Salmonella as a Promising Curative Tool against Cancer
Abstract
:1. Introduction
2. Bacterial Application for Cancer Therapy
3. Attenuated Bacteria for Cancer Therapy
4. Why Is Salmonella the Best Option?
4.1. High Tumor Colonization
4.2. Non-Specific Tumor Target
4.3. Inherent Anti-Tumor Character
4.4. Engineering Plasticity
5. Salmonella enterica Serovar Typhimurium
6. ST VNP20009 Strain
7. ST A1-R Strain
8. Other Auxotrophic Mutant Strains
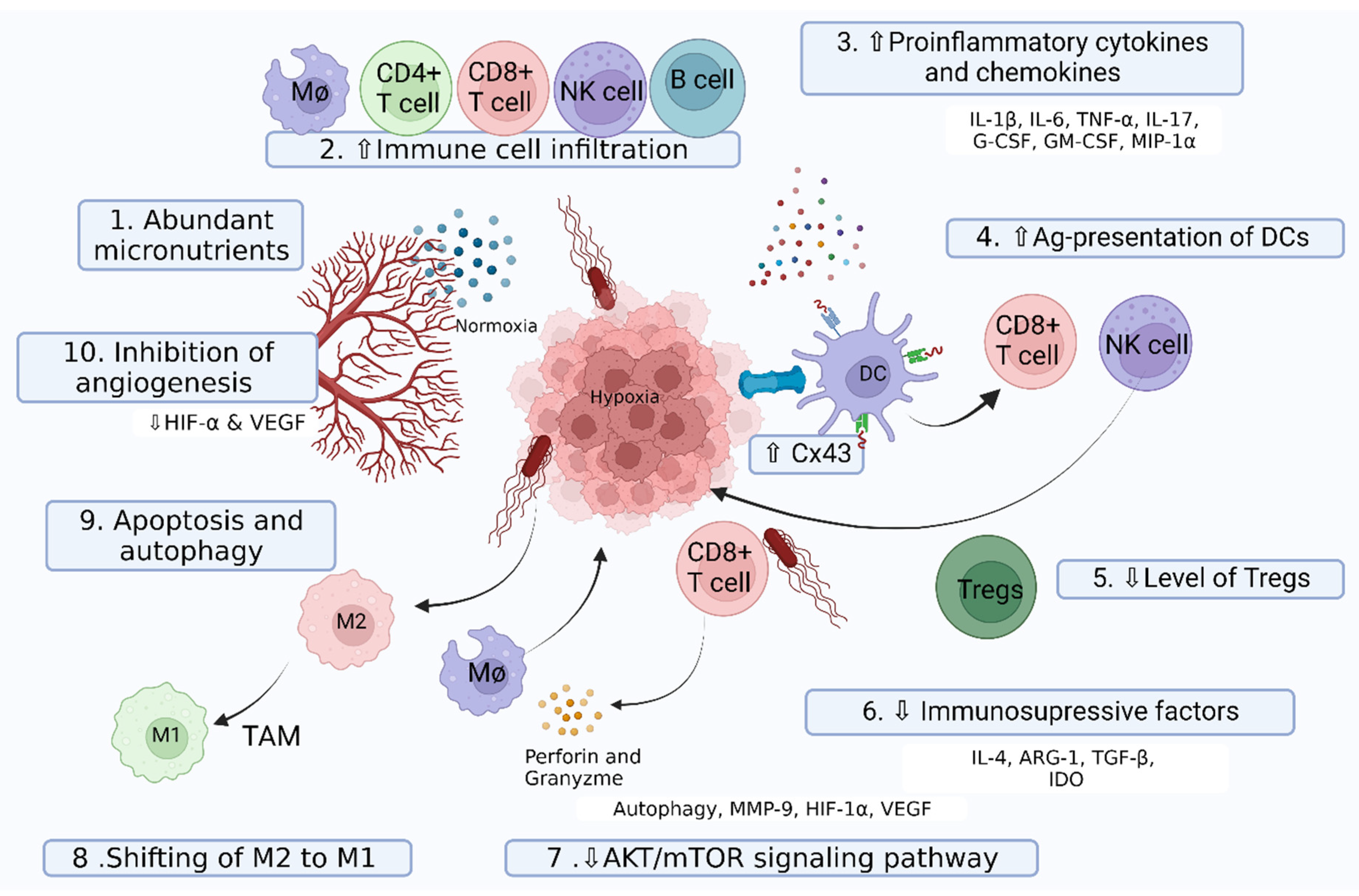
9. Anti-Tumor Mechanism of Salmonella
9.1. Hypoxic Environment and Tumor Vasculature
9.2. Abundant Nutrients and Competitive Nature of Salmonella
9.3. Tumor Penetration
9.4. Apoptosis and Autophagy-Inducing Intrinsic Anti-Tumor Action
9.5. Inhibition of Angiogenesis
9.6. Immunomodulation in Tumor Tissue
9.7. Orchestration of TAM Function and Polarization
9.8. Release of Cytotoxic Chemicals
9.9. Role of the Type III Secretion System
10. Current Approach with Combination Therapy
11. Cancer Vaccines Delivered by Salmonella
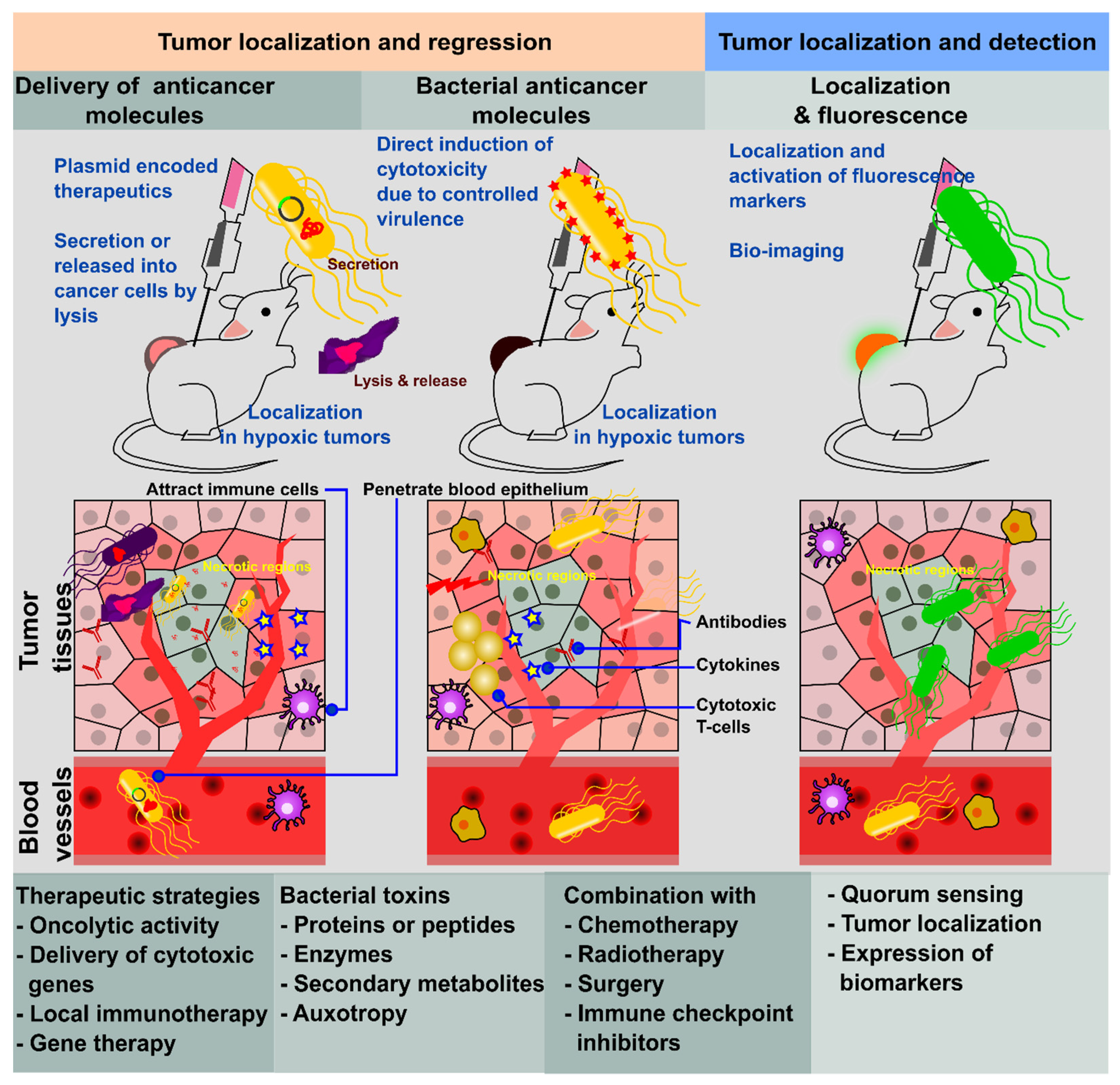
12. Application of Salmonella in Tumor Targeting and Detection
13. Clinical Trials
14. Limitations of Bacteria-Mediated Cancer Therapy
15. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- St Jean, A.T.; Zhang, M.; Forbes, N.S. Bacterial therapies: Completing the cancer treatment toolbox. Curr. Opin. Biotechnol. 2008, 19, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Keung, E.Z.; Fairweather, M.; Raut, C.P. Surgical Management of Metastatic Disease. Surg. Clin. North Am. 2016, 96, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Ahmed, M.M.; Loo, B.W., Jr.; Strober, S. Novel Radiation Therapy Paradigms and Immunomodulation: Heresies and Hope. Semin. Radiat. Oncol. 2020, 30, 194–200. [Google Scholar] [CrossRef]
- Kocakavuk, E.; Anderson, K.J.; Varn, F.S.; Johnson, K.C.; Amin, S.B.; Sulman, E.P.; Lolkema, M.P.; Barthel, F.P.; Verhaak, R.G.W. Radiotherapy is associated with a deletion signature that contributes to poor outcomes in patients with cancer. Nat. Genet. 2021, 53, 1088–1096. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [Green Version]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updat. 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Dang, L.H.; Bettegowda, C.; Huso, D.L.; Kinzler, K.W.; Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 15155–15160. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.H.; Min, J.J. Salmonella-Mediated Cancer Therapy: Roles and Potential. Nucl. Med. Mol. Imaging 2017, 51, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tjuvajev, J.; Blasberg, R.; Luo, X.; Zheng, L.M.; King, I.; Bermudes, D. Salmonella-based tumor-targeted cancer therapy: Tumor amplified protein expression therapy (TAPET) for diagnostic imaging. J. Control. Release 2001, 74, 313–315. [Google Scholar] [CrossRef]
- Jawalagatti, V.; Kirthika, P.; Lee, J.H. Targeting primary and metastatic tumor growth in an aggressive breast cancer by engineered tryptophan auxotrophic Salmonella Typhimurium. Mol. Ther. Oncolytics 2022, 25, 350–363. [Google Scholar] [CrossRef]
- McCarthy, E.F.; The Toxins of William, B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar] [PubMed]
- Yu, Y.A.; Shabahang, S.; Timiryasova, T.M.; Zhang, Q.; Beltz, R.; Gentschev, I.; Goebel, W.; Szalay, A.A. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat. Biotechnol. 2004, 22, 313–320. [Google Scholar] [CrossRef]
- Parker, R.C.; Plummer, H.C.; Siebenmann, C.O.; Chapman, M.G. Effect of histolyticus infection and toxin on transplantable mouse tumors. Proc. Soc. Exp. Biol. Med. 1947, 66, 461–467. [Google Scholar] [CrossRef]
- Malmgren, R.A.; Flanigan, C.C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955, 15, 473–478. [Google Scholar]
- Kohwi, Y.; Imai, K.; Tamura, Z.; Hashimoto, Y. Antitumor effect of Bifidobacterium infantis in mice. Gan 1978, 69, 613–618. [Google Scholar]
- Maletzki, C.; Linnebacher, M.; Kreikemeyer, B.; Emmrich, J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut 2008, 57, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Sugiura, K.; Reilly, H.C.; Stock, C.C. Oncolytic effect of Proteus mirabilis upon tumor-bearing animals. II. Effect on transplantable mouse and rat tumors. Gan 1968, 59, 117–122. [Google Scholar] [PubMed]
- Moese, J.R.; Moese, G. Oncolysis by Clostridia. I. Activity of Clostridium Butyricum (M-55) and Other Nonpathogenic Clostridia against the Ehrlich Carcinoma. Cancer Res. 1964, 24, 212–216. [Google Scholar] [PubMed]
- Mohr, U.; Hondius Boldingh, W.; Emminger, A.; Behagel, H.A. Oncolysis by a new strain of Clostridium. Cancer Res. 1972, 32, 1122–1128. [Google Scholar] [PubMed]
- Iftekhar, A.; Berger, H.; Bouznad, N.; Heuberger, J.; Boccellato, F.; Dobrindt, U.; Hermeking, H.; Sigal, M.; Meyer, T.F. Genomic aberrations after short-term exposure to colibactin-producing E. coli transform primary colon epithelial cells. Nat. Commun. 2021, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Kwong, T.N.Y.; Wang, X.; Nakatsu, G.; Chow, T.C.; Tipoe, T.; Dai, R.Z.W.; Tsoi, K.K.K.; Wong, M.C.S.; Tse, G.; Chan, M.T.V.; et al. Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology 2018, 155, 383–390.e8. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 2010, 57, 1411–1415. [Google Scholar]
- Caygill, C.P.; Braddick, M.; Hill, M.J.; Knowles, R.L.; Sharp, J.C. The association between typhoid carriage, typhoid infection and subsequent cancer at a number of sites. Eur. J. Cancer Prev. 1995, 4, 187–193. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Vijayagomaran, P.A.; Lian, P.N.P.; Ning, T.J.; Wai, N.Z.; Xian-Zhuang, N.; Le Er, C.; Rahmah, N.S.N.; Kamaruzzaman, N.D.B.; et al. Recent Update on Bacteria as a Delivery Carrier in Cancer Therapy: From Evil to Allies. Pharm. Res. 2022, 39, 1115–1134. [Google Scholar] [CrossRef]
- Jawalagatti, V.; Kirthika, P.; Lee, J.H. Oral mRNA Vaccines against Infectious Diseases—A Bacterial Perspective [Invited]. Front. Immunol. 2022, 13, 884862. [Google Scholar] [CrossRef]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Sun, Y.; Nabel, G.J. Regulation of the proinflammatory effects of Fas ligand (CD95L). Science 1998, 282, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Stritzker, J.; Weibel, S.; Seubert, C.; Gotz, A.; Tresch, A.; van Rooijen, N.; Oelschlaeger, T.A.; Hill, P.J.; Gentschev, I.; Szalay, A.A. Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int. J. Med. Microbiol. 2010, 300, 449–456. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007, 67, 3201–3209. [Google Scholar] [CrossRef] [Green Version]
- Tu, D.G.; Chang, W.W.; Lin, S.T.; Kuo, C.Y.; Tsao, Y.T.; Lee, C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget 2016, 7, 37513–37523. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, L.; Hoffman, R.M.; Zhao, M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010, 9, 4518–4524. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.; Poole, S.; Wilson, M. Microbial/host interactions in health and disease: Who controls the cytokine network? Immunopharmacology 1996, 35, 1–21. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef] [PubMed]
- al-Ramadi, B.K.; Fernandez-Cabezudo, M.J.; El-Hasasna, H.; Al-Salam, S.; Bashir, G.; Chouaib, S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin. Immunol. 2009, 130, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Duong, M.T.; Zuo, C.; Qin, Y.; Zhang, Y.; Guo, Y.; Hong, Y.; Zheng, J.H.; Min, J.J. Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium. Mol. Ther. 2022, 30, 662–671. [Google Scholar] [CrossRef] [PubMed]
- de Lima Fragelli, B.D.; Camillo, L.; de Almeida Rodolpho, J.M.; de Godoy, K.F.; de Castro, C.A.; Brassolatti, P.; da Silva, A.J.; Borra, R.C.; de Freitas Anibal, F. Antitumor Effect of IL-2 and TRAIL Proteins Expressed by Recombinant Salmonella in Murine Bladder Cancer Cells. Cell. Physiol. Biochem. 2021, 55, 460–476. [Google Scholar]
- Guan, G.F.; Zhao, M.; Liu, L.M.; Jin, C.S.; Sun, K.; Zhang, D.J.; Yu, D.J.; Cao, H.W.; Lu, Y.Q.; Wen, L.J. Salmonella typhimurium mediated delivery of Apoptin in human laryngeal cancer. Int. J. Med. Sci. 2013, 10, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.; Kim, K.S.; Kim, H.; Ko, K.C.; Song, J.J.; Choi, J.H.; Shin, M.; Min, J.J.; Jeong, J.H.; Choy, H.E. Anti-tumor activity of an immunotoxin (TGFalpha-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget 2017, 8, 37550–37560. [Google Scholar] [CrossRef] [Green Version]
- Blache, C.A.; Manuel, E.R.; Kaltcheva, T.I.; Wong, A.N.; Ellenhorn, J.D.; Blazar, B.R.; Diamond, D.J. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012, 72, 6447–6456. [Google Scholar] [CrossRef] [Green Version]
- Raman, V.; Van Dessel, N.; Hall, C.L.; Wetherby, V.E.; Whitney, S.A.; Kolewe, E.L.; Bloom, S.M.K.; Sharma, A.; Hardy, J.A.; Bollen, M.; et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat. Commun. 2021, 12, 6116. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Jiang, Z.; Yang, M.; Li, H.; Wang, J. Enhancement of ovarian cancer chemotherapy by delivery of multidrug-resistance gene small interfering RNA using tumor targeting Salmonella. J. Obs. Gynaecol. Res. 2015, 41, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.V.; Zheng, J.H.; Yun, M.; Park, S.G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef] [Green Version]
- Akoachere, J.F.; Tanih, N.F.; Ndip, L.M.; Ndip, R.N. Phenotypic characterization of Salmonella typhimurium isolates from food-animals and abattoir drains in Buea, Cameroon. J. Health Popul. Nutr. 2009, 27, 612–618. [Google Scholar]
- Semenov, A.V.; van Overbeek, L.; Termorshuizen, A.J.; van Bruggen, A.H. Influence of aerobic and anaerobic conditions on survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in Luria-Bertani broth, farm-yard manure and slurry. J. Env. Manag. 2011, 92, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Inhibition of tumor growth using salmonella expressing Fas ligand. J. Natl. Cancer Inst. 2008, 100, 1113–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganai, S.; Arenas, R.B.; Forbes, N.S. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br. J. Cancer 2009, 101, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- King, I.; Bermudes, D.; Lin, S.; Belcourt, M.; Pike, J.; Troy, K.; Le, T.; Ittensohn, M.; Mao, J.; Lang, W.; et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum. Gene 2002, 13, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Friedlos, F.; Lehouritis, P.; Ogilvie, L.; Hedley, D.; Davies, L.; Bermudes, D.; King, I.; Martin, J.; Marais, R.; Springer, C.J. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin. Cancer Res. 2008, 14, 4259–4266. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Hiroshima, Y.; Zhang, Y.; Zhao, M.; Kiyuna, T.; Hwang, H.K.; Miyake, K.; Homma, Y.; Mori, R.; Matsuyama, R.; et al. Tumor-Targeting Salmonella typhimurium A1-R Promotes Tumoricidal CD8(+) T Cell Tumor Infiltration and Arrests Growth and Metastasis in a Syngeneic Pancreatic-Cancer Orthotopic Mouse Model. J. Cell Biochem. 2018, 119, 634–639. [Google Scholar] [CrossRef]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Li, S.; Han, Q.; Tan, Y.; Zhao, M.; Li, Y.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: Decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle 2018, 17, 801–809. [Google Scholar]
- Murakami, T.; DeLong, J.; Eilber, F.C.; Zhao, M.; Zhang, Y.; Zhang, N.; Singh, A.; Russell, T.; Deng, S.; Reynoso, J.; et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016, 7, 12783–12790. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Chmielowski, B.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Singh, A.; Unno, M.; Nelson, S.D.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016, 7, 85929–85936. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; Singh, A.S.; et al. Salmonella typhimurium A1-R targeting of a chemotherapy-resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozolomide. Cell Cycle 2017, 16, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.G.; Chen, Y.; Wu, Z.P.; Liu, X.; Liu, C.; Zhou, Y.C.; Wu, C.L.; Jin, C.G.; Gu, Y.L.; Li, J.; et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol. Pharm. Bull. 2010, 33, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yang, B.; Cheng, X.; Qiao, Y.; Tang, B.; Chen, G.; Wei, J.; Liu, X.; Cheng, W.; Du, P.; et al. Salmonella-mediated tumor-targeting TRAIL gene therapy significantly suppresses melanoma growth in mouse model. Cancer Sci. 2012, 103, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, X.; Chen, L.; Li, S.; Ren, D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol. 2008, 7, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, R.M.; Green, J.; Williams, P.J.; Tazzyman, S.; Hunt, S.; Harmey, J.H.; Kehoe, S.C.; Lewis, C.E. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene 2009, 16, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene 2008, 15, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Hoiseth, S.K.; Stocker, B.A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 1981, 291, 238–239. [Google Scholar] [CrossRef]
- Low, K.B.; Ittensohn, M.; Le, T.; Platt, J.; Sodi, S.; Amoss, M.; Ash, O.; Carmichael, E.; Chakraborty, A.; Fischer, J.; et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat. Biotechnol. 1999, 17, 37–41. [Google Scholar] [CrossRef]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Curtiss, R., 3rd; Erhardt, M.; Weiss, S. Optimizing Salmonella enterica serovar Typhimurium for bacteria-mediated tumor therapy. Gut Microbes 2016, 7, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Thamm, D.H.; Kurzman, I.D.; King, I.; Li, Z.; Sznol, M.; Dubielzig, R.R.; Vail, D.M.; MacEwen, E.G. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clin. Cancer Res. 2005, 11, 4827–4834. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.; Nemunaitis, J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum. Gene 2001, 12, 1594–1596. [Google Scholar]
- Luo, X.; Li, Z.; Lin, S.; Le, T.; Ittensohn, M.; Bermudes, D.; Runyab, J.D.; Shen, S.Y.; Chen, J.; King, I.C.; et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol. Res. 2001, 12, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Lu, M.; Lai, Y.; Zhang, X.; Li, Y.; Mao, P.; Liang, Z.; Mu, Y.; Lin, Y.; Zhao, A.Z.; et al. Inhibition of acute leukemia with attenuated Salmonella typhimurium strain VNP20009. Biomed. Pharm. 2020, 129, 110425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.M.; Luo, X.; Feng, M.; Li, Z.; Le, T.; Ittensohn, M.; Trailsmith, M.; Bermudes, D.; Lin, S.L.; King, I.C. Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncol. Res. 2000, 12, 127–135. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Gu, M.; Li, G.; Ni, J.; Fan, M. Antitumor Effect of Cycle Inhibiting Factor Expression in Colon Cancer via Salmonella VNP20009. Anticancer Agents Med. Chem. 2020, 20, 1722–1727. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Zhou, Y.; Zhang, C.; Hua, Z.C. A Salmonella Typhimurium mutant strain capable of RNAi delivery: Higher tumor-targeting and lower toxicity. Cancer Biol. 2014, 15, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Broadway, K.M.; Modise, T.; Jensen, R.V.; Scharf, B.E. Complete genome sequence of Salmonella enterica serovar Typhimurium VNP20009, a strain engineered for tumor targeting. J. Biotechnol. 2014, 192 Pt A, 177–178. [Google Scholar] [CrossRef]
- Liu, R.; Cao, Z.P.; Wang, L.; Wang, X.Y.; Lin, S.S.; Wu, F.; Pang, Y.; Liu, J.Y. Multimodal oncolytic bacteria by coating with tumor cell derived nanoshells. Nano Today 2022, 45, 101537. [Google Scholar] [CrossRef]
- Hoffman, R.M. Tumor-targeting amino acid auxotrophic Salmonella typhimurium. Amino Acids 2009, 37, 509–521. [Google Scholar] [CrossRef]
- Zhao, M.; Geller, J.; Ma, H.; Yang, M.; Penman, S.; Hoffman, R.M. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 10170–10174. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Miwa, S.; Zhang, Y.; Hiroshima, Y.; Yano, S.; Uehara, F.; Yamamoto, M.; Toneri, M.; Bouvet, M.; Matsubara, H.; et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J. Cell. Biochem. 2014, 115, 1996–2003. [Google Scholar] [PubMed]
- Miwa, S.; Yano, S.; Zhang, Y.; Matsumoto, Y.; Uehara, F.; Yamamoto, M.; Hiroshima, Y.; Kimura, H.; Hayashi, K.; Yamamoto, N.; et al. Tumor-targeting Salmonella typhimurium A1-R prevents experimental human breast cancer bone metastasis in nude mice. Oncotarget 2014, 5, 7119–7125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Zhang, Y.; Zhao, M.; Hiroshima, Y.; Miwa, S.; Uehara, F.; Kishimoto, H.; Tazawa, H.; Bouvet, M.; Fujiwara, T.; et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014, 13, 3958–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.; Zhang, R.; Luo, H.; Zhang, J.; Tian, Z.; Zhang, X.; Zhang, Y.; Ali, M.K.; Kong, Q. Optimized Attenuated Salmonella Typhimurium Suppressed Tumor Growth and Improved Survival in Mice. Front. Microbiol. 2021, 12, 774490. [Google Scholar] [CrossRef]
- Fensterle, J.; Bergmann, B.; Yone, C.L.; Hotz, C.; Meyer, S.R.; Spreng, S.; Goebel, W.; Rapp, U.R.; Gentschev, I. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene 2008, 15, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhou, H.; Krueger, J.; Kaplan, C.; Lee, S.H.; Dolman, C.; Markowitz, D.; Wu, W.; Liu, C.; Reisfeld, R.A.; et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Investig. 2006, 116, 2132–2141. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 2019, 8, e44235. [Google Scholar] [CrossRef]
- Kaimala, S.; Al-Sbiei, A.; Cabral-Marques, O.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Attenuated Bacteria as Immunotherapeutic Tools for Cancer Treatment. Front. Oncol. 2018, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Grille, S.; Moreno, M.; Bascuas, T.; Marques, J.M.; Munoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica serovar Typhimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, H.C.; Shiau, A.L. B cells are required for tumor-targeting Salmonella in host. Appl. Microbiol. Biotechnol. 2011, 92, 1251–1260. [Google Scholar] [CrossRef]
- Raymond, B.; Young, J.C.; Pallett, M.; Endres, R.G.; Clements, A.; Frankel, G. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol. 2013, 21, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010, 2, 44ra57. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Chang, S.Y.; Lee, B.R.; Pyun, A.R.; Kim, J.W.; Kweon, M.N.; Ko, H.J. Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine 2013, 31, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chopra, A.K. An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: The critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene 2010, 17, 97–108. [Google Scholar] [CrossRef]
- Kuan, Y.D.; Lee, C.H. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget 2016, 7, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [Green Version]
- Renner, C.; Held, G.; Ohnesorge, S.; Bauer, S.; Gerlach, K.; Pfitzenmeier, J.P.; Pfreundschuh, M. Role of perforin, granzymes and the proliferative state of the target cells in apoptosis and necrosis mediated by bispecific-antibody-activated cytotoxic T cells. Cancer Immunol. Immunother. 1997, 44, 70–76. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Ganai, S.; Arenas, R.B.; Sauer, J.P.; Bentley, B.; Forbes, N.S. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene 2011, 18, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.W.; Lee, C.H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014, 15, 14546–14554. [Google Scholar] [CrossRef] [Green Version]
- Uchugonova, A.; Zhao, M.; Zhang, Y.; Weinigel, M.; Konig, K.; Hoffman, R.M. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012, 32, 4331–4337. [Google Scholar] [PubMed]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.S.; Munn, L.L.; Fukumura, D.; Jain, R.K. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 2003, 63, 5188–5193. [Google Scholar]
- Zhao, M.; Yang, M.; Li, X.M.; Jiang, P.; Baranov, E.; Li, S.; Xu, M.; Penman, S.; Hoffman, R.M. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2005, 102, 755–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toley, B.J.; Forbes, N.S. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. 2012, 4, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Westphal, K.; Leschner, S.; Jablonska, J.; Loessner, H.; Weiss, S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008, 68, 2952–2960. [Google Scholar] [CrossRef] [Green Version]
- Mi, Z.; Feng, Z.C.; Li, C.; Yang, X.; Ma, M.T.; Rong, P.F. Salmonella-Mediated Cancer Therapy: An Innovative Therapeutic Strategy. J. Cancer 2019, 10, 4765–4776. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Lin, S.T.; Liu, J.J.; Chang, W.W.; Hsieh, J.L.; Wang, W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene 2014, 21, 309–316. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Kuo, C.Y.; Cheng, S.P.; Lee, C.H. Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. Int. J. Mol. Sci. 2018, 19, 1630. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.K.; Chen, M.C.; Leong, H.F.; Kuo, Y.L.; Kuo, C.Y.; Lee, C.H. Connexin 43 suppresses tumor angiogenesis by down-regulation of vascular endothelial growth factor via hypoxic-induced factor-1alpha. Int. J. Mol. Sci. 2014, 16, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyuna, T.; Tome, Y.; Uehara, F.; Murakami, T.; Zhang, Y.; Zhao, M.; Kanaya, F.; Hoffman, R.M. Tumor-targeting Salmonella typhimurium A1-R Inhibits Osteosarcoma Angiogenesis in the In Vivo Gelfoam(R) Assay Visualized by Color-coded Imaging. Anticancer. Res. 2018, 38, 159–164. [Google Scholar] [PubMed]
- Galdiero, M.R.; Bonavita, E.; Barajon, I.; Garlanda, C.; Mantovani, A.; Jaillon, S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Varela-Vazquez, A.; Guitian-Caamano, A.; Carpintero-Fernandez, P.; Fonseca, E.; Sayedyahossein, S.; Aasen, T.; Penuela, S.; Mayan, M.D. Emerging functions and clinical prospects of connexins and pannexins in melanoma. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188380. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Biswas, S.K.; Gangi, L.; Paul, S.; Schioppa, T.; Saccani, A.; Sironi, M.; Bottazzi, B.; Doni, A.; Vincenzo, B.; Pasqualini, F.; et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 2006, 107, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- De Palma, M.; Mazzieri, R.; Politi, L.S.; Pucci, F.; Zonari, E.; Sitia, G.; Mazzoleni, S.; Moi, D.; Venneri, M.A.; Indraccolo, S.; et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008, 14, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Duluc, D.; Corvaisier, M.; Blanchard, S.; Catala, L.; Descamps, P.; Gamelin, E.; Ponsoda, S.; Delneste, Y.; Hebbar, M.; Jeannin, P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer 2009, 125, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Pangilinan, C.R.; Wu, L.H.; Lee, C.H. Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma. Cancers 2021, 13, 2894. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xu, J.; Wang, Q.; Zhang, A.Q.; Wang, K. An obligatory anaerobic Salmonella typhimurium strain redirects M2 macrophages to the M1 phenotype. Oncol. Lett. 2018, 15, 3918–3922. [Google Scholar] [PubMed] [Green Version]
- Bernsmeier, C.; van der Merwe, S.; Perianin, A. Innate immune cells in cirrhosis. J. Hepatol. 2020, 73, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Buttner, M.; Fabri, M.; Mougiakakos, D.; Bittenbring, J.T.; Hoffmann, M.H.; Beier, F.; Pasemann, S.; Jitschin, R.; Hofmann, A.D.; et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci. Transl. Med. 2015, 7, 282ra47. [Google Scholar] [CrossRef]
- Lewen, S.; Zhou, H.; Hu, H.D.; Cheng, T.; Markowitz, D.; Reisfeld, R.A.; Xiang, R.; Luo, Y. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol. Immunother. 2008, 57, 507–515. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kim, H.S.; Ha, J.M.; Hong, Y.; Choy, H.E.; Min, J.J. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010, 70, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, B.S.; Banton, K.L.; Frykman, N.L.; Leonard, A.S.; Saltzman, D.A. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin. Orthop. Relat. Res. 2008, 466, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef]
- Galan, J.E.; Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 2006, 444, 567–573. [Google Scholar] [CrossRef]
- Rajashekar, R.; Liebl, D.; Seitz, A.; Hensel, M. Dynamic remodeling of the endosomal system during formation of Salmonella-induced filaments by intracellular Salmonella enterica. Traffic 2008, 9, 2100–2116. [Google Scholar] [CrossRef] [PubMed]
- Liss, V.; Swart, A.L.; Kehl, A.; Hermanns, N.; Zhang, Y.; Chikkaballi, D.; Bohles, N.; Deiwick, J.; Hensel, M. Salmonella enterica Remodels the Host Cell Endosomal System for Efficient Intravacuolar Nutrition. Cell Host Microbe 2017, 21, 390–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Novel tools to analyze the function of Salmonella effectors show that SvpB ectopic expression induces cell cycle arrest in tumor cells. PLoS ONE 2013, 8, e78458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercado-Lubo, R.; Zhang, Y.; Zhao, L.; Rossi, K.; Wu, X.; Zou, Y.; Castillo, A.; Leonard, J.; Bortell, R.; Greiner, D.L.; et al. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat. Commun. 2016, 7, 12225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siccardi, D.; Mumy, K.L.; Wall, D.M.; Bien, J.D.; McCormick, B.A. Salmonella enterica serovar Typhimurium modulates P-glycoprotein in the intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1392–G1400. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, B.; Kanneganti, T.D. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int. J. Mol. Sci. 2021, 22, 1048. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sato, E.; Briones, G.; Chen, L.M.; Matsuo, M.; Nagata, Y.; Ritter, G.; Jager, E.; Nomura, H.; Kondo, S.; et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Investig. 2006, 116, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Roider, E.; Jellbauer, S.; Kohn, B.; Berchtold, C.; Partilla, M.; Busch, D.H.; Russmann, H.; Panthel, K. Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer. Cancer Immunol. Immunother. 2011, 60, 371–380. [Google Scholar] [CrossRef]
- Xiong, G.; Husseiny, M.I.; Song, L.; Erdreich-Epstein, A.; Shackleford, G.M.; Seeger, R.C.; Jackel, D.; Hensel, M.; Metelitsa, L.S. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int. J. Cancer 2010, 126, 2622–2634. [Google Scholar]
- Panthel, K.; Meinel, K.M.; Sevil Domenech, V.E.; Trulzsch, K.; Russmann, H. Salmonella type III-mediated heterologous antigen delivery: A versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int. J. Med. Microbiol. 2008, 298, 99–103. [Google Scholar] [CrossRef]
- Jia, L.J.; Wei, D.P.; Sun, Q.M.; Jin, G.H.; Li, S.F.; Huang, Y.; Hua, Z.C. Tumor-targeting Salmonella typhimurium improves cyclophosphamide chemotherapy at maximum tolerated dose and low-dose metronomic regimens in a murine melanoma model. Int. J. Cancer 2007, 121, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, J.; Cheng, H.; Zhu, Z.; Xu, H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J. Exp. Clin. Cancer Res. 2016, 35, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroshima, Y.; Zhang, Y.; Murakami, T.; Maawy, A.; Miwa, S.; Yamamoto, M.; Yano, S.; Sato, S.; Momiyama, M.; Mori, R.; et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget 2014, 5, 12346–12357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, J.; Sodi, S.; Kelley, M.; Rockwell, S.; Bermudes, D.; Low, K.B.; Pawelek, J. Antitumour effects of genetically engineered Salmonella in combination with radiation. Eur. J. Cancer 2000, 36, 2397–2402. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Piao, L.; Yuan, F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp. Anim. 2016, 65, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.S.; Kim, S.; Seo, S.; Park, Y. Salmonella typhimurium with gamma-radiation induced H2AX phosphorylation and apoptosis in melanoma. Biosci. Biotechnol. Biochem. 2014, 78, 1082–1085. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Miwa, S.; Zhang, N.; Hoffman, R.M.; Zhao, M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015, 6, 2615–2622. [Google Scholar] [CrossRef] [Green Version]
- Dresselaers, T.; Theys, J.; Nuyts, S.; Wouters, B.; de Bruijn, E.; Anne, J.; Lambin, P.; Van Hecke, P.; Landuyt, W. Non-invasive 19F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br. J. Cancer 2003, 89, 1796–1801. [Google Scholar] [CrossRef] [Green Version]
- Roland, K.L.; Brenneman, K.E. Salmonella as a vaccine delivery vehicle. Expert Rev. Vaccines 2013, 12, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorobik, P.; Czaplicki, D.; Ossysek, K.; Bereta, J. Salmonella and cancer: From pathogens to therapeutics. Acta Biochim. Pol. 2013, 60, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Bolhassani, A.; Zahedifard, F. Therapeutic live vaccines as a potential anticancer strategy. Int. J. Cancer 2012, 131, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Guzman, C.A.; Staendner, L.H.; Colombo, M.P.; Paglia, P. Salmonella vaccine carrier strains: Effective delivery system to trigger anti-tumor immunity by oral route. Eur. J. Immunol. 1999, 29, 693–699. [Google Scholar] [CrossRef]
- Xu, X.; Hegazy, W.A.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.R.; Diamond, D.J.; et al. Effective cancer vaccine platform based on attenuated salmonella and a type III secretion system. Cancer Res. 2014, 74, 6260–6270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhou, P.; Cai, J.; Yang, G.; Liang, S.; Ren, D. Tumor antigen delivered by Salmonella III secretion protein fused with heat shock protein 70 induces protection and eradication against murine melanoma. Cancer Sci. 2010, 101, 2621–2628. [Google Scholar] [CrossRef]
- Stegantseva, M.V.; Shinkevich, V.A.; Tumar, E.M.; Meleshko, A.N. Multi-antigen DNA vaccine delivered by polyethylenimine and Salmonella enterica in neuroblastoma mouse model. Cancer Immunol. Immunother. 2020, 69, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Ding, J.; Pan, B.R.; Yu, Z.C.; Han, Q.L.; Meng, F.P.; Liu, N.; Fan, D.M. Development of an oral DNA vaccine against MG7-Ag of gastric cancer using attenuated salmonella typhimurium as carrier. World J. Gastroenterol. 2003, 9, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bai, T.; Tian, Y.; Zhou, B.; Wang, Y.; Yang, L. H2O2-Inactivated Salmonella typhimurium RE88 Strain as a New Cancer Vaccine Carrier: Evaluation in a Mouse Model of Cancer. Drug Des. Devel. 2021, 15, 209–222. [Google Scholar] [CrossRef]
- Revaz, V.; Benyacoub, J.; Kast, W.M.; Schiller, J.T.; De Grandi, P.; Nardelli-Haefliger, D. Mucosal vaccination with a recombinant Salmonella typhimurium expressing human papillomavirus type 16 (HPV16) L1 virus-like particles (VLPs) or HPV16 VLPs purified from insect cells inhibits the growth of HPV16-expressing tumor cells in mice. Virology 2001, 279, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Baud, D.; Ponci, F.; Bobst, M.; De Grandi, P.; Nardelli-Haefliger, D. Improved efficiency of a Salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J. Virol. 2004, 78, 12901–12909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echchannaoui, H.; Bianchi, M.; Baud, D.; Bobst, M.; Stehle, J.C.; Nardelli-Haefliger, D. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect. Immun. 2008, 76, 1940–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soghomonyan, S.A.; Doubrovin, M.; Pike, J.; Luo, X.; Ittensohn, M.; Runyan, J.D.; Balatoni, J.; Finn, R.; Tjuvajev, J.G.; Blasberg, R.; et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene 2005, 12, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panteli, J.T.; Forkus, B.A.; Van Dessel, N.; Forbes, N.S. Genetically modified bacteria as a tool to detect microscopic solid tumor masses with triggered release of a recombinant biomarker. Integr. Biol. (Camb) 2015, 7, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Nemunaitis, J.; Cunningham, C.; Senzer, N.; Kuhn, J.; Cramm, J.; Litz, C.; Cavagnolo, R.; Cahill, A.; Clairmont, C.; Sznol, M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene 2003, 10, 737–744. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 2018, 7, e1303584. [Google Scholar] [CrossRef] [Green Version]
- Heimann, D.M.; Rosenberg, S.A. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 2003, 26, 179–180. [Google Scholar] [CrossRef] [Green Version]
- Leong, M.L.; Hampl, J.; Liu, W.; Mathur, S.; Bahjat, K.S.; Luckett, W.; Dubensky, T.W., Jr.; Brockstedt, D.G. Impact of preexisting vector-specific immunity on vaccine potency: Characterization of listeria monocytogenes-specific humoral and cellular immunity in humans and modeling studies using recombinant vaccines in mice. Infect. Immun. 2009, 77, 3958–3968. [Google Scholar] [CrossRef] [Green Version]
| Bacteria (Strain) | Strategy/Gene of Interest | Tumor Model | Results/Mode of Action | References |
|---|---|---|---|---|
| S. Typhimurium VNP20009 TAPET-CD | Cytosine deaminase expression from E. coli that converts non-toxic 5-fluorocytosine to the active anti-tumor agent 5-fluorouracil | Mice colon tumors | Mice treated with 5-FC inhibited tumor growth by 88−96% compared to TAPET-CD alone, which inhibited tumor growth by 38−79% | [55] |
| S. Typhimurium VNP20009 | Carboxypeptidase G2 (CPG2) | Murine models of breast and colon cancer | Prodrug-based suicide gene therapy | [56] |
| S. Typhimurium VNP20009 | msbB and purI mutations | melanomas | Attenuated strain preferentially accumulates in tumors and is rapidly cleared from other organs | [32] |
| S. Typhimurium | Deletion of ppGpp | CT26 tumor | Tumor suppression via IL-1β. | [50] |
| S. Typhimurium A1-R | leucine-arginine auxotroph | Pancreatic cancer orthotopic mouse model | Promotes CD8+ T cell infiltration and arrests tumor growth and metastasis. | [57] |
| S. Typhimurium A1-R | Combination with recombinant methioninase or doxorubin or temozolomide | Osteosarcoma, sarcoma, melanoma | Eradicate osteosarcoma and soft tissue sarcoma; regresses malignant melanoma | [58,59,60] |
| S. Typhimurium A1-R | Combination with temozolomide or vemurafenib | Melanoma in patient-derived orthotopic xenograft (PDOX) model | Combinatorial anti-tumor effect and drugs promoted targeting of S. Typhimurium A1-R | [61] |
| S. Typhimurium (ST2514P3) | Tryptophan auxotroph (trpA trpE deletion) | Breast cancer (4T1) | Suppressed the primary tumor growth and pulmonary metastasis | [13] |
| S. Typhimurium | Vascular endothelial growth factor receptor 2 | Lewis lung carcinoma | Tumor suppression and inhibition of pulmonary metastasis | [62] |
| S. Typhimurium VNP20009 | TNF-related apoptosis-inducing ligand (TRAIL) | Mammary tumor, melanoma | Caspase-3-mediated apoptosis in cancer cells | [54,63] |
| S. Typhimurium | Fas ligand (FasL) | breast carcinoma and CT-26 colon carcinoma cells | Tumor growth inhibition by 59% for breast tumors and 82% for colon carcinoma | [53] |
| S. Typhimurium | shRNA- expressing vectors targeting bcl2 | Melanoma | Delayed tumor growth and prolonged survival | [64] |
| S. Typhimurium | Cytotoxic protein (HlyE) | Mammary tumor | Increased tumor necrosis and reduced tumor growth | [65] |
| S. Typhimurium msbB- and purI- | IL-18 | Lewis lung carcinoma | Inhibit the growth of primary subcutaneous tumors and pulmonary metastases | [66] |
| S. Typhimurium | Herpes simplex virus thymidine kinase (HSV TK) | Melanoma | Suppressed tumor growth and a prolonged average survival | [15] |
| Bacteria | Strain | Cancer Model | Result | Phase | References |
|---|---|---|---|---|---|
| S. Typhimurium | VNP20009 | Metastatic melanoma and renal cell carcinoma | Focal tumor colonization was recorded without tumor regression | I | [33,167] |
| S. Typhimurium | VNP20009 expressing TAPET-CD (Cytosine deaminase) | Squamous cell carcinoma and adenocarcinoma | Intratumoral bacterial colonization in 2 out of 3 patients | I | [165] |
| S. Typhimurium | VNP20009 | Solid tumors | Not provided | I | https://clinicaltrials.gov/ct2/show/NCT00006254 (accessed on 1 September 2022) |
| Salmonella typhi (Express VEGFR2) | Ty21a (VXM01) | Pancreatic cancer | VXM01 vaccination increased vaccine-specific T cell responses | I | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aganja, R.P.; Sivasankar, C.; Senevirathne, A.; Lee, J.H. Salmonella as a Promising Curative Tool against Cancer. Pharmaceutics 2022, 14, 2100. https://doi.org/10.3390/pharmaceutics14102100
Aganja RP, Sivasankar C, Senevirathne A, Lee JH. Salmonella as a Promising Curative Tool against Cancer. Pharmaceutics. 2022; 14(10):2100. https://doi.org/10.3390/pharmaceutics14102100
Chicago/Turabian StyleAganja, Ram Prasad, Chandran Sivasankar, Amal Senevirathne, and John Hwa Lee. 2022. "Salmonella as a Promising Curative Tool against Cancer" Pharmaceutics 14, no. 10: 2100. https://doi.org/10.3390/pharmaceutics14102100







