Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Vector Design and Purification
- Plasmids carrying different variants of IRES to ensure simultaneous expression of HGF and VEGF165 genes (general structure schemes: “pCMV_HGF-[IRES]–VEGF165”). We used IRES sequences cloned from EMCV, binding immunoglobulin protein (Bip), FGF1 and eukaryotic translation initiation factor 4 G (elF4G) genes (see Supplementary S1).
- A vector with the bidirectional cytomegalovirus (CMV) promoter common for the HGF and VEGF165 genes (bi-HGF/VEGF).
- A vector with two independent promotors: CMV for HGF and CAG for VEGF165 genes (pHGF/VEGF).
2.2. Cell Culture and Ca2+/Phosphate Transfection
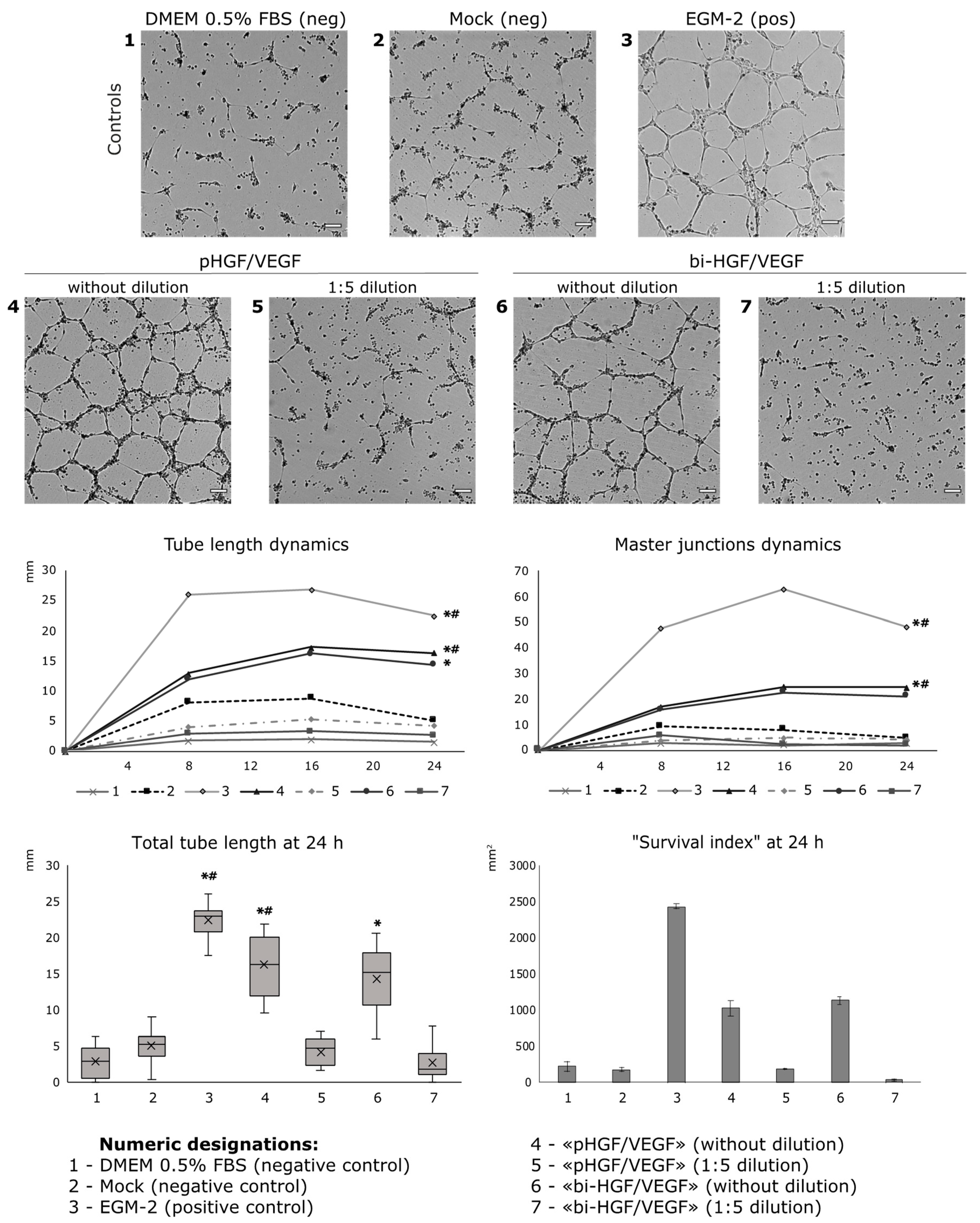
2.2.1. Tube Formation Assay
2.2.2. Tube Assay with Assessment of Cells Mortality and Survival
2.2.3. Tube Assay with Assessment of Cells Apoptosis
2.2.4. Animal Strain and Ethical Approval
2.3. Plasmid Injection and Low-Voltage Electroporation
2.4. Ex Vivo Analysis of HGF and VEGF165 Production by Explanted Muscle
2.5. Mouse Hind Limb Ischemia Model and Injection of Plasmid Solutions
2.6. Laser Doppler Perfusion Measurement
2.7. Muscle Harvest and Histological Analysis
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Protein Expression after Delivery of Plasmid DNA Encoding HGF and VEGF165 Genes Separated by IRES
3.1.1. IRES Sequences from Mammalian Genes Show Different Production of HGF and VEGF165 in HEK293T Cells
- EMCV of encephalomyocarditis virus (HGF-EMCV_IRES-VEGF);
- Bip of immunoglobulin heavy chain chaperone protein gene (HGF-Bip_IRES-VEGF);
- FGF1 of acidic fibroblast growth factor (FGF1) gene (HGF-FGF1_IRES-VEGF);
- ElF4G of eukaryotic translation initiation factor 4G gene (HGF-ElF4G_IRES-VEGF).
3.1.2. Conditioned Medium from HGF/VEGF165-Expressing HEK293T Induces Angiogenesis in HUVEC
3.1.3. Assessment of IRES-Based Plasmids in Skeletal Muscle Explants Demonstrates Efficient HGF but Not VEGF165 Expression
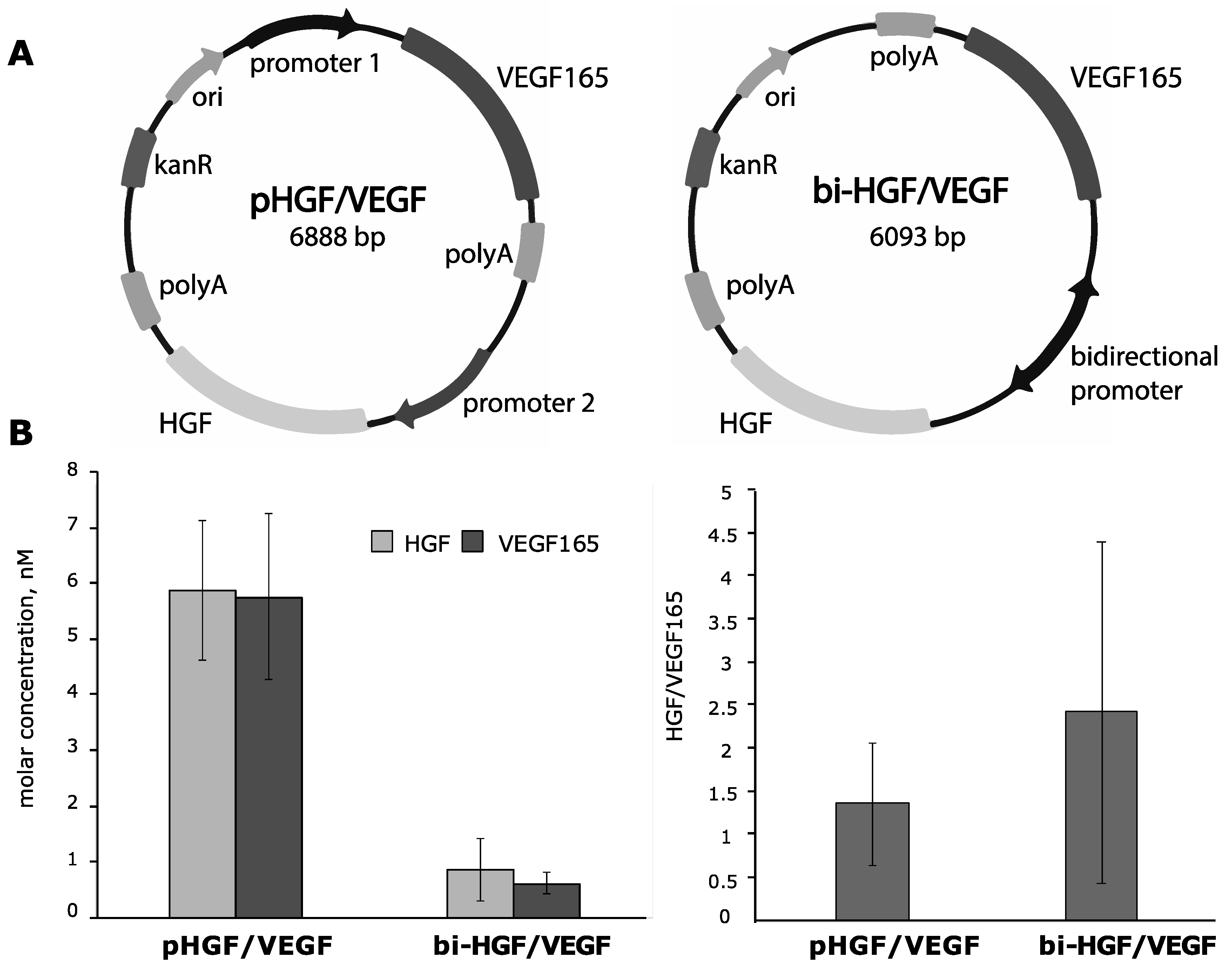
3.2. Construction of Bicistronic Plasmids with HGF and VEGF165 Genes and Evaluation of Their Expression Activity
- -
- pHGF/VEGF—plasmid with CMV and CAG promotors driving HGF and VEGF165 expression, respectively (Figure S2);
- -
- bi-HGF/VEGF—plasmid with bidirectional CMV promotor for both genes.
3.2.1. In Vitro Efficacy of Plasmid Vectors with Bidirectional CMV Promotor or with Independent CMV and CAG Promotors for Simultaneous Delivery of HGF or VEGF165 Genes
3.2.2. Conditioned Medium from HGF/VEGF165 Expressing HEK293 Renders Potent Angiogenic Effect In Vitro
3.2.3. Ex Vivo Assessment of HGF and VEGF165 Production by Skeletal Muscle Explants Shows Dramatic Difference of Bicistronic Vectors
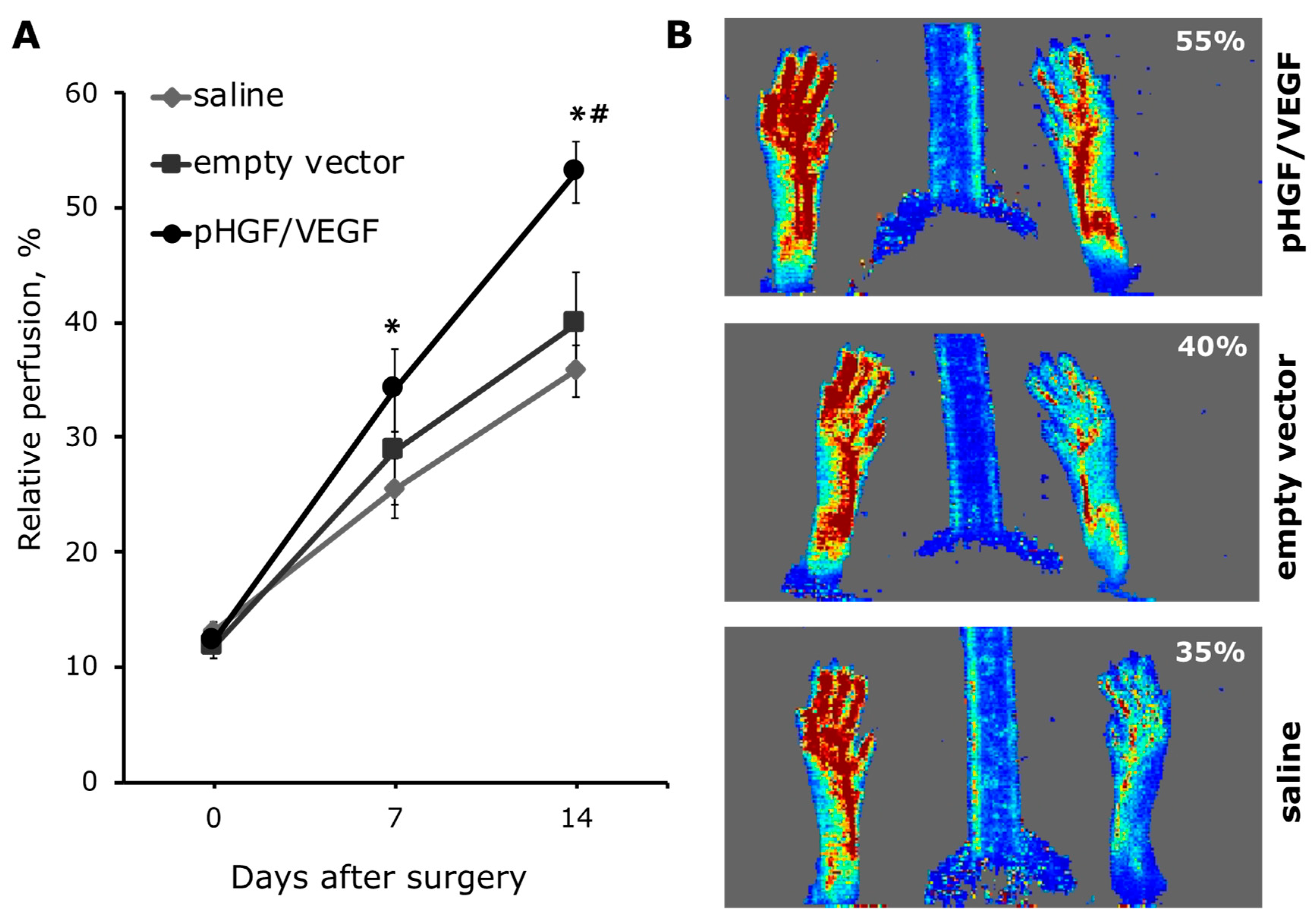
3.3. Effect of Angiogenic Bicistronic Plasmid Transfer in Mouse Hind Limb Ischemia Model
- (1)
- “pHGF/VEGF”—single delivery of pHGF/VEGF (150 μg)*, n = 9;
- (2)
- “empty vector”—negative control group with single delivery of empty pDNA backbone (commercially available pVAX2) (150 μg)*, n = 7;
- (3)
- “saline”—vehicle negative control with 0.9% NaCl injection (150 μL), n = 8.
3.3.1. Single pHGF/VEGF Plasmid Injection Induces Effective Restoration of Blood Flow in Ischemic Limb
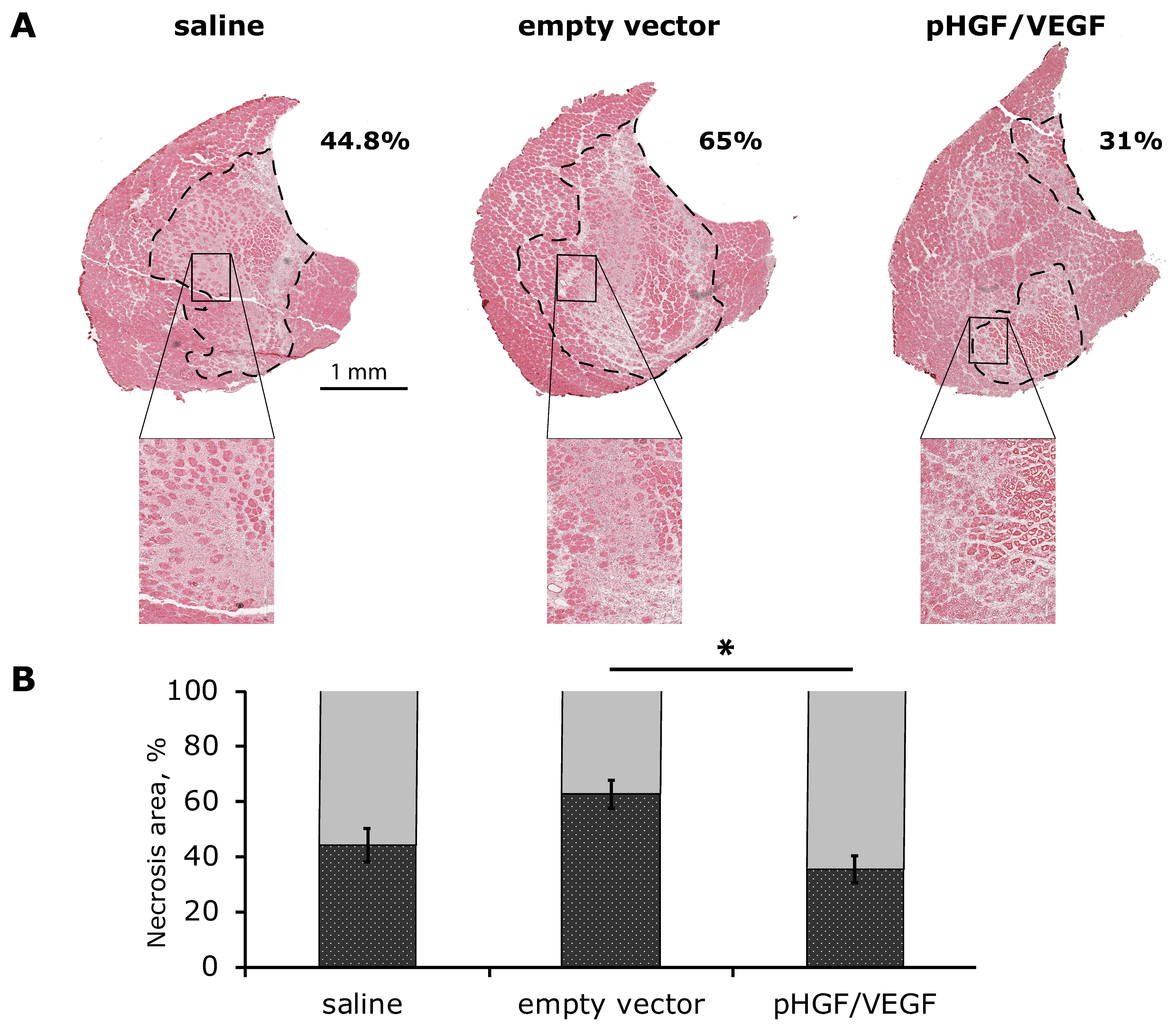
3.3.2. Histological Analysis of Necrosis Span in Ischemic Skeletal Muscle
3.3.3. Increased Vascularization of Ischemic Skeletal Muscle after HGF and VEGF165 Gene Delivery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-SMA | α-smooth muscle actin |
| AAV | Adeno-associated virus |
| AGF | Angiogenic growth factors |
| Akt | Alpha serine/threonine-protein kinase |
| Bip | Binding immunoglobulin protein |
| BP | Branching point |
| CAG | Chicken β-actin |
| cDNA | Complementary DNA |
| c-Met | Mesenchymal-epithelial transition factor |
| CMV | Cytomegalovirus |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EEF1A1 | Elongation factor 1 human alpha |
| elF4G | Eukaryotic translation initiation factor 4 G |
| EMCV | Encephalomyocarditis virus |
| ERK | Extracellular signal-regulated kinase |
| FBS | Fetal bovine serum |
| FGF | Fibroblast growth factor |
| FOV | Field of view |
| GFP | green fluorescent protein |
| HGF | Hepatocyte growth factor |
| HUVEC | Human umbilical vein endothelial cell |
| ICAM-1 | Inter-cellular adhesion molecule 1 |
| IRES | Internal ribosomal entry site |
| MAPK | Mitogen-activated protein kinase |
| pDNA | Plasmid DNA |
| PGK1 | Phosphoglycerate kinase |
| RFP | Red fluorescent protein |
| SD | Standard deviation |
| SDF | Stromal-derived factor |
| SEM | Standard error of the mean |
| uPA | Urokinase plasminogen activator |
| VCAM | Vascular cell adhesion molecule |
| VEGF | Vascular endothelial growth factor |
References
- Isner, J.; Pieczek, A.; Schainfeld, R.; Blair, R.; Haley, L.; Asahara, T.; Rosenfield, K.; Razvi, S.; Walsh, K.; Symes, J. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 1996, 10, 370–374. [Google Scholar] [CrossRef]
- Shigematsu, H.; Yasuda, K.; Sasajima, T.; Takano, T.; Miyata, T.; Ohta, T.; Tanemoto, K.; Obitsu, Y.; Iwai, T.; Ozaki, S.; et al. Transfection of human HGF plasmid DNA improves limb salvage in Buerger’s disease patients with critical limb ischemia. Int. Angiol. 2011, 30, 140–149. [Google Scholar] [PubMed]
- Nikol, S.; Baumgartner, I.; Van Belle, E.; Diehm, C.; Visoná, A.; Capogrossi, M.; Ferreira-Maldent, N.; Gallino, A.; Wyatt, M.; Wijesinghe, L.; et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol. Ther. 2008, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Zhang, F.; Chen, L.; Ha, T.; Gao, X.; Li, C. Synergistically therapeutic effects of VEGF165 and angiopoietin-1 on ischemic rat myocardium. Scand. Cardiovasc. J. SCJ 2007, 41, 95–101. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, K.; Jang, H.; Shin, I.; Jeon, E.; Suh, W.; Byun, J.; Kim, D. Combined administration of naked DNA vectors encoding VEGF and bFGF enhances tissue perfusion and arteriogenesis in ischemic hindlimb. Biochem. Biophys. Res. Commun. 2007, 360, 752–758. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Tsokolaeva, Z.I.; Shevelev, A.A.; Talitskiy, K.A.; Stepanova, V.V.; Johnstone, B.H.; Rahmat-zade, T.M.; Kapustin, A.N.; Tkachuk, V.A.; March, K.L.; et al. Urokinase Gene Transfer Augments Angiogenesis in Ischemic Skeletal and Myocardial Muscle. Mol. Ther. 2007, 15, 1939–1946. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Lv, W.; Ye, C.; Peng, X.; Zhang, H.; Xiao, L.; Wang, S. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J. Vasc. Surg. 2009, 50, 608–616. [Google Scholar] [CrossRef]
- Makarevich, P.; Tsokolaeva, Z.; Shevelev, A.; Rybalkin, I.; Shevchenko, E.; Beloglazova, I.; Vlasik, T.; Tkachuk, V.; Parfyonova, Y. Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarevich, P.I.; Dergilev, K.V.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Shevchenko, E.K.; Gluhanyuk, E.V.; Gallinger, J.O.; Menshikov, M.Y.; Parfyonova, Y.V. Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene therapy in a rat model of myocardial infarction. PLoS ONE 2018, 13, e0197566. [Google Scholar] [CrossRef]
- Gam, J.J.; Weiss, R.; Diandreth, B.; Jones, R.D. A ‘poly-transfection’ method for rapid, one-pot characterization and optimization of genetic systems. Nucleic Acids Res. 2019, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belousova, N.; Harris, R.; Zinn, K.; Rhodes-selser, M.A.; Kotov, A.; Kotova, O.; Wang, M.; Aurigemma, R.; Zhu, Z.B.; Curiel, D.T.; et al. Circumventing Recombination Events Encountered with Production of a Clinical-Grade Adenoviral Vector with a Double-Expression Cassette. Mol. Pharmacol. 2006, 70, 1488–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerman, M.; Temin, H. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell 1984, 39, 449–467. [Google Scholar] [CrossRef]
- Emerman, M.; Temin, H. Comparison of promoter suppression in avian and murine retrovirus vectors. Nucleic Acids Res. 1986, 14, 9381–9396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, N.; Lieber, M.R. Bidirectional gene organization: A common architectural feature of the human genome. Cell 2002, 109, 807–809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Frost, A.R.; Blundell, M.P.; Bales, O.; Antoniou, M.N.; Thrasher, A.J. A Ubiquitous Chromatin Opening Element (UCOE) confers resistance to DNA methylation-mediated silencing of lentiviral vectors. Mol. Ther. 2010, 18, 1640–1649. [Google Scholar] [CrossRef]
- Golding, M.C.; Mann, M.R.W. ORIGINAL ARTICLE A bidirectional promoter architecture enhances lentiviral transgenesis in embryonic and extraembryonic stem cells. Gene Ther. 2011, 18, 817–826. [Google Scholar] [CrossRef]
- Zhou, Y.; Aran, J.; Gottesman, M.; Pastan, I. Co-expression of human adenosine deaminase and multidrug resistance using a bicistronic retroviral vector. Hum. Gene Ther. 1998, 9, 287–289. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Xu, Z.; Ishii-watabe, A.; Uchida, E. IRES-Dependent Second Gene Expression Is Significantly Lower Than Cap-Dependent First Gene Expression in a Bicistronic Vector AND. Mol. Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef]
- Wong, E.-T.; Ngoi, S.-M.; Lee, C. Improved co-expression of multiple genes in vectors containing internal ribosome entry sites (IRESes) from human genes. Gene Ther. 2002. [Google Scholar] [CrossRef] [Green Version]
- Hennecke, M.; Kwissa, M.; Metzger, K.; Oumard, A.; Kröger, A.; Schirmbeck, R.; Reimann, J.; Hauser, H. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res. 2001, 29, 3327–3334. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.R.; Li, L.H.; Park, H.J.; Park, J.H.; Lee, K.Y.; Kim, M.K.; Shin, B.A.; Choi, S.Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoi, S.M.; Chien, A.C.; Lee, C.G.L. Exploiting Internal Ribosome Entry Sites in Gene Therapy Vector Design. Curr. Gene Ther. 2004, 4, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.D.; Lynch, G.S. Plasmid-based gene transfer in mouse skeletal muscle by electroporation. Methods Mol. Biol. 2008, 433, 15–125. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, H.J.; Kim, J.M.; Lee, Y.S.; Kim, K.L.; Kim, J.A.; Lee, J.Y.; Suh, W.; Choi, J.H.; Jeon, E.S.; et al. A novel ex vivo angiogenesis assay based on electroporation-mediated delivery of naked plasmid DNA to skeletal muscle. Mol. Ther. 2004, 9, 464–474. [Google Scholar] [CrossRef]
- Boldyreva, M.A.; Shevchenko, E.K.; Molokotina, Y.D.; Makarevich, P.I.; Beloglazova, I.B.; Zubkova, E.S.; Dergilev, K.V.; Tsokolaeva, Z.I.; Penkov, D.; Hsu, M.N.; et al. Transplantation of adipose stromal cell sheet producing hepatocyte growth factor induces pleiotropic effect in ischemic skeletal muscle. Int. J. Mol. Sci. 2019, 20, 3088. [Google Scholar] [CrossRef] [Green Version]
- Makarevich, P.I.; Dergilev, K.V.; Tsokolaeva, Z.I.; Gluhanyuk, E.V.; Parfyonova, Y.E.V. Combined gene delivery of VEGF and HGF induces angiogenesis and proliferation in ischemic rat myocardium. Eur. J. Heart Fail. 2013, 12, 44. [Google Scholar]
- Licursi, M.; Christian, S.L.; Pongnopparat, T.; Hirasawa, K. In vitro and in vivo comparison of viral and cellular internal ribosome entry sites for bicistronic vector expression. Gene Ther. 2011. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.C.; Tretiakova, M.S.; Nallasura, V.; Jagadeeswaran, R.; Husain, A.N. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: Implications for tumour invasion. Br. J. Cancer 2007, 97, 368–377. [Google Scholar] [CrossRef]
- Yamamoto, K.; Morishita, R.; Hayashi, S.; Matsushita, H.; Nakagami, H.; Moriguchi, A.; Matsumoto, K.; Nakamura, T. Contribution of Bcl-2, but Not Bcl-xL and Bax, to Antiapoptotic Actions of Hepatocyte Growth Factor in Hypoxia-Conditioned Human Endothelial Cells. Cell 2001, 37, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Barć, P.; Antkiewicz, M.; Śliwa, B.; Baczyńska, D.; Witkiewicz, W.; Skóra, J.P. Treatment of Critical Limb Ischemia by pIRES/VEGF165/HGF Administration. Ann. Vasc. Surg. 2019, 60, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, P.; Kelly, J.F.; Balazsi, K.; Slack, R.S.; Megeney, L.A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 11025–11030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svandova, E.; Vesela, B.; Tucker, A.S.; Matalova, E. Activation of pro-apoptotic caspases in non-apoptotic cells during odontogenesis and related osteogenesis. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Connolly, P.F.; Jäger, R.; Fearnhead, H.O. New roles for old enzymes: Killer caspases as the engine of cell behavior changes. Front. Physiol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Krieg, A. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 2000, 12, 35–43. [Google Scholar] [CrossRef]
- Häcker, G.; Redecke, R.; Häcker, H. Activation of the immune system by bacterial CpG-DNA. Immunology 2002, 105, 245–251. [Google Scholar] [CrossRef]
- Paludan, S.R. Activation and Regulation of DNA-Driven Immune Responses. Microbiol. Mol. Biol. Rev. 2015, 79, 225–241. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef]
- Mukwaya, A.; Peebo, B.; Xeroudaki, M.; Ali, Z.; Lennikov, A.; Jensen, L.; Lagali, N. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Sci. Rep. 2016, 6, 32137. [Google Scholar] [CrossRef] [Green Version]
- Bosnjak, M.; Jesenko, T.; Kamensek, U.; Sersa, G.; Lavrencak, J.; Heller, L.; Cemazar, M. Electrotransfer of different control plasmids elicits different antitumor effectiveness in B16.F10 melanoma. Cancers 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.A.; Budker, V. The mechanism of naked DNA uptake and expression. Adv. Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Rostovtseva, A.; Semina, E.; Klimovich, P.; Balabanyan, V.; Makarevich, P.; Popov, V.; Stambolsky, D.; Tkachuk, V. A bicistronic plasmid encoding brain-derived neurotrophic factor and urokinase plasminogen activator stimulates peripheral nerve regeneration after injury. J. Pharmacol. Exp. Ther. 2020, 372, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Golocheikine, A.; Tiriveedhi, V.; Angaswamy, N.; Benshoff, N.; Sabarinathan, R.; Mohanakumar, T. Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 2010, 90, 725–731. [Google Scholar] [CrossRef]
- Taher, T.; Derksen, P.; Boer, O.; Spaargaren, M.; Teeling, P.; Wal, A.; Pals, S. Hepatocyte growth factor triggers signaling cascades mediating vascular smooth muscle cell migration. Biochem. Biophys. Res. Commun. 2002, 298, 80–86. [Google Scholar] [CrossRef]
- Ma, H.; Calderon, T.; Kessel, T.; Ashton, A.; Berman, J. Mechanisms of hepatocyte growth factor-mediated vascular smooth muscle cell migration. Circ. Res. 2003, 93, 1066–1073. [Google Scholar] [CrossRef] [Green Version]
- Boldyreva, M.; Bondar, I.V.; Stafeev, I.S.; Makarevich, P.I.; Beloglazova, I.B.; Zubkova, E.S.; Shevchenko, E.K.; Molokotina, Y.D.; Karagyaur, M.N.; Ratner, I.; et al. Plasmid-based gene therapy with hepatocyte growth factor stimulates peripheral nerve regeneration after traumatic injury. Biomed. Pharmacother. 2018, 101, 682–690. [Google Scholar] [CrossRef]
- Kibbe, M.; Hirsch, A.; Mendelsohn, F.; Davies, M.; Pham, H.; Saucedo, J.; Marston, W.; Pyun, W.; Min, S.; Peterson, B.; et al. Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Ther. 2016, 23, 306–312. [Google Scholar] [CrossRef]
- Kessler, J.A.; Smith, A.G.; Cha, B.-S.; Choi, S.H.; Wymer, J.; Shaibani, A.; Ajroud-Driss, S.; Vinik, A. Double-blind, placebo-controlled study of HGF gene therapy in diabetic neuropathy. Ann. Clin. Transl. Neurol. 2015, 2, 465–478. [Google Scholar] [CrossRef]
- Gene Therapy Phase 3 Trial for Painful Diabetic Peripheral Neuropathy Using Engensis (or VM202), a Plasmid DNA Engineered to Express Human HGF Proteins by Simple Intramuscular Injections. n.d. Available online: https://pipelinereview.com/index.php/2020021973821/DNA-RNA-and-Cells/Gene-Therapy-Phase-3-Trial-for-Painful-Diabetic-Peripheral-Neuropathy-Using-Engensis-or-VM202-a-plasmid-DNA-engineered-to-express-human-HGF-proteins-by-simple-intramusc.html (accessed on 19 February 2020).
- David, R.M.; Doherty, A.T. Viral Vectors: The Road to Reducing Genotoxicity. Toxicol. Sci. 2016, 155, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Flotte, T.R. Moving Forward After Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum. Gene Ther. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I. Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 2019, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodkina, E.; Boldyreva, M.; Karagyaur, M.; Eremichev, R.; Alexandrushkina, N.; Balabanyan, V.; Akopyan, Z.; Parfyonova, Y.; Tkachuk, V.; Makarevich, P. Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle. Pharmaceutics 2020, 12, 1231. https://doi.org/10.3390/pharmaceutics12121231
Slobodkina E, Boldyreva M, Karagyaur M, Eremichev R, Alexandrushkina N, Balabanyan V, Akopyan Z, Parfyonova Y, Tkachuk V, Makarevich P. Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle. Pharmaceutics. 2020; 12(12):1231. https://doi.org/10.3390/pharmaceutics12121231
Chicago/Turabian StyleSlobodkina, Ekaterina, Maria Boldyreva, Maxim Karagyaur, Roman Eremichev, Natalia Alexandrushkina, Vadim Balabanyan, Zhanna Akopyan, Yelena Parfyonova, Vsevolod Tkachuk, and Pavel Makarevich. 2020. "Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle" Pharmaceutics 12, no. 12: 1231. https://doi.org/10.3390/pharmaceutics12121231






