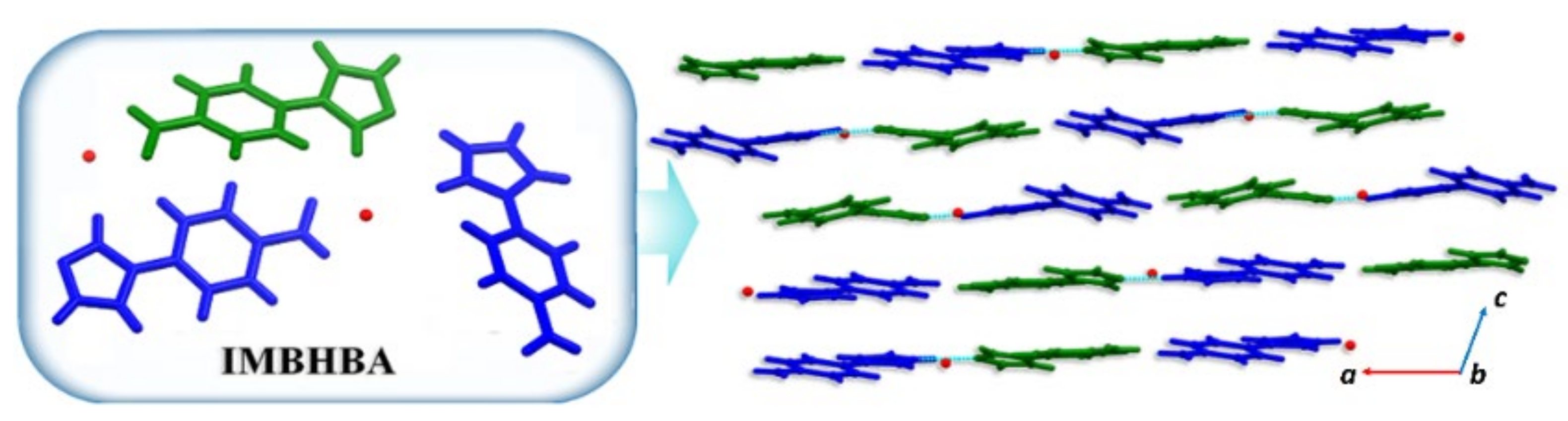
3.3.3. ICC Containing 4-(1H-Imidazol-1-yl) Benzaldehyde, IMB, 8
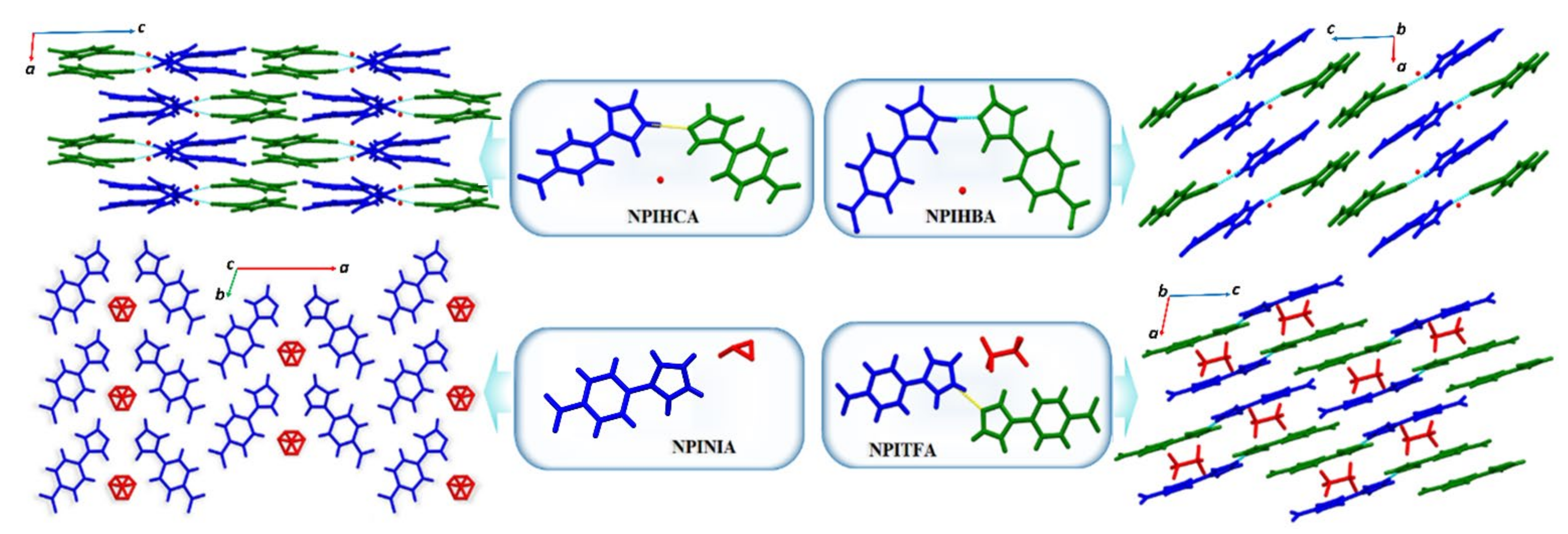
3.3.4. ICCs Containing 1-(4-Nitrophenyl)-1H-Imidazole, NPI, 9–12
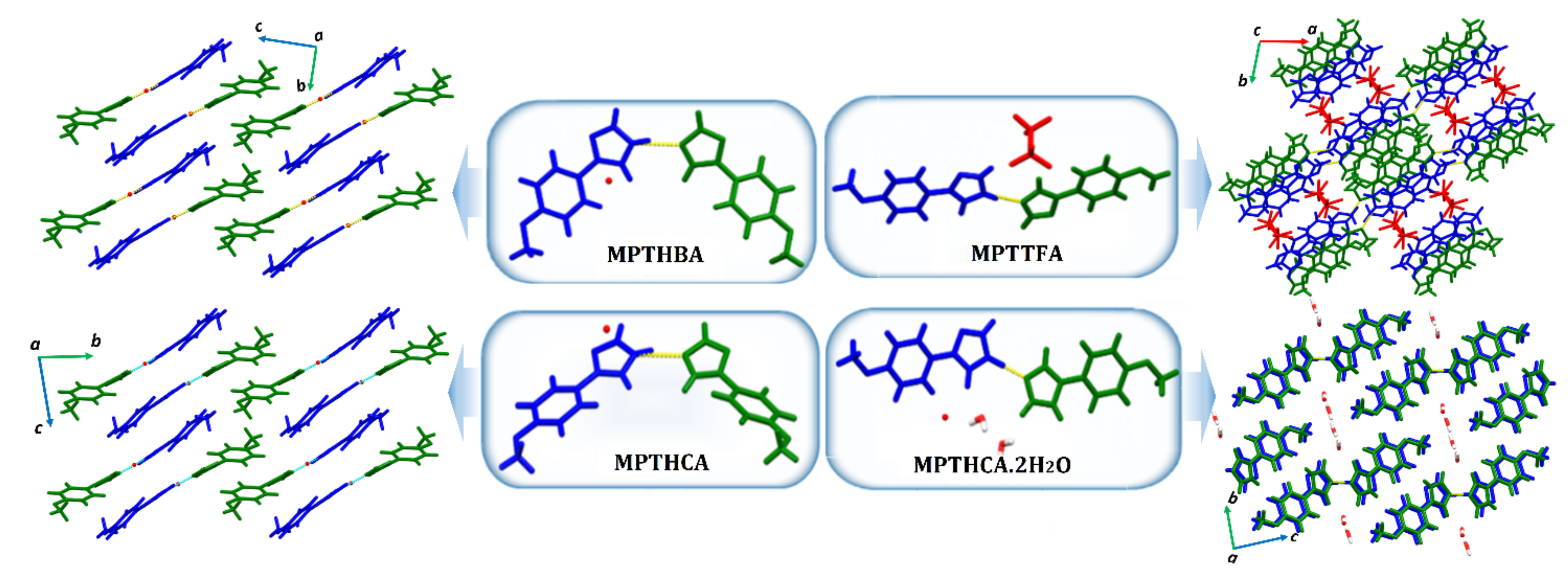
3.3.5. ICCs Containing 1-(4-Methoxyphenyl)-1H-1,2,4-Triazole, MPT, 13–16
3.3.6. A+B−C Type ICCs
3.4. Overall Analysis of Crystal Structures
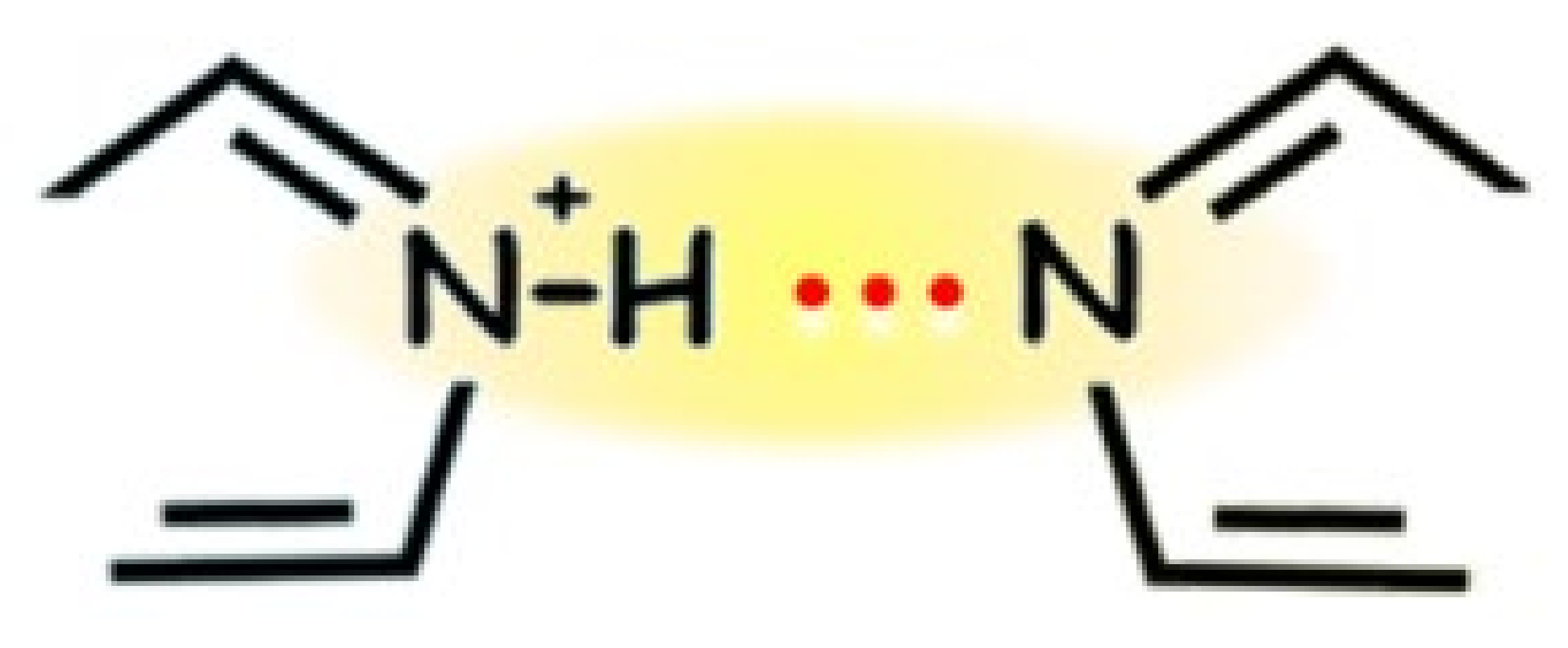
3.5. Hydrogen-Bond Strengths
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Peraka, K.S.; Zaworotko, M.J. The role of hydrogen bonding in co-crystals. In Co-Crystals: Preparation, Characterization and Applications; The Royal Society of Chemistry: Croydon, UK, 2018; pp. 33–79. [Google Scholar]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zaworotko, M.J. Crystal Engineering of Hybrid Coordination Networks: From Form to Function. Trends Chem. 2020, 2, 506–518. [Google Scholar] [CrossRef]
- Gao, M.-Y.; Song, B.-Q.; Sensharma, D.; Zaworotko, M.J. Crystal engineering of porous coordination networks for C3 hydrocarbon separation. SmartMat 2021, 2, 38–55. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Crystal Engineering of Nonlinear Optical Materials Based on Interpenetrated Diamondoid Coordination Networks. Chem. Mater. 2001, 13, 2705–2712. [Google Scholar] [CrossRef]
- Wong, M.S.; Bosshard, C.; Günter, P. Crystal engineering of molecular NLO materials. Adv. Mater. 1997, 9, 837–842. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of Quercetin with Improved Solubility and Oral Bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Bhattacharya, B.; Roy, P.; Michalchuk, A.A.L.; Emmerling, F.; Ghosh, A. Enhancing the Pharmaceutical Properties of Pirfenidone by Mechanochemical Cocrystallization. Cryst. Growth Des. 2019, 19, 6482–6492. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Smith, A.J.; Wojtas, Ł.; Shytle, R.D.; Zaworotko, M.J. Physical Stability Enhancement and Pharmacokinetics of a Lithium Ionic Cocrystal with Glucose. Cryst. Growth Des. 2014, 14, 6135–6142. [Google Scholar] [CrossRef]
- Shiraki, K.; Takata, N.; Takano, R.; Hayashi, Y.; Terada, K. Dissolution Improvement and the Mechanism of the Improvement from Cocrystallization of Poorly Water-soluble Compounds. Pharm. Res. 2008, 25, 2581–2592. [Google Scholar] [CrossRef]
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical cocrystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef]
- Nangia, A.K.; Desiraju, G.R. Heterosynthons, Solid Form Design and Enhanced Drug Bioavailability. Angew. Chem. 2022, 134, e202207484. [Google Scholar] [CrossRef]
- Remenar, J.F.; Morissette, S.L.; Peterson, M.L.; Moulton, B.; MacPhee, J.M.; Guzmán, H.R.; Almarsson, Ö. Crystal engineering of novel cocrystals of a triazole drug with 1, 4-dicarboxylic acids. J. Am. Chem. Soc. 2003, 125, 8456–8457. [Google Scholar] [CrossRef]
- Walsh, R.D.B.; Bradner, M.W.; Fleischman, S.; Morales, L.A.; Moulton, B.; Rodríguez-Hornedo, N.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Chem. Commun. 2003, 2, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Smolenskaya, V.N.; Stahly, B.C.; Stahly, G.P. Crystal Engineering Approach To Forming Cocrystals of Amine Hydrochlorides with Organic Acids. Molecular Complexes of Fluoxetine Hydrochloride with Benzoic, Succinic, and Fumaric Acids. J. Am. Chem. Soc. 2004, 126, 13335–13342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischman, S.G.; Kuduva, S.S.; McMahon, J.A.; Moulton, B.; Bailey Walsh, R.D.; Rodríguez-Hornedo, N.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases: Multiple-Component Crystalline Solids Involving Carbamazepine. Cryst. Growth Des. 2003, 3, 909–919. [Google Scholar] [CrossRef]
- Salaman, C.R.P.; Tesson, N. Co-Crystals of Tramadol and Coxibs. U.S. Patent 8598152B2, 21 April 2015. [Google Scholar]
- Christopherson, J.C.; Topić, F.; Barrett, C.J.; Friščić, T. Halogen-Bonded Cocrystals as Optical Materials: Next-Generation Control over Light–Matter Interactions. Cryst. Growth Des. 2018, 18, 1245–1259. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Prosperi, S.; Gobetto, R.; Chierotti, M.R. From unexpected reactions to a new family of ionic co-crystals: The case of barbituric acid with alkali bromides and caesium iodide. Chem. Commun. 2010, 46, 7715–7717. [Google Scholar] [CrossRef]
- Sanii, R.; Andaloussi, Y.H.; Patyk-Kaźmierczak, E.; Zaworotko, M.J. Polymorphism in Ionic Cocrystals Comprising Lithium Salts and l-Proline. Cryst. Growth Des. 2022, 22, 3786–3794. [Google Scholar] [CrossRef]
- de l’Isle, J.R. Cristallographie, ou Description des Forms Propres À Tous les Corps du Regne Minéral, 2nd ed.; Édition de L’imprimerie de Monsieur Paris: Paris, France, 1783; Volume 2, pp. 29–50. [Google Scholar]
- Kobell, F. Krystallographische Beobachtungen. J. Für. Prakt. Chem. 1843, 28, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Gill, C.H. XVI.—On some saline compounds of cane-sugar. J. Chem. Soc. 1871, 24, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.A.; James, V.J.; Mills, J.A. 2,5-O-Methylene-D-Mannitol Sodium-Chloride, C7H14O6·Nacl. Cryst. Struct. Commun. 1976, 5, 207–210. [Google Scholar]
- Ferguson, G.; Kaitner, B.; Connett, B.E.; Rendle, D.F. Structure of the [alpha]-d-glucose-sodium chloride-water complex (2/1/1). Acta Crystallogr. Sect. B 1991, 47, 479–484. [Google Scholar] [CrossRef]
- Palm, J.H.; MacGillavry, C.H. Habit modification in the system rocksalt-urea-water. Acta Crystallogr. 1963, 16, 963–968. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef] [Green Version]
- Grifasi, F.; Chierotti, M.R.; Gaglioti, K.; Gobetto, R.; Maini, L.; Braga, D.; Dichiarante, E.; Curzi, M. Using Salt Cocrystals to Improve the Solubility of Niclosamide. Cryst. Growth Des. 2015, 15, 1939–1948. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt−Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbas, R.; Font-Bardia, M.; Paradkar, A.; Hunter, C.A.; Prohens, R. Combined Virtual/Experimental Multicomponent Solid Forms Screening of Sildenafil: New Salts, Cocrystals, and Hybrid Salt–Cocrystals. Cryst. Growth Des. 2018, 18, 7618–7627. [Google Scholar] [CrossRef]
- Clare Speakman, J. Acid salts of carboxylic acids, crystals with some “very short” hydrogen bonds. In Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1972; pp. 141–199. [Google Scholar]
- Jin, S.; Sanii, R.; Song, B.-Q.; Zaworotko, M.J. Crystal Engineering of Ionic Cocrystals Sustained by the Phenol–Phenolate Supramolecular Heterosynthon. Cryst. Growth Des. 2022, 22, 4582–4591. [Google Scholar] [CrossRef]
- Haskins, M.M.; Lusi, M.; Zaworotko, M.J. Supramolecular Synthon Promiscuity in Phosphoric Acid–Dihydrogen Phosphate Ionic Cocrystals. Cryst. Growth Des. 2022, 22, 3333–3342. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Desai, A.S.; McMurray, J.J.V.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Chen, F.; Gong, J.; Rizkala, A.R.; Brahimi, A.; Claggett, B.; et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur. Heart J. 2015, 36, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Cebrecos, J.; Carlson, J.D.; Encina, G.; Lahjou, M.; Sans, A.; Sust, M.; Vaqué, A.; Morte, A.; Gascón, N.; Plata-Salamán, C. Celecoxib-tramadol co-crystal: A Randomized 4-Way Crossover Comparative Bioavailability Study. Clin. Ther. 2021, 43, 1051–1065. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Khan, M.; Enkelmann, V.; Brunklaus, G. Heterosynthon mediated tailored synthesis of pharmaceutical complexes: A solid-state NMR approach. CrystEngComm 2011, 13, 3213–3223. [Google Scholar] [CrossRef]
- Li, C.; Campillo-Alvarado, G.; Swenson, D.C.; MacGillivray, L.R. Photoreactive salt cocrystal: N+–H⋯N hydrogen bond and cation–π interactions support a cascade-like photodimerization of a 4-stilbazole. CrystEngComm 2021, 23, 1071–1074. [Google Scholar] [CrossRef]
- Fabry, J. A long symmetric N…H…N hydrogen bond in bis(4-aminopyridinium)(1+) azide(1-): Redetermination from the original data. Acta Crystallogr. Sect. E 2017, 73, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Prasher, P. An epigrammatic status of the ‘azole’-based antimalarial drugs. RSC Med. Chem. 2020, 11, 184–211. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Klose, W.; Boettcher, I. Antiinflammatory imidazole derivatives. U.S. Patent US4585771A, 29 April 1986. [Google Scholar]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [CrossRef] [Green Version]
- Maertens, J.A. History of the development of azole derivatives. Clin. Microbiol. Infect. 2004, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maddila, S.; B Jonnalagadda, S. New class of triazole derivatives and their antimicrobial activity. Lett. Drug Des. Discov. 2012, 9, 687–693. [Google Scholar] [CrossRef]
- El-Sebaey, S. Recent Advances in 1, 2, 4-Triazole Scaffolds as Antiviral Agents. ChemistrySelect 2020, 5, 11654–11680. [Google Scholar] [CrossRef]
- Banfi, E.; Scialino, G.; Zampieri, D.; Mamolo, M.G.; Vio, L.; Ferrone, M.; Fermeglia, M.; Paneni, M.S.; Pricl, S. Antifungal and antimycobacterial activity of new imidazole and triazole derivatives. A combined experimental and computational approach. J. Antimicrob. Chemother. 2006, 58, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Mojaddami, A.; Sakhteman, A.; Fereidoonnezhad, M.; Faghih, Z.; Najdian, A.; Khabnadideh, S.; Sadeghpour, H.; Rezaei, Z. Binding mode of triazole derivatives as aromatase inhibitors based on docking, protein ligand interaction fingerprinting, and molecular dynamics simulation studies. Res. Pharm. Sci. 2017, 12, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Gould, P.L. Salt selection for basic drugs. Int. J. Pharm. 1986, 33, 201–217. [Google Scholar] [CrossRef]
- Bharate, S.S. Recent developments in pharmaceutical salts: FDA approvals from 2015 to 2019. Drug Discov. Today 2021, 26, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.A.; Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Perlovich, G.L. Novel cocrystals of itraconazole: Insights from phase diagrams, formation thermodynamics and solubility. Int. J. Pharm. 2021, 599, 120441. [Google Scholar] [CrossRef]
- Martin, F.; Pop, M.; Kacso, I.; Grosu, I.G.; Miclăuş, M.; Vodnar, D.; Lung, I.; Filip, G.A.; Olteanu, E.D.; Moldovan, R.; et al. Ketoconazole-p-aminobenzoic Acid Cocrystal: Revival of an Old Drug by Crystal Engineering. Mol. Pharm. 2020, 17, 919–932. [Google Scholar] [CrossRef]
- Chen, J.-M.; Wang, Z.-Z.; Wu, C.-B.; Li, S.; Lu, T.-B. Crystal engineering approach to improve the solubility of mebendazole. CrystEngComm 2012, 14, 6221–6229. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Novel pharmaceutical salts of albendazole. CrystEngComm 2018, 20, 6394–6405. [Google Scholar] [CrossRef] [Green Version]
- Sanphui, P.; Mishra, M.K.; Ramamurty, U.; Desiraju, G.R. Tuning Mechanical Properties of Pharmaceutical Crystals with Multicomponent Crystals: Voriconazole as a Case Study. Mol. Pharm. 2015, 12, 889–897. [Google Scholar] [CrossRef]
- Vlahakis, J.Z.; Lazar, C.; Crandall, I.E.; Szarek, W.A. Anti-Plasmodium activity of imidazolium and triazolium salts. Bioorg. Med. Chem. 2010, 18, 6184–6196. [Google Scholar] [CrossRef] [PubMed]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium salts as antifungal agents: Activity against emerging yeast pathogens, without human leukocyte toxicity. MedChemComm 2013, 4, 1457–1460. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.K.; Singh, N.J.; Kim, K.S. Imidazolium receptors for the recognition of anions. Chem. Soc. Rev. 2006, 35, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Kim, S.K.; Yoon, J. Revisit to imidazolium receptors for the recognition of anions: Highlighted research during 2006–2009. Chem. Soc. Rev. 2010, 39, 1457–1466. [Google Scholar] [CrossRef]
- Byrne, S.; Mullen, K.M. Sensing anions on surfaces: Tethering triazolium based anion receptors to polymer resins. RSC Adv. 2016, 6, 33880–33887. [Google Scholar] [CrossRef]
- Vaishya, V.; Patider, S.; Pilania, M. Imidazolium/triazolium based NHC–Palladium complexes and their application in catalysis. Mater. Today Proc. 2021, 43, 3181–3187. [Google Scholar] [CrossRef]
- Comès, A.; Poncelet, R.; Pescarmona, P.P.; Aprile, C. Imidazolium-based titanosilicate nanospheres as active catalysts in carbon dioxide conversion: Towards a cascade reaction from alkenes to cyclic carbonates. J. CO2 Util. 2021, 48, 101529. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Rogers, R.D.; Dixon, D.A. Accurate Thermochemical Properties for Energetic Materials Applications. II. Heats of Formation of Imidazolium-,1,2,4-Triazolium-, and Tetrazolium-Based Energetic Salts from Isodesmic and Lattice Energy Calculations. J. Phys. Chem. B 2007, 111, 4788–4800. [Google Scholar] [CrossRef]
- Singh, R.; Singh, H.J.; Sengupta, S.K. Computational studies on 1,2,4-Triazolium-based salts as energetic materials. J. Chem. Sci. 2015, 127, 1099–1107. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, W.; Zhang, T.; Li, Z.; Zeng, Q.; Zhang, T. A simple and efficient strategy for constructing nitrogen-rich isomeric salts and cocrystal through pKa calculation. J. Mol. Struct. 2021, 1223, 128955. [Google Scholar] [CrossRef]
- Fu, X.; Liu, X.; Sun, P.; Zhang, S.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.; Fan, X. A new family of insensitive energetic copolymers composed of nitro and nitrogen-rich energy components: Structure, physicochemical property and density functional theory. J. Anal. Appl. Pyrolysis 2015, 114, 79–90. [Google Scholar] [CrossRef]
- Bruker, A. SAINT Software Reference Manual; Scientific Research Publishing: Madison, WI, USA, 1998; p. 5465. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the p K a rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Turner, M.; McKinnon, J.; Wolff, S.; Grimwood, D.; Spackman, P.; Jayatilaka, D.; Spackman, M. CrystalExplorer17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Mandel, G.S.; Marsh, R.E. 9-Ethylguanine hemihydrochloride: A short asymmetric N-H…N hydrogen bond. Acta Crystallogr. Sect. B 1975, 31, 2862–2867. [Google Scholar] [CrossRef]
- Kelley, S.P.; Narita, A.; Holbrey, J.D.; Green, K.D.; Reichert, W.M.; Rogers, R.D. Understanding the Effects of Ionicity in Salts, Solvates, Co-Crystals, Ionic Co-Crystals, and Ionic Liquids, Rather than Nomenclature, Is Critical to Understanding Their Behavior. Cryst. Growth Des. 2013, 13, 965–975. [Google Scholar] [CrossRef]
- Bis, J.A.; Zaworotko, M.J. The 2-Aminopyridinium-carboxylate Supramolecular Heterosynthon: A Robust Motif for Generation of Multiple-Component Crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- Easton, M.E.; Li, K.; Titi, H.M.; Kelley, S.P.; Rogers, R.D. Controlling the Interface between Salts, Solvates, Co-crystals, and Ionic Liquids with Non-stoichiometric Protic Azolium Azolates. Cryst. Growth Des. 2020, 20, 2608–2616. [Google Scholar] [CrossRef]

| ICC ID | DMIHBA | BEPTEX | MPIHBA·2H2O | MPINIA | MPINIA·2H2O | MPITSA |
| formula | C10H17BrN4 | C10H17ClN4 | C20H24BrN4O4 | C20H21.10N5O5.04 | C21H25N4O7 | C27H28N4O5S |
| crystal system | orthorhombic | orthorhombic | monoclinic | monoclinic | monoclinic | monoclinic |
| space group | Pca21 | Pca21 | P21/c | C2/c | P21/n | P21/n |
| a (Å) | 13.7773(5) | 13.6634(7) | 8.292(1) | 19.3498(8) | 9.6840(3) | 13.9274(18) |
| b (Å) | 9.9093(4) | 10.0618(5) | 12.0213(16) | 8.1285(3) | 9.1315(2) | 9.6125(8) |
| c (Å) | 9.2692(4) | 8.8159(4) | 21.786(3) | 12.4460(5) | 23.9779(7) | 19.599(3) |
| α (deg) | 90 | 90 | 90 | 90 | 90 | 90 |
| β (deg) | 90 | 90 | 91.467(4) | 91.468(1) | 94.445(1) | 103.497(3) |
| γ (deg) | 90 | 90 | 90 | 90 | 90 | 90 |
| vol (Å3) | 1265.46(9) | 1214.81(10) | 2170.94(5) | 1956.92(13) | 2113.97(10) | 2551.39(8) |
| Z | 4 | 4 | 4 | 4 | 4 | 4 |
| T (K) | 150(2) | 150(2) | 150(2) | 150(2) | 150(2) | 173(2) |
| R1 | 0.0201 | 0.0532 | 0.0445 | 0.0392 | 0.0487 | 0.0681 |
| wR2 | 0.0516 | 0.1050 | 0.1106 | 0.0960 | 0.1464 | 0.1330 |
| GOF | 1.059 | 1.235 | 1.009 | 1.023 | 1.023 | 1.062 |
| CCDC# | 2190303 | BEPTEX | 2190306 | 2190307 | 2190308 | 2190310 |
| ICC ID | MPITFA | IMBHBA | NPIHBA | NPIHCA | NPINIA | NPITFA |
| formula | C21H21F3N4O5S | C40H34Br2N8O4 | C18H15BrN6O4 | C18H15ClN6O4 | C18H14N7O7 | C19H15F3N6O7S |
| crystal system | monoclinic | monoclinic | triclinic | orthorhombic | monoclinic | triclinic |
| space group | P21/c | C2/c | P | Pbca | C2 | P |
| a (Å) | 13.8953(4) | 32.9927(6) | 8.0379(5) | 7.0102(3) | 32.372(2) | 8.3095(3) |
| b (Å) | 10.2973(3) | 8.1315(2) | 8.1504(5) | 14.9429(8) | 7.8860(5) | 9.9778(3) |
| c (Å) | 15.4543(4) | 21.2172(5) | 15.4708(9) | 35.3795(17) | 3.7236(2) | 13.1516(4) |
| α (deg) | 90 | 90 | 87.586(2) | 90 | 90 | 93.2280(10) |
| β (deg) | 98.310(2) | 110.838(2) | 88.707(2) | 90 | 95.797(2) | 100.0570(10) |
| γ (deg) | 90 | 90 | 65.284(2) | 90 | 90 | 94.8960(10) |
| vol (Å3) | 2188.05(11) | 5319.83(7) | 919.85(10) | 3706.12(3) | 945.71(10) | 1066.91(6) |
| Z | 4 | 8 | 2 | 8 | 4 | 2 |
| T (K) | 150(2) | 150(2) | 150(2) | 186(2) | 293(2) | 100(2) |
| R1 | 0.0564 | 0.0431 | 0.0258 | 0.0828 | 0.0476 | 0.0360 |
| wR2 | 0.1485 | 0.1125 | 0.0603 | 0.1252 | 0.1132 | 0.1021 |
| GOF | 1.051 | 1.046 | 1.090 | 1.022 | 1.168 | 1.034 |
| CCDC# | 2190309 | 2190304 | 2190315 | 2190316 | 2190317 | 2190318 |
| ICC ID | MPTHBA | MPTHCA | MPTHCA·2H2O | MPTTFA | MPIBMPT | |
| formula | C18H19BrN6O2 | C18H19ClN6O2 | C18H24ClN6O4 | C19H19F3N6O5S | C38H38Cl2N10O4 | |
| crystal system | triclinic | triclinic | triclinic | monoclinic | monoclinic | |
| space group | P | P | P | C2/c | Cc | |
| a (Å) | 7.9413(13) | 7.7313(4) | 8.3338(2) | 15.521(3) | 7.0520(8) | |
| b (Å) | 8.6863(13) | 8.5007(3) | 11.5631(3) | 21.124(3) | 19.680(2) | |
| c (Å) | 14.233(2) | 14.3868(7) | 11.6282(3) | 15.615(3) | 13.6463(16) | |
| α (deg) | 83.211(5) | 82.761(2) | 73.6850(10) | 90 | 90 | |
| β (deg) | 83.831(6) | 83.458(2) | 69.9830(10) | 117.825(7) | 104.322(3) | |
| γ (deg) | 81.315(5) | 81.233(2) | 82.9500(10) | 90 | 90 | |
| vol (Å3) | 959.72(3) | 922.69(7) | 1010.02(4) | 4527.81(4) | 1835.13(4) | |
| Z | 2 | 2 | 2 | 8 | 2 | |
| T (K) | 150(2) | 150(2) | 150(2) | 303(2) | 150(2) | |
| R1 | 0.0411 | 0.0663 | 0.0505 | 0.0981 | 0.0664 | |
| wR2 | 0.0936 | 0.1010 | 0.1352 | 0.3173 | 0.1577 | |
| GOF | 0.995 | 0.959 | 1.076 | 1.206 | 1.033 | |
| CCDC# | 2190311 | 2190312 | 2190313 | 2190314 | 2190305 |
| No | Code | d(N-H)/Å | d(H···N)/Å | d(N···N)/Å | α/° | θ1/° | θ2/° |
|---|---|---|---|---|---|---|---|
| 1 | DMIHBA | 0.936(33) | 1.787(34) | 2.712(3) | 169.4(30) | 106.02(23) | 108.54(24) |
| 2 | BEPTEX | 0.880(3) | 1.829(3) | 2.699(4) | 169.4(2) | 106.17(36) | 108.83(37) |
| 3 | MPIHBA·2H2O | 0.791(66) | 1.872(66) | 2.662(4) | 178.2(68) | 107.50(24) | 107.50(24) |
| 2.644(3) | 105.88(27) | 105.88(27) | |||||
| 4 | MPINIA | 0.907(38) | 1.745(38) | 2.652(1) | 178.7(36) | 107.29(12) | 107.68(12) |
| 5 | MPINIA·2H2O | 1.030(19) | 1.648(19) | 2.678(1) | 177.3(18) | 105.79(10) | 108.67(11) |
| 6 | MPITSA | 0.924(39) | 1.877(39) | 2.791(4) | 169.4(36) | 104.90(31) | 109.00(26) |
| 7 | MPITFA | 0.997(45) | 1.762(45) | 2.748(3) | 169.6(38) | 105.44(22) | 108.70(23) |
| 8 | IMBHBA | 0.880(3) | 1.823(3) | 2.678(4) | 163.2(2) | 107.29(30) | 108.25(30) |
| 2.658(4) | 107.05(31) | 107.04(31) | |||||
| 9 | NPIHBA | 0.880(1) | 1.839(1) | 2.686(2) | 161.0(1) | 106.08(17) | 109.23(17) |
| 10 | NPIHCA | 1.059(26) | 1.634(24) | 2.691(2) | 175.3(25) | 106.47(17) | 108.70(16) |
| 11 | NPINIA | 0.887(48) | 1.798(48) | 2.678(3) | 171.1(47) | 107.83(23) | 107.83(23) |
| 12 | NPITFA | 0.922(22) | 1.911(21) | 2.808(1) | 163.6(18) | 105.30(12) | 109.11(12) |
| 13 | MPTHBA | 1.038(43) | 1.665(45) | 2.690(3) | 168.3(37) | 103.41(24) | 105.41(24) |
| 14 | MPTHCA | 0.880(1) | 1.814(1) | 2.663(2) | 161.2(1) | 103.44(16) | 106.36(16) |
| 15 | MPTHCA·2H2O | 1.046(39) | 1.677(39) | 2.723(3) | 178.7(29) | 103.42(20) | 106.04(20) |
| 16 | MPTTFA | 0.860(4) | 1.844(4) | 2.694(6) | 169.1(3) | 101.71(54) | 105.70(52) |
| Code | Eele | Epol | Edis | Erep | Etot |
|---|---|---|---|---|---|
| DMIHBA | −74.9 | −13.8 | −13.9 | 71.6 | −31.0 |
| BEPTEX | −77.9 | −14.5 | −13.9 | 74.2 | −32.1 |
| MPINIA·2H2O | −88.8 | −15.9 | −9.2 | 74.8 | −39.1 |
| MPITST | −66.3 | −12.5 | −8.6 | 47.9 | −39.5 |
| MPITFA | −74.5 | −13.7 | −8.8 | 58.3 | −41.4 |
| IMBHBA | −85.1 | −16.4 | −10.4 | 67.1 | −44.8 |
| NPIHBA | −83.8 | −16.4 | −9.8 | 65.0 | −45.0 |
| NPIHCA | −88.1 | −17.4 | −9.5 | 69.4 | −45.6 |
| NPITFA | −64.6 | −13.5 | −8.5 | 42.1 | −44.5 |
| MPTHBA | −84.3 | −14.9 | −9.4 | 67.0 | −41.6 |
| MPTHCA | −85.4 | −15.0 | −9.6 | 70.6 | −39.4 |
| MPTHCA·2H2O | −79.6 | −14.3 | −8.4 | 62.2 | −40.1 |
| MPTTFA | −85.5 | −15.6 | −8.7 | 68.3 | −41.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, M.; Kumar, V.; Bruno-Colmenarez, J.; Zaworotko, M.J. Crystal Engineering of Ionic Cocrystals Sustained by Azolium···Azole Heterosynthons. Pharmaceutics 2022, 14, 2321. https://doi.org/10.3390/pharmaceutics14112321
Rahmani M, Kumar V, Bruno-Colmenarez J, Zaworotko MJ. Crystal Engineering of Ionic Cocrystals Sustained by Azolium···Azole Heterosynthons. Pharmaceutics. 2022; 14(11):2321. https://doi.org/10.3390/pharmaceutics14112321
Chicago/Turabian StyleRahmani, Maryam, Vijith Kumar, Julia Bruno-Colmenarez, and Michael J. Zaworotko. 2022. "Crystal Engineering of Ionic Cocrystals Sustained by Azolium···Azole Heterosynthons" Pharmaceutics 14, no. 11: 2321. https://doi.org/10.3390/pharmaceutics14112321






