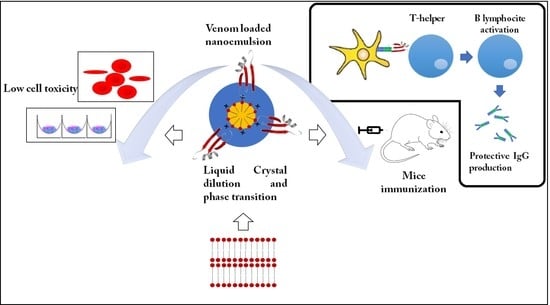
Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Nanoemulsions and Liquid Crystals
2.2.1. Preparation of Emulsions
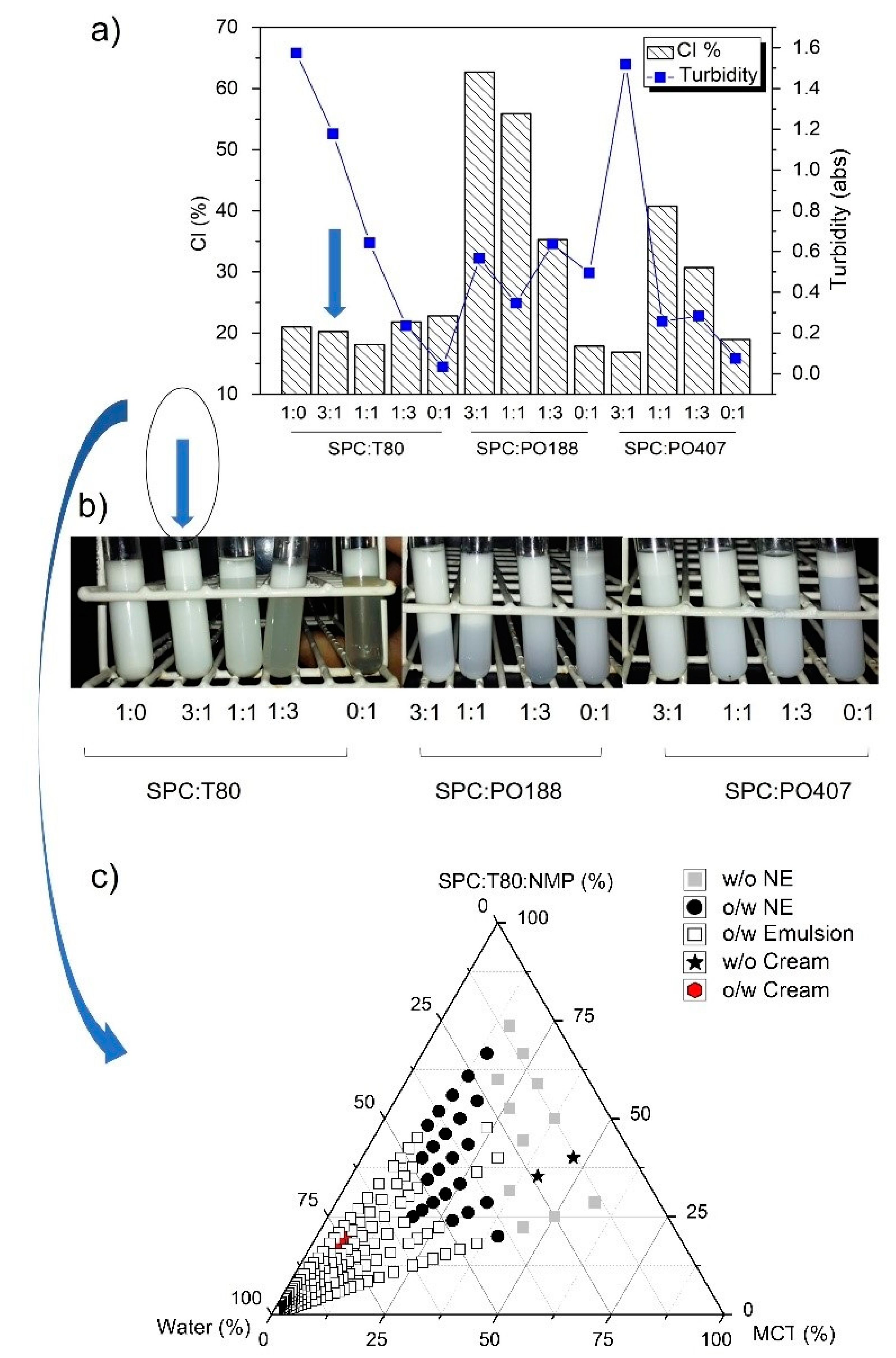
2.2.2. Pseudo-Ternary Phase Diagrams (PTPDs)
2.2.3. Droplet Size and Zeta Potential Measurements and Identification of Liquid Crystals
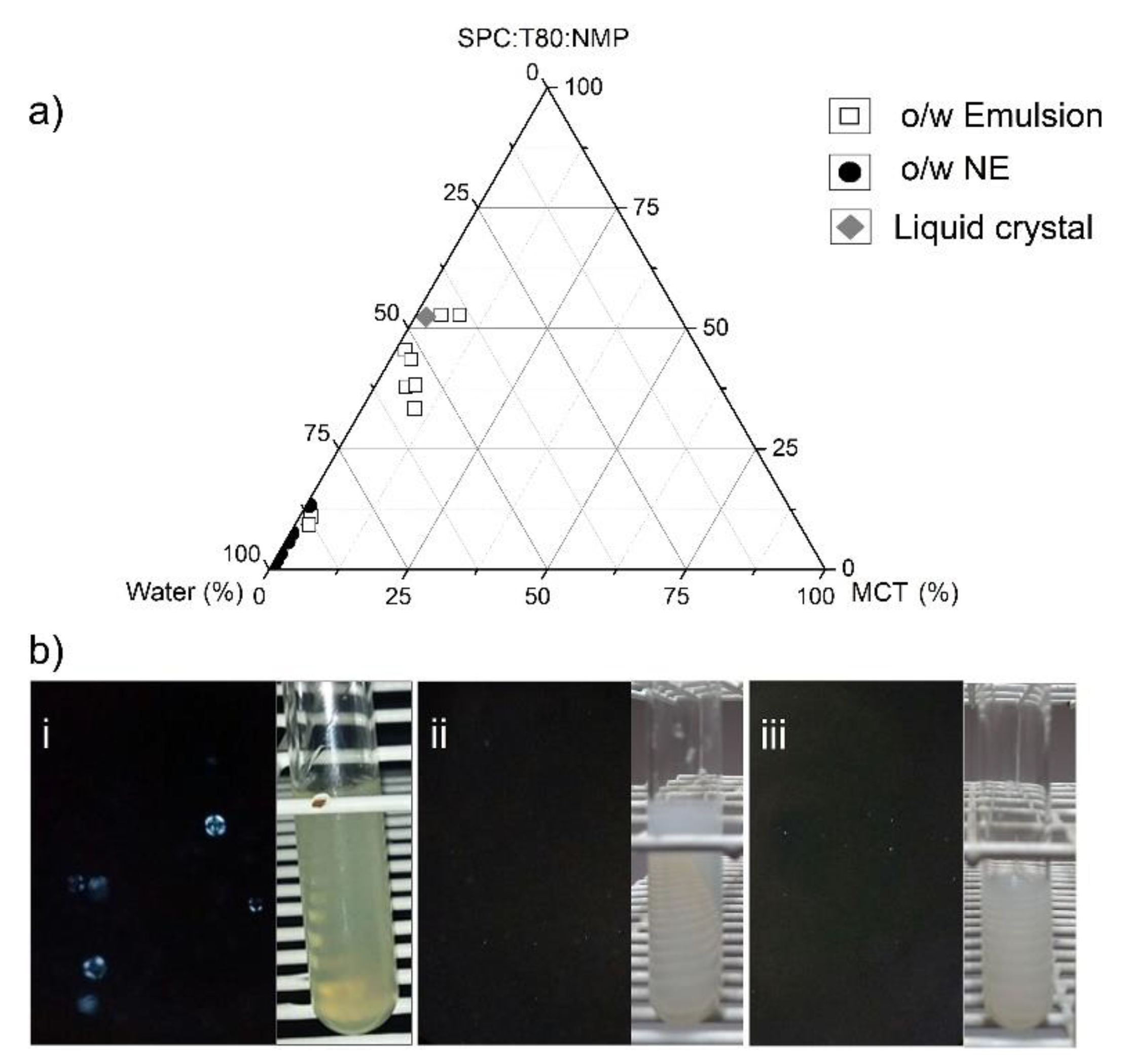
2.2.4. Polarized Light Microscopy (PLM) and Identification of Liquid Crystals
2.2.5. Preparation of Cationic Hyper-Branched Poly(Ethyleneimine) (PEI) Covered Nanoemulsions
2.3. Physical Stability of NE Formulations Against pH and Saline Content
2.4. Preparation of TsV-Loaded Nanoemulsions
2.5. Fourier Transformed Infrared Spectroscopy (FTIR)
2.6. Cell Viability and Hemolysis Tests
2.6.1. Cell Culture
2.6.2. MTT Reduction Assay
2.6.3. Hemolysis Test
2.7. In Vivo Immunization Performance
2.7.1. Animals
2.7.2. Immunization Protocol
2.7.3. IgG Titration by ELISA
2.8. Statistical
3. Results
3.1. Design of Nanoemulsions and Liquid Crystal
3.2. Physicochemical Properties of Anionic and Cationic NE Formulations
3.3. Physical Stability of NE Formulations Against pH and Saline Content
3.4. In Vitro Cell Viability and Hemolytic Tests
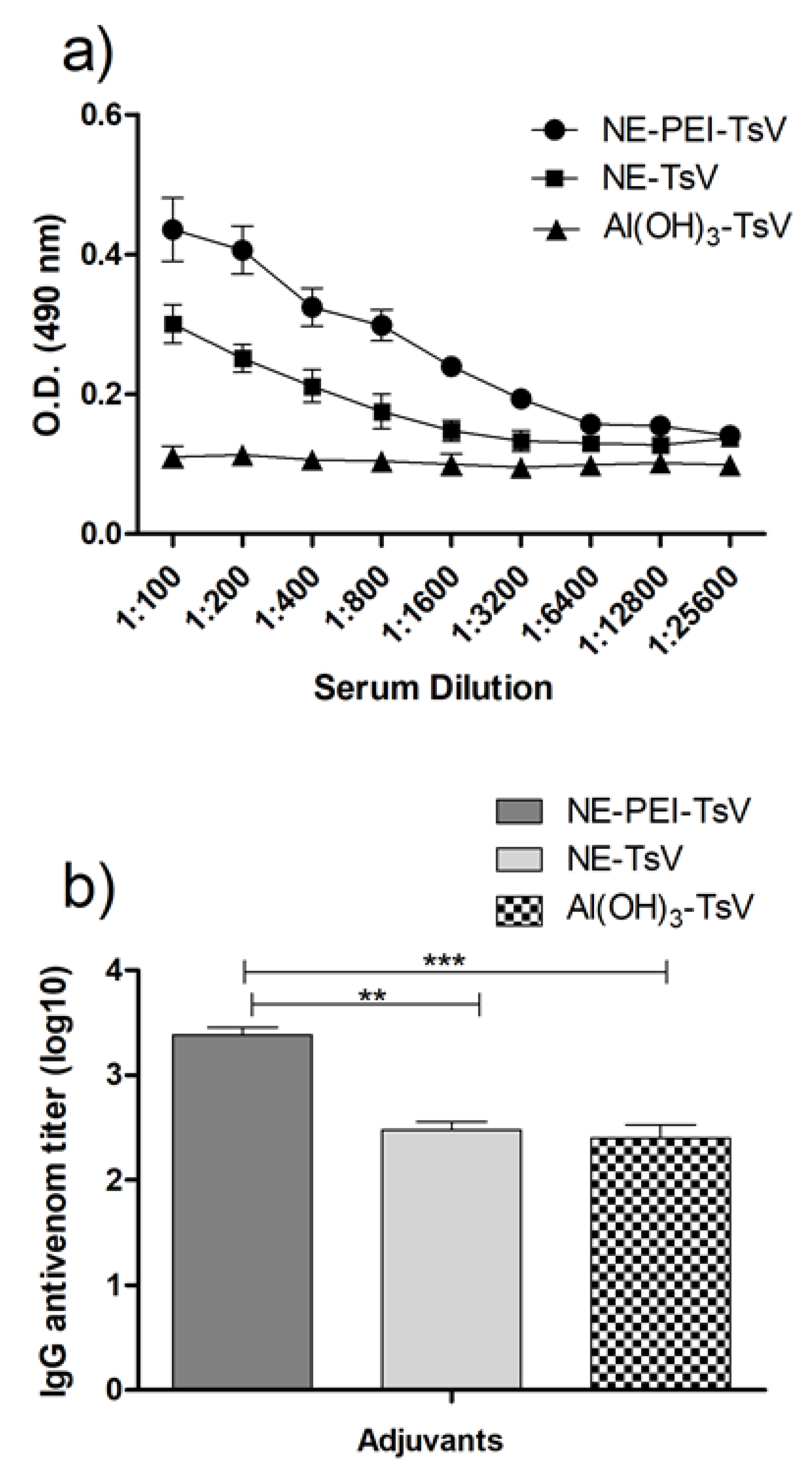
3.5. Antivenom IgG Titration
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bucaretchi, F.; Fernandes, L.C.R.; Fernandes, C.B.; Branco, M.M.; Prado, C.C.; Vieira, R.J.; De Capitani, E.M.; Hyslop, S. Clinical consequences of Tityus bahiensis and Tityus serrulatus scorpion stings in the region of Campinas, southeastern Brazil. Toxicon 2014, 89, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.L.A.; Neto, E.B.; de Freitas, L.A.; Dorce, V.A.C. Effects of Brazilian scorpion venoms on the central nervous system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Eauclaire, M.-F.; Pimenta, A.M.C.; Bougis, P.E.; De Lima, M.-E. Potassium channel blockers from the venom of the Brazilian scorpion Tityus serrulatus (Lutz and Mello, 1922). Toxicon 2016, 119, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.G.; Longhim, H.T.; Cologna, C.T.; Degueldre, M.; De Pauw, E.; Quinton, L.; Arantes, E.C. Proteome of fraction from Tityus serrulatus venom reveals new enzymes and toxins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Rates, B.; Ferraz, K.K.F.; Borges, M.H.; Richardson, M.; De Lima, M.E.; Pimenta, A.M.C. Tityus serrulatus venom peptidomics: Assessing venom peptide diversity. Toxicon 2008, 52, 611–618. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Laraba-Djebari, F. Development and characterization of a new carrier for vaccine delivery based on calcium-alginate nanoparticles: Safe immunoprotective approach against scorpion envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef]
- Nouri, A.; Laraba-Djebari, F. Enhancement of long-lasting immunoprotective effect against Androctonus australis hector envenomation using safe antigens: Comparative role of MF59 and Alum adjuvants. Vaccine 2015, 33, 5756–5763. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- WHO. W.H.O. Global Vaccine Action Plan 2011–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Barnowski, C.; Kadzioch, N.; Damm, D.; Yan, H.; Temchura, V. Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics 2019, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccin. Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef] [Green Version]
- Powell, B.S.; Andrianov, A.K.; Fusco, P.C. Polyionic vaccine adjuvants: Another look at aluminum salts and polyelectrolytes. Clin. Exp. Vaccine Res. 2015, 4, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R.J. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, J.; Smith, D.; Cao, Z.; Acosta, H.; Fan, Y.; Ciotti, S.; Fattom, A.; Baker, J. A novel combination of intramuscular vaccine adjuvants, nanoemulsion and CpG produces an effective immune response against influenza A virus. Vaccine 2020, 38, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Ahmad, M.Z.; Syed, R.; Alsenaidy, M.A.; Albraiki, S.A. Lignin nanoparticles as a promising vaccine adjuvant and delivery system for ovalbumin. Int. J. Biol. Macromol. 2020, 163, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Sunay, M.M.E.; Martins, K.A.O.; Steffens, J.T.; Gregory, M.; Vantongeren, S.A.; Van Hoeven, N.; Garnes, P.G.; Bavari, S. Glucopyranosyl lipid adjuvant enhances immune response to Ebola virus-like particle vaccine in mice. Vaccine 2019, 37, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhu, Z.; Ma, L.; Li, Y. O/W Nanoemulsion as an Adjuvant for an Inactivated H3N2 Influenza Vaccine: Based on Particle Properties and Mode of Carrying. Int. J. Nanomed. 2020, 15, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Wong, P.T.; Leroueil, P.R.; Smith, D.M.; Ciotti, S.; Bielinska, A.U.; Janczak, K.W.; Mullen, C.H.; Groom, J.V., II; Taylor, E.M.; Passmore, C.; et al. Formulation, High Throughput In Vitro Screening and In Vivo Functional Characterization of Nanoemulsion-Based Intranasal Vaccine Adjuvants. PLoS ONE 2015, 10, e0126120. [Google Scholar] [CrossRef] [Green Version]
- Stelzner, J.J.; Behrens, M.; Behrens, S.-E.; Mader, K. Squalene containing solid lipid nanoparticles, a promising adjuvant system for yeast vaccines. Vaccine 2018, 36, 2314–2320. [Google Scholar] [CrossRef]
- Cao, W.; Davis, W.G.; Kim, J.H.; De La Cruz, J.A.; Taylor, A.; Hendrickson, G.R.; Kumar, A.; Ranjan, P.; Lyon, L.A.; Katz, J.M.; et al. An oil-in-water nanoemulsion enhances immunogenicity of H5N1 vaccine in mice. Nanomedicine 2016, 12, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Myc, A.; Kukowska-Latallo, J.F.; Smith, D.M.; Passmore, C.; Pham, T.; Wong, P.; Bielinska, A.U.; Baker, J.R., Jr. Nanoemulsion nasal adjuvant W₈₀5EC induces dendritic cell engulfment of antigen-primed epithelial cells. Vaccine 2013, 31, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Streck, L.; Sarmento, V.H.V.; de Menezes, R.P.R.P.B.; Fernandes-Pedrosa, M.F.; Martins, A.M.C.; da Silva-Júnior, A.A. Tailoring microstructural, drug release properties, and antichagasic efficacy of biocompatible oil-in-water benznidazol-loaded nanoemulsions. Int. J. Pharm. 2019, 555, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.L.; Taira, T.C.; Di Filippo, L.D.; Fonseca-Santos, B.; Pinto, M.C.; Chorilli, M. Novel bioadhesive polycarbophil-based liquid crystal systems containing Melaleuca alternifolia oil as potential repellents against Aedes aegypti. J. Mol. Liq. 2020, 314, 113626. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.C.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Somwongin, S.; Marsup, P.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C. Dermal Delivery Enhancement of Natural Anti-Ageing Compounds from Ocimum sanctum Linn. Extract by Nanostructured Lipid Carriers. Pharmaceutics 2020, 12, 309. [Google Scholar] [CrossRef] [Green Version]
- Yukuyama, M.N.; Kato, E.T.M.; de Araujo, G.L.B.; Löbenberg, R.; Monteiro, L.M.; Lourenço, F.R.; Bou-Chacra, N.A. Olive oil nanoemulsion preparation using high-pressure homogenization and d-phase emulsification—A design space approach. J. Drug Deliv. Sci. Technol. 2019, 49, 622–631. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Tong, K.; Zhao, C.; Sun, D. Formation of nanoemulsion with long chain oil by W/O microemulsion dilution method. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 497, 101–108. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Wang, Y.; Liu, T.; Gu, X. Microstructural transitions in β-carotene loaded nonionic microemulsions upon aqueous phase dilution. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 567, 288–296. [Google Scholar] [CrossRef]
- Streck, L.; Sarmento, V.; Machado, P.; Farias, K.; Fernandes-Pedrosa, M.; da Silva-Júnior, A. Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading. Int. J. Mol. Sci. 2016, 17, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Feng, J.; Rodríguez-Abreu, C.; Esquena, J.; Solans, C. A Concise Review on Nano-emulsion Formation by the Phase Inversion Composition (PIC) Method. J. Surfactants Deterg. 2020, 23. [Google Scholar] [CrossRef]
- Jafari, S.M.; Paximada, P.; Mandala, I.; Assadpour, E.; Mehrnia, M.A. Nanoencapsulation Technologies for the Food and Nutraceutical Industries, Chapter 2—Encapsulation by Nanoemulsions; Adadmic Press: Cambridge, MA, USA, 2017; pp. 36–73. [Google Scholar]
- Perazzo, A.; Preziosi, V. Nanoemulsions, Chapter 3—Catastrophic Phase Inversion Techniques for Nanoemulsification; Adadmic Press: Cambridge, MA, USA, 2018; pp. 36–73. [Google Scholar]
- Lefebvre, G.; Riou, J.; Bastiat, G.; Roger, E.; Frombach, K.; Gimel, J.-C.; Saulnier, P.; Calvignac, B. Spontaneous nano-emulsification: Process optimization and modeling for the prediction of the nanoemulsion’s size and polydispersity. Int. J. Pharm. 2017, 534, 220–228. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Lara-Sanchez, A.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol. Food Chem. 2020, 328, 127131. [Google Scholar] [CrossRef]
- Gupta, R.; Tandon, A.; Hansen, E.T.; Cebulko, T.C.; Hemmat, Y.J.; Fortune, J.A.; Klibanov, A.M.; Mohan, R.R. Rapid And Substantial Gene Delivery Into Cornea In Vivo And In Vitro With Linearized Polyethyleneimine Nanoparticles. Investig. Ophtalmol. Vis. Sci. 2011, 52, 494. [Google Scholar]
- Mukalel, A.J.; Riley, R.S.; Zhang, R.; Mitchell, M.J. Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy. Cancer Lett. 2019, 458, 102–112. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions by spontaneous emulsification: Effect of propylene glycol and ethanol on formation, stability, and properties. Food Res. Int. 2013, 54, 812–820. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Da Silva, G.B.R.F.; Scarpa, M.V.; Rossanezi, G.; do Egito, E.S.T.; de Oliveira, A.G. Development and characterization of biocompatible isotropic and anisotropic oil-in-water colloidal dispersions as a new delivery system for methyl dihydrojasmonate antitumor drug. Int. J. Nanomed. 2014, 9, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Si, L.; Zhai, X.; Fan, Z.; Ma, Y.; Zhang, R.; Yang, X. The influence of co-solvents on the stability and bioavailability of rapamycin formulated in self-microemulsifying drug delivery systems. Drug Dev. Ind. Pharm. 2011, 37, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Herz, E.; McClements, D.J.; Weiss, J. Impact of alcohols on the formation and stability of protein-stabilized nanoemulsions. J. Colloid Interface Sci. 2014, 433, 196–203. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, A.S.A.; Zoppi, A.; Barbosa, E.G.; Oliveira, J.I.N.; Fernandes-Pedrosa, M.F.; Longhi, M.R.; da Silva-Júnior, A.A. Supramolecular aggregates of oligosaccharides with co-solvents in ternary systems for the solubilizing approach of triamcinolone. Carbohydr. Polym. 2016, 151. [Google Scholar] [CrossRef]
- Solè, I.; Maestro, A.; González, C.; Solans, C.; Gutiérrez, J.M. Optimization of Nano-emulsion Preparation by Low-Energy Methods in an Ionic Surfactant System. Langmuir 2006, 22, 8326–8332. [Google Scholar] [CrossRef]
- Maestro, A.; Sole, I.; Gonzalez, C.; Solans, C.; Gutierrez, J.M. Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method. J. Colloid Interface Sci. 2008, 327, 433–439. [Google Scholar] [CrossRef]
- Rocha Soares, K.S.; Cardozo Fonseca, J.L.; Oliveira Bitencourt, M.A.; Santos, K.S.C.R.; Silva, A.A.J.; Fernandes-Pedrosa, M.F. Serum production against Tityus serrulatus scorpion venom using cross-linked chitosan nanoparticles as immunoadjuvant. Toxicon 2012, 60, 1349–1354. [Google Scholar] [CrossRef]
- Rocha Soares, K.S.; Oliveira, A.R.; Daniele-Silva, A.; Glaucia-Silva, F.; Caroni, A.L.P.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Self-assembled scorpion venom proteins cross-linked chitosan nanoparticles for use in the immunotherapy. J. Mol. Liq. 2017, 241, 540–548. [Google Scholar] [CrossRef]
- Soares, K.S.R.; Glaucia-Silva, F.; Daniele-Silva, A.; Torres-Rego, M.; de Araujo, N.K.; de Menezes, Y.A.S.; Damasceno, I.Z.; Tambourgi, D.V.; da Silva-Junior, A.A.; Fernandes-Pedrosa, M.d.F. Antivenom Production against Bothrops jararaca and Bothrops erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant. Toxins 2018, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaucia-Silva, F.; Torres-Rego, M.; Rocha Soares, K.S.; Damasceno, I.Z.; Tambourgi, D.V.; da Silva-Junior, A.A.; Fernandes-Pedrosa, M.d.F. A biotechnological approach to immunotherapy: Antivenom against Crotalus durissus cascavella snake venom produced from biodegradable nanoparticles. Int. J. Biol. Macromol. 2018, 120, 1917–1924. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-Y.; Feng, M.-Q.; Wang, M.; Wang, P.; Sun, J.; Xu, X.-L.; Zhou, G.-H. Influence of flaxseed gum and NaCl concentrations on the stability of oil-in-water emulsions. Food Hydrocoll. 2018, 79, 371–381. [Google Scholar] [CrossRef]
- Dewey, D.C.; Strulson, C.A.; Cacace, D.N.; Bevilacqua, P.C.; Keating, C.D. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat. Commun. 2014, 5, 4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, P.C.; dos Santos-Silva, E.; Streck, L.; Damasceno, I.Z.; Maia, A.M.S.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Cationic functionalized biocompatible polylactide nanoparticles for slow release of proteins. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 442–451. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, B.; Zhu, Y.; Lan, F.; Wu, Y.; Gu, Z. Conformational changes of adsorbed and free proteins on magnetic nanoclusters. Colloids Surf. B Biointerfaces 2018, 170, 664–672. [Google Scholar] [CrossRef]
- Van de Weert, M.; Haris, P.I.; Hennink, W.E.; Crommelin, D.J.A. Fourier Transform Infrared Spectrometric Analysis of Protein Conformation: Effect of Sampling Method and Stress Factors. Anal. Biochem. 2001, 297, 160–169. [Google Scholar] [CrossRef]
- Almeida, C.P.; Vital, C.G.; Contente, T.C.; Maria, D.A.; Maranhao, R.C. Modification of composition of a nanoemulsion with different cholesteryl ester molecular species: Effects on stability, peroxidation, and cell uptake. Int. J. Nanomed. 2010, 5, 679–686. [Google Scholar]
- Chang, H.-B.; Chen, B.-H. Inhibition of lung cancer cells A549 and H460 by curcuminoid extracts and nanoemulsions prepared from Curcuma longa Linnaeus. Int. J. Nanomed. 2015, 10, 5059–5080. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Hung, C.-F.; Chen, B.-H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouri, A.; Diat, O.; Lerner, D.A.; Ghzaoui, A.E.; Ajovalasit, A.; Dorandeu, C.; Maurel, J.-C.; Devoisselle, J.-M.; Legrand, P. Water solubilization capacity of pharmaceutical microemulsions based on Peceol®, lecithin and ethanol. Int. J. Pharm. 2014, 475, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, M.M.; Sampaio, S.V.; Lopes, R.A.; Mancuso, L.C.; Cunha, O.A.B.; Franco, J.J.; Giglio, J.R. Biochemical and histopathological alterations induced in rats by Tityus serrulatus scorpion venom and its major neurotoxin tityustoxin-I. Toxicon 1997, 35, 1053–1067. [Google Scholar] [CrossRef]
- Bertazzi, D.T.; Isabel de Assis-Pandochi, A.; Elisa Caleiro Seixas Azzolini, A.; Talhaferro, V.L.; Lazzarini, M.; Arantes, E.C. Effect of Tityus serrulatus scorpion venom and its major toxin, TsTX-I, on the complement system in vivo. Toxicon 2003, 41, 501–508. [Google Scholar] [CrossRef]
- Venancio, E.J.; Portaro, F.C.V.; Kuniyoshi, A.K.; Carvalho, D.C.; Pidde-Queiroz, G.; Tambourgi, D.V. Enzymatic properties of venoms from Brazilian scorpions of Tityus genus and the neutralisation potential of therapeutical antivenoms. Toxicon 2013, 69, 180–190. [Google Scholar] [CrossRef]
- Verdier, F.; Burnett, R.; Michelet-Habchi, C.; Moretto, P.; Fievet-Groyne, F.; Sauzeat, E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine 2005, 23, 1359–1367. [Google Scholar] [CrossRef]
- Morefield, G.L.; Sokolovska, A.; Jiang, D.; HogenEsch, H.; Robinson, J.P.; Hem, S.L. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 2005, 23, 1588–1595. [Google Scholar] [CrossRef]
- Azmi, F.; Ahmad Fuaad, A.A.H.; Skwarczynski, M.; Toth, I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccines Immunother. 2014, 10, 778–796. [Google Scholar] [CrossRef] [Green Version]


| Ingredient Amount | Formulations | ||||
|---|---|---|---|---|---|
| Gel-Like System | Blank Anionic NE | Tsv-Loaded Anionic NE | Blank Cationic NE | Tsv-Loaded Cationic NE | |
| TsV (µg/mL) | 0.0 | 0.0 | 18.0 | 0.0 | 18.0 |
| MCT (% w/w) | 2.00 | 0.9 | 0.9 | 0.9 | 0.9 |
| T80 (% w/w) | 4.55 | 2.0 | 2.0 | 2.0 | 2.0 |
| SPC (% w/w) | 13.65 | 6.0 | 6.0 | 6.0 | 6.0 |
| NMP (% w/w) | 34.2 | 15 | 15 | 15 | 15 |
| PEI (% w/w) | 0.0 | 0.0 | 0.0 | 0.02 | 0.02 |
| Water (% w/w) | 45.6 | 76.1 | 76.1 | 76.08 | 76.08 |
| Formulation | Mean Size | Pdi | Zeta Potential (Mv) |
|---|---|---|---|
| NE | 125.7 ± 0.3 | 0.13 ± 0.03 | −18.3 ± 1.0 |
| TsV-loaded NE | 126.3 ± 1.3 | 0.13 ± 0.01 | −13.3 ± 0.6 |
| NE-PEI | 165.2 ± 0.5 | 0.13 ± 0.01 | 8.4 ± 1.4 |
| TsV-loaded NE-PEI | 167.1 ± 0.5 | 0.13 ± 0.01 | 5.04 ± 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.S.A.d.; Torres-Rêgo, M.; Lacerda, A.F.; Rocha, H.A.O.; Egito, E.S.T.d.; Cornélio, A.M.; Tambourgi, D.V.; Fernandes-Pedrosa, M.d.F.; da Silva-Júnior, A.A. Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom. Pharmaceutics 2020, 12, 927. https://doi.org/10.3390/pharmaceutics12100927
Medeiros ASAd, Torres-Rêgo M, Lacerda AF, Rocha HAO, Egito ESTd, Cornélio AM, Tambourgi DV, Fernandes-Pedrosa MdF, da Silva-Júnior AA. Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom. Pharmaceutics. 2020; 12(10):927. https://doi.org/10.3390/pharmaceutics12100927
Chicago/Turabian StyleMedeiros, Arthur Sérgio Avelino de, Manoela Torres-Rêgo, Ariane Ferreira Lacerda, Hugo Alexandre Oliveira Rocha, Eryvaldo Sócrates Tabosa do Egito, Alianda Maira Cornélio, Denise V. Tambourgi, Matheus de Freitas Fernandes-Pedrosa, and Arnóbio Antônio da Silva-Júnior. 2020. "Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom" Pharmaceutics 12, no. 10: 927. https://doi.org/10.3390/pharmaceutics12100927
APA StyleMedeiros, A. S. A. d., Torres-Rêgo, M., Lacerda, A. F., Rocha, H. A. O., Egito, E. S. T. d., Cornélio, A. M., Tambourgi, D. V., Fernandes-Pedrosa, M. d. F., & da Silva-Júnior, A. A. (2020). Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom. Pharmaceutics, 12(10), 927. https://doi.org/10.3390/pharmaceutics12100927