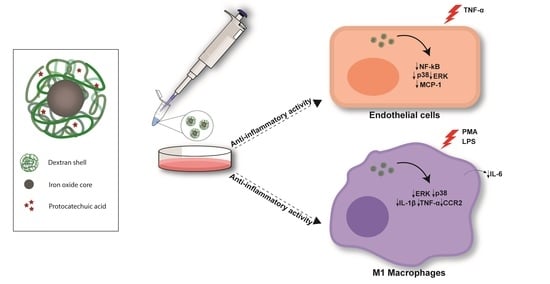
Development of Dextran-Coated Magnetic Nanoparticles Loaded with Protocatechuic Acid for Vascular Inflammation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading of PCA on Magnetic Nanoparticles
2.2.1. MNP Synthesis
2.2.2. Synthesis of Dextran-Coated MNP (MNP-Dex)
2.2.3. Synthesis of PCA-Loaded Dextran-Coated MNP (MNP-Dex/PCA)
2.3. Physico-Chemical Characterization of PCA-Loaded Magnetic Nanoparticles
2.3.1. Morphological Analysis of MNP-Dex/PCA
2.3.2. Size and Zeta Potential
2.3.3. PCA Loading on MNP-Dex
2.4. In Vitro Evaluation of PCA-Loaded Magnetic Nanoparticles
2.4.1. Cell Culture
2.4.2. Cell Cytotoxicity Assays
Colorimetric Assay
Bioluminescent Assay
2.4.3. Magnetic Nanoparticles Internalization
2.4.4. Monocyte Adhesion Assay
2.4.5. Immunological Detection of Proteins Involved in the Inflammatory Process
Immunoblotting Assay
Flow Cytometry-Based Immunoassay
2.5. Statistical Analysis
3. Results
3.1. Physico-Chemical Characterization of PCA-Loaded Magnetic Nanoparticles
3.1.1. Morphological Analysis of Nanoparticles by TEM
3.1.2. Size and Zeta Potential
3.1.3. PCA Loading Efficiency
3.2. In Vitro Evaluation of PCA-Loaded Magnetic Nanoparticles Cytotoxicity
3.3. Intracellular Localization of MNP-Dex/PCA
3.4. Anti-Inflammatory Effect of MNP-Dex/PCA
3.4.1. Anti-Inflammatory Activity of MNP-Dex/PCA on Activated EC
3.4.2. Anti-Inflammatory Activity of MNP-Dex/PCA on M1 Macrophages
3.4.3. The Effect of MNP-Dex/PCA on Key Proteins of MAPKs Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McAloon, C.J.; Osman, F.; Glennon, P.; Lim, P.B.; Hayat, S.A. Global Epidemiology and Incidence of Cardiovascular Disease. In Cardiovascular Diseases; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–96. ISBN 9780128033135. [Google Scholar]
- Sako, Y. Effects of Turbulent Blood Flow and Hypertension on Experimental Atherosclerosis. JAMA 1962, 179, 36–40. [Google Scholar] [CrossRef]
- Simionescu, M. Implications of Early Structural-Functional Changes in the Endothelium for Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, T.; Shimokado, A.; Sun, Y.; Akasaka, T.; Muragaki, Y. Diverse Roles of Macrophages in Atherosclerosis: From Inflammatory Biology to Biomarker Discovery. Mediat. Inflamm. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Li, X.; Zhou, H. Inflammation: A Novel Therapeutic Target/Direction in Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-κB system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Veeraragavan, V. Natural products as antiinflammatory agents. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 269–306. [Google Scholar] [CrossRef]
- Fuior, E.-V.; Calin, M. Nanoparticle-based delivery of polyphenols for the treatment of inflammation-associated diseases. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–382. [Google Scholar] [CrossRef]
- Calin, M.; Manduteanu, I. Emerging Nanocarriers-based Approaches to Diagnose and Red uce Vascular Inflammation in Atherosclerosis. Curr. Med. Chem. 2017, 24, 550–567. [Google Scholar] [CrossRef]
- Pillai, G.; Ceballos-Coronel, M.L. Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Med. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C. Magnetic Nanoparticles and Their Bioapplications. In Smart Nanoparticles for Biomedicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–142. ISBN 9780128141571. [Google Scholar]
- Pankhurst, Q.A.; Thanh, N.K.T.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef] [Green Version]
- Mulens-Arias, V.; Rojas, J.M.; Barber, D.F. The Intrinsic Biological Identities of Iron Oxide Nanoparticles and Their Coatings: Unexplored Territory for Combinatorial Therapies. Nanomaterials 2020, 10, 837. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef] [Green Version]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Lungoci, A.L.; Pinteala, M.; Petrovici, A.R.; Rosca, I.; Turin-Moleavin, I.A.; Fifere, A. Biosynthesized dextran coated magnetic nanoparticles with antifungal activity. Rev. Roum. Chim. 2018, 63, 497–503. [Google Scholar]
- Lungoci, A.L.; Turin-Moleavin, I.A.; Corciova, A.; Mircea, C.; Arvinte, A.; Fifere, A.; Marangoci, N.L.; Pinteala, M. Multifunctional magnetic cargo-complexes with radical scavenging properties. Mater. Sci. Eng. C 2019, 94, 608–618. [Google Scholar] [CrossRef]
- Constantin, M.; Bucatariu, S.; Sacarescu, L.; Daraba, O.M.; Anghelache, M.; Fundueanu, G. Pullulan derivative with cationic and hydrophobic moieties as an appropriate macromolecule in the synthesis of nanoparticles for drug delivery. Int. J. Biol. Macromol. 2020, 164, 4487–4498. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Bucatariu, S.-M.; Deleanu, M.; Voicu, G.; Safciuc, F.; Manduteanu, I.; Fundueanu, G.; Simionescu, M.; Calin, M. A novel platform for drug testing: Biomimetic three-dimensional hyaluronic acid-based scaffold seeded with human hepatocarcinoma cells. Int. J. Biol. Macromol. 2021, 185, 604–619. [Google Scholar] [CrossRef]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.C.; Hsu, S.H.; Karabanovas, V.; Rotomskis, R. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuior, E.V.; Deleanu, M.; Ana Constantinescu, C.; Rebleanu, D.; Voicu, G.; Simionescu, M.; Calin, M. Functional role of VCAM-1 targeted flavonoid-loaded lipid nanoemulsions in reducing endothelium inflammation. Pharmaceutics 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Maxim, C.; Manduteanu, I.; Calin, M.; Popescu, D.L. Synthesis, characterization, and in vitro insulin-mimetic activity evaluation of valine schiff base coordination compounds of oxidovanadium(v). Biomedicines 2021, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.N.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418–431. [Google Scholar] [CrossRef]
- Moleavin, I.A.T.; Fifere, A.; Lungoci, A.L.; Rosca, I.; Coroaba, A.; Peptanariu, D.; Nastasa, V.; Pasca, S.A.; Bostanaru, A.C.; Mares, M.; et al. In vitro and in vivo antioxidant activity of the new magnetic-cerium oxide nanoconjugates. Nanomaterials 2019, 9, 1565. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.F.; An, L.J.; Jiang, B.; Guan, S.; Bao, Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Young, B.D.; Bender, J.R. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol. Cell. Endocrinol. 2014, 389, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Brahma, P.K.; Wang, C.; Kallen, C.B. Evidence that neither THP-1 monocytes nor THP-1 macrophages have functional estrogen receptor-α. Fertil. Steril. 2009, 92, S20. [Google Scholar] [CrossRef]
- Martelli, A.; Citi, V.; Calderone, V. Recent efforts in drug discovery on vascular inflammation and consequent atherosclerosis. Expert Opin. Drug Discov. 2021, 16, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Manduteanu, I.; Simionescu, M. Inflammation in atherosclerosis: A cause or a result of vascular disorders? J. Cell. Mol. Med. 2012, 16, 1978–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’Archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Wu, X.; Cao, G.; Prior, R.L. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J. Nutr. 2002, 132, 1865–1871. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Stetsyshyn, Y.; Donchak, V.; Harhay, K.; Voronov, S.; Raczkowska, J.; Budkowski, A. Modification of poly(ethylene terephthalate) surface with attached dextran macromolecules. Polym. Int. 2009, 58, 1034–1040. [Google Scholar] [CrossRef]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554. [Google Scholar] [CrossRef] [Green Version]
- Matuszak, J.; Dörfler, P.; Zaloga, J.; Unterweger, H.; Lyer, S.; Dietel, B.; Alexiou, C.; Cicha, I. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin. Hemorheol. Microcirc. 2015, 61, 259–277. [Google Scholar] [CrossRef]
- Watanabe, M.; Yoneda, M.; Morohashi, A.; Hori, Y.; Okamoto, D.; Sato, A.; Kurioka, D.; Nittami, T.; Hirokawa, Y.; Shiraishi, T.; et al. Effects of Fe3O4 magnetic nanoparticles on A549 cells. Int. J. Mol. Sci. 2013, 14, 5546. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, M.; Mafakheri, H.; Khoshgard, K.; Montazerabadi, A.; Mohammadbeigi, A.; Oubari, F. The Cytotoxicity of Dextran-coated Iron Oxide Nanoparticles on Hela and MCF-7 Cancerous Cell Lines. Iran. J. Toxicol. 2017, 11, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.S.; Bastianetto, S.; Dumont, Y.; Quirion, R. Specific plasma membrane binding sites for polyphenols, including resveratrol, in the rat brain. J. Pharmacol. Exp. Ther. 2006, 318, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds1. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.Y.; Ham, S.K.; Shigenaga, M.K.; Han, O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Pang, J.; Zhang, H.; Luo, J.; Qian, X.; Chen, Q.; Ling, W. Attenuation of Atherosclerosis by Protocatechuic Acid via Inhibition of M1 and Promotion of M2 Macrophage Polarization. J. Agric. Food Chem. 2019, 67, 807–818. [Google Scholar] [CrossRef]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Q.; He, L.; Weng, H.; Su, D.; Liu, X.; Ling, W.; Wang, D. Protocatechuic acid inhibits vulnerable atherosclerotic lesion progression in older apoe −/− mice. J. Nutr. 2020, 150, 1167–1177. [Google Scholar] [CrossRef]
- Chang, W.C.; Hsu, F.L. Inhibition of platelet activation and endothelial cell injury by polyphenolic compounds isolated from Lonicera japonica Thunb. Prostaglandins Leukot. Essent. Fat. Acids 1992, 45, 307–312. [Google Scholar] [CrossRef]
- Zhou-Stache, J.; Buettner, R.; Artmann, G.; Mittermayer, C.; Bosserhoff, A.K. Inhibition of TNF-α induced cell death in human umbilical vein endothelial cells and Jurkat cells by protocatechuic acid. Med. Biol. Eng. Comput. 2002, 40, 698–703. [Google Scholar] [CrossRef]
- Nehmé, A.; Edelman, J. Dexamethasone inhibits high glucose-, TNF-α- And IL-1β-induced secretion of inflammatory and angiogenic mediators from retinal microvascular pericytes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2030–2038. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Malemud, C.J. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int. J. Mol. Sci. 2019, 20, 3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.X.; Zhang, Y.; Dong, X.; Zhang, L.; Liu, M.D.; Li, B.; Zhang, M.K.; Feng, J.; Zhang, X.Z. Artificially Reprogrammed Macrophages as Tumor-Tropic Immunosuppression-Resistant Biologics to Realize Therapeutics Production and Immune Activation. Adv. Mater. 2019, 31, 1807211. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, K.; Bloch, D.B.; Graw, J.A.; Malhotra, R.; Ichinose, F.; Bagchi, A. Iron Loading Exaggerates the Inflammatory Response to the Toll-like Receptor 4 Ligand Lipopolysaccharide by Altering Mitochondrial Homeostasis. Anesthesiology 2017, 127, 121–135. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghelache, M.; Turtoi, M.; Petrovici, A.R.; Fifere, A.; Pinteala, M.; Calin, M. Development of Dextran-Coated Magnetic Nanoparticles Loaded with Protocatechuic Acid for Vascular Inflammation Therapy. Pharmaceutics 2021, 13, 1414. https://doi.org/10.3390/pharmaceutics13091414
Anghelache M, Turtoi M, Petrovici AR, Fifere A, Pinteala M, Calin M. Development of Dextran-Coated Magnetic Nanoparticles Loaded with Protocatechuic Acid for Vascular Inflammation Therapy. Pharmaceutics. 2021; 13(9):1414. https://doi.org/10.3390/pharmaceutics13091414
Chicago/Turabian StyleAnghelache, Maria, Mihaela Turtoi, Anca Roxana Petrovici, Adrian Fifere, Mariana Pinteala, and Manuela Calin. 2021. "Development of Dextran-Coated Magnetic Nanoparticles Loaded with Protocatechuic Acid for Vascular Inflammation Therapy" Pharmaceutics 13, no. 9: 1414. https://doi.org/10.3390/pharmaceutics13091414