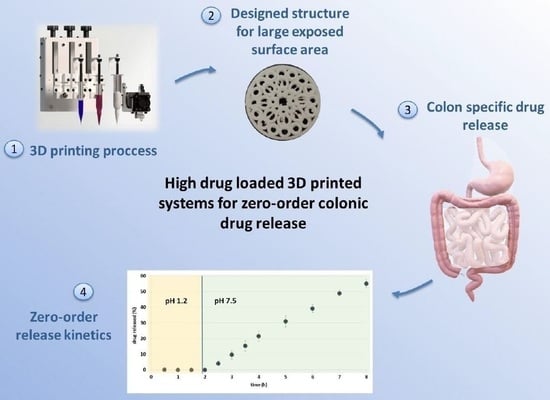
3D Printed Fractal-like Structures with High Percentage of Drug for Zero-Order Colonic Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Physical Mixture
2.2.2. Extrusion Process
2.2.3. Mechanical Property Testing of Filaments
2.2.4. Fractal Analysis
2.2.5. 3D Printing Process
2.2.6. Physical Tests of Printed Systems
2.2.7. Thermal Analysis of Filaments
Differential Scanning Calorimetry
Thermogravimetric Analysis
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. X-ray Tomography
2.2.10. Dissolution Testing of Filaments
2.2.11. Dissolution Testing of 3D Printed Tablets
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awad, A.; Madla, C.M.; McCoubrey, L.E.; Ferraro, F.; Gavins, F.K.H.; Buanz, A.; Gaisford, S.; Orlu, M.; Siepmann, F.; Siepmann, J.; et al. Clinical Translation of Advanced Colonic Drug Delivery Technologies. Adv. Drug Deliv. Rev. 2022, 181, 114076. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent Advances in Colon Drug Delivery Systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Kurakula, M.; Gorityala, S.; Moharir, K. Recent Trends in Design and Evaluation of Chitosan-Based Colon Targeted Drug Delivery Systems: Update 2020. J. Drug Deliv. Sci. Technol. 2021, 64, 102579. [Google Scholar] [CrossRef]
- García, M.A.; Varum, F.; Al-Gousous, J.; Hofmann, M. In Vitro Methodologies for Evaluating Colon-Targeted Pharmaceutical Products and Industry Perspectives for Their Applications. Pharmaceutics 2022, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M. Colon: A Gateway for Chronotherapeutic Drug Delivery Systems Mayur M Patel (Associate Professor). Expert Opin. Drug Deliv. 2015, 12, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varum, F.; Thorne, H.; Bravo, R.; Gilgen, D.; Hartig, C.; Nicolas, G.P.; Wild, D.; Liakoni, E.; Haschke, M. Targeted Colonic Release Formulations of Mesalazine—A Clinical Pharmaco-Scintigraphic Proof-of-Concept Study in Healthy Subjects and Patients with Mildly Active Ulcerative Colitis. Int. J. Pharm. 2022, 625, 122055. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Ong, J.J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected Healthcare: Improving Patient Care Using Digital Health Technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low Temperature Fused Deposition Modeling (FDM) 3D Printing of Thermolabile Drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-De-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong Tan, D.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, J.; Deon, M.; da Silva, G.S.; Beck, R.C.R. Multiple Variable Effects in the Customisation of Fused Deposition Modelling 3D-Printed Medicines: A Design of Experiments (DoE) Approach. Int. J. Pharm. 2021, 597, 120331. [Google Scholar] [CrossRef]
- Mora-Castaño, G.; Millán-Jiménez, M.; Linares, V.; Caraballo, I. Assessment of the Extrusion Process and Printability of Suspension-Type Drug-Loaded Affinisol TM Filaments for 3D Printing. Pharmaceutics 2022, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Charbe, N.B.; Mccarron, P.A.; Lane, M.E.; Tambuwala, M.M. Application of Three-Dimensional Printing for Colon Targeted Drug Delivery Systems. Int. J. Pharm. Investig. 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Uboldi, M.; Briatico-Vangosa, F.; Moutaharrik, S.; Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Gazzaniga, A. The ChronotopicTM System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM). Pharmaceutics 2021, 13, 759. [Google Scholar] [CrossRef]
- Mirdamadian, S.Z.; Varshosaz, J.; Minaiyan, M.; Taheri, A. 3D Printed Tablets Containing Oxaliplatin Loaded Alginate Nanoparticles for Colon Cancer Targeted Delivery. An in Vitro/in Vivo Study. Int. J. Biol. Macromol. 2022, 205, 90–109. [Google Scholar] [CrossRef]
- Linares, V.; Casas, M.; Caraballo, I. Printfills: 3D Printed Systems Combining Fused Deposition Modeling and Injection Volume Filling. Application to Colon-Specific Drug Delivery. Eur. J. Pharm. Biopharm. 2019, 134, 138–143. [Google Scholar] [CrossRef]
- Patel, S.K.; Khoder, M.; Peak, M.; Alhnan, M.A. Controlling Drug Release with Additive Manufacturing-Based Solutions. Adv Drug Deliv. Rev. 2021, 174, 369–386. [Google Scholar] [CrossRef]
- Almeida, A.; Linares, V.; Mora-Castaño, G.; Casas, M.; Caraballo, I.; Sarmento, B. 3D Printed Systems for Colon-Specific Delivery of Camptothecin-Loaded Chitosan Micelles. Eur. J. Pharm. Biopharm. 2021, 167, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the New Asset for the Production of Tailored Medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of Modified Release 3D Printed Tablets (Printlets) with Pharmaceutical Excipients Using Additive Manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Galdón, E.; Casas, M.; Caraballo, I. Critical Points for Predicting 3D Printable Filaments Behaviour. J. Drug Deliv. Sci. Technol. 2021, 66, 102933. [Google Scholar] [CrossRef]
- Galdón, E.; Casas, M.; Caraballo, I. Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression. Pharmaceutics 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Li, J.; Meda, A.; Osei-Yeboah, F.; Peterson, M.L.; Repka, M.; Zhan, X. Development of a Quantitative Method to Evaluate the Printability of Filaments for Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2020, 588, 119760. [Google Scholar] [CrossRef]
- Fuertes, I.; Caraballo, I.; Miranda, A.; Millán, M. Study of Critical Points of Drugs with Different Solubilities in Hydrophilic Matrices. Int. J. Pharm. 2010, 383, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, R.C.A.; Stuurman, F.E.; van der Weert, F.H.J.; Kosterink, J.G.W.; Frijlink, H.W. A Novel Dissolution Method Relevant to Intestinal Release Behaviour and Its Application in the Evaluation of Modified Release Mesalazine Products. Eur. J. Pharm. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Dillon, C.G.; Carey, P.F.; Worden, R.H. Fractscript: A Macro for Calculating the Fractal Dimension of Object Perimeters in Images of Multiple Objects. Comput. Geosci. 2001, 27, 787–794. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Xu, X.; Wen, N.; Song, R.; Meng, Q.; Guan, Y.; Cheng, S.; Cao, D.; Dong, Y.; Qie, J.; et al. A Drug Carrier for Sustained Zero-Order Release of Peptide Therapeutics. Sci. Rep. 2017, 7, 5524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerea, M.; Maroni, A.; Palugan, L.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Oral Hydrophilic Matrices Having Non Uniform Drug Distribution for Zero-Order Release: A Literature Review. J. Control. Release 2020, 325, 72–83. [Google Scholar] [CrossRef]
- Janusziewicz, R.; Olson, K.R.; Benhabbour, S.R. Design and Characterization of a Novel Series of Geometrically Complex Intravaginal Rings with Digital Light Synthesis. Adv. Mater. Technol. 2020, 5, 2000261. [Google Scholar] [CrossRef]
- Laracuente, M.L.; Yu, M.H.; McHugh, K.J. Zero-Order Drug Delivery: State of the Art and Future Prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]









| Batch | Theophylline Anhydrous (AT) (%) | Thermoplastic Polyurethane (TPU) (%) | Magnesium Stearate (%) |
|---|---|---|---|
| B20 | 20 | 80 | 0 |
| B30 | 30 | 70 | 0 |
| B40 | 40 | 60 | 0 |
| B50 | 50 | 50 | 0 |
| B60 | 60 | 40 | 0 |
| T20 | 20 | 75 | 5 |
| T30 | 30 | 65 | 5 |
| T40 | 40 | 55 | 5 |
| T50 | 50 | 45 | 5 |
| T60 | 60 | 35 | 5 |
| Experimental (n = 6) | Theoretical | ||||
|---|---|---|---|---|---|
| Height (mm) | Diameter (mm) | Weight (mg) | Height (mm) | Diameter (mm) | |
| Mean | 1.22 | 15.02 | 120.32 | 1 | 15 |
| SD | 0.04 | 0.05 | 4.26 | ||
| Higuchi | Korsmeyer | Zero Order | Peppas and Sahlin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| b a (min−0.5) | r2 e | K a (min−n) | n b | r2 e | K a (min−1) | r2 e | kd c (min−0.425) | kr d (min−0.850) | r2 e |
| 0.0331 | 0.9433 | 0.00147 | 1.0166 | 0.9917 | 0.0016 | 0.9947 | 0.0065 | 0.0012 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares, V.; Aguilar-de-Leyva, Á.; Casas, M.; Caraballo, I. 3D Printed Fractal-like Structures with High Percentage of Drug for Zero-Order Colonic Release. Pharmaceutics 2022, 14, 2298. https://doi.org/10.3390/pharmaceutics14112298
Linares V, Aguilar-de-Leyva Á, Casas M, Caraballo I. 3D Printed Fractal-like Structures with High Percentage of Drug for Zero-Order Colonic Release. Pharmaceutics. 2022; 14(11):2298. https://doi.org/10.3390/pharmaceutics14112298
Chicago/Turabian StyleLinares, Vicente, Ángela Aguilar-de-Leyva, Marta Casas, and Isidoro Caraballo. 2022. "3D Printed Fractal-like Structures with High Percentage of Drug for Zero-Order Colonic Release" Pharmaceutics 14, no. 11: 2298. https://doi.org/10.3390/pharmaceutics14112298