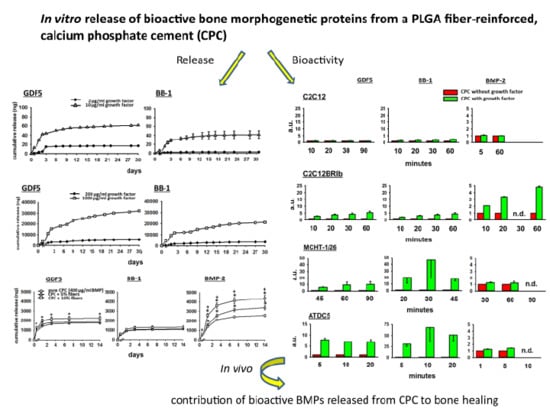
In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of PLGA Fibers and PLGA Fiber-Reinforced Cement
2.2. Production of Recombinant GDF5, BB-1, and BMP-2
2.3. Release of Low-Dose GDF5 and BB-1 from the CPC
2.4. Release of High-Dose GDF5 and BB-1 from the CPC
2.5. Release of GDF5, BB-1, and BMP-2 from PLGA Fiber-Reinforced CPC
2.6. Alkaline Phosphatase (ALP) Activity of the C2C12, C2C12BRlb, MCHT -1/26, and ATDC-5 Cell Lines Following Exposure to the Extracts of BMP-Loaded, PLGA Fiber-Reinforced CPC
2.7. Isolation of Human Adipose Tissue-Derived Mesenchymal Stem Cells (hASCs)
2.8. Extraction of GDF5 from the CPC, Exposure of hASCS to the Extracts, RNA Isolation, cDNA Synthesis, and RT-PCR
2.9. Protein Extraction from hASCs and Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
3.1. In Vitro Release of GDF5 from the CPC
3.2. In Vitro Release of BB-1 from the CPC
3.3. Influence of PLGA Fibers on the In Vitro Release of BMPs from the CPC
3.3.1. GDF5
3.3.2. BB-1
3.3.3. BMP-2
3.4. Bioactivity of the BMPs Released from the CPC
3.4.1. Fold-Change Effects of GDF5, BB-1, and BMP-2 Extracts on the ALP Activity in the Cell Line C2C12
3.4.2. Fold-Change Effects of GDF5, BB-1, and BMP-2 Extracts on the ALP Activity in the Cell Line C2C12BRIb
3.4.3. Fold-Change Effects of GDF5, BB-1, and BMP-2 Extracts on the ALP Activity in the Cell Line MCHT-1/26
3.4.4. Fold-Change Effects of GDF5, BB-1, and BMP-2 Extracts on the ALP Activity in the Cell Line ATDC5
3.4.5. Effects of GDF5 Extracts on the Gene Expression in hASCs
3.4.6. Effects of GDF5, BB-1, and BMP-2 Extracts on the Protein Expression in hASCs
4. Discussion
4.1. Kinetics of BMP Release
4.2. Differences among the Three BMPs
4.3. Bioactivity of the Released BMPs
4.4. Long-Term Retention of a Depot of Therapeutically Applied BMP
4.5. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wolke, J.G.C.; Jansen, J.A.; Leeuwenburgh, S.C.G. Influence of polymeric additives on the cohesion and mechanical properties of calcium phosphate cements. J. Mater. Sci. Mater. Electron. 2016, 27, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, X.; Feng, S.; Yang, S.; Zeng, R.; Tu, M. The fabrication of biomineralized fiber-aligned PLGA scaffolds and their effect on enhancing osteogenic differentiation of UCMSC cells. J. Mater. Sci. Mater. Electron. 2018, 29, 117. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.; Magri, A.; Kido, H.; Parisi, J.; Assis, L.; Fernandes, K.; Mesquita-Ferrari, R.; Martins, V.; Plepis, A.; Zanotto, E.; et al. Biosilicate/PLGA osteogenic effects modulated by laser therapy: In vitro and in vivo studies. J. Photochem. Photobiol. B Boil. 2017, 173, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Bai, H.; Hu, Q.; Gao, T.; Bai, Y. Enhanced proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts on graphene oxide-impregnated PLGA–gelatin nanocomposite fibrous membranes. RSC Adv. 2017, 7, 8886–8897. [Google Scholar] [CrossRef]
- Naumenko, E.A.; Guryanov, I.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube-biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef] [PubMed]
- Kunisch, E.; Maenz, S.; Knoblich, M.; Ploeger, F.; Jandt, K.D.; Bossert, J.; Kinne, R.W.; Alsalameh, S. Short-time pre-washing of brushite-forming calcium phosphate cement improves its in vitro cytocompatibility. Tissue Cell 2017, 49, 697–710. [Google Scholar] [CrossRef]
- Kunisch, E.G.F.; Wagner, S.; Dees, F.; Maenz, S.; Bossert, J.; Jandt, K.D.; Kinne, R.W. The PGLA fiber component of brushite-forming calcium phosphate cement induces the osteogenic differentiation of human adipose tissue-derived stem cells. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef]
- Maenz, S.; Brinkmann, O.; Kunisch, E.; Horbert, V.; Gunnella, F.; Bischoff, S.; Schubert, H.; Sachse, A.; Xin, L.; Günster, J.; et al. Enhanced bone formation in sheep vertebral bodies after minimally invasive treatment with a novel, PLGA fiber-reinforced brushite cement. Spine J. 2017, 17, 709–719. [Google Scholar] [CrossRef]
- Bungartz, M.; Kunisch, E.; Maenz, S.; Horbert, V.; Xin, L.; Gunnella, F.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. GDF5 significantly augments the bone formation induced by an injectable, PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1685–1698. [Google Scholar] [CrossRef]
- Gunnella, F.; Kunisch, E.; Maenz, S.; Horbert, V.; Xin, L.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; Sachse, A.; et al. The GDF5 mutant BB-1 enhances the bone formation induced by an injectable, poly(l-lactide-co-glycolide) acid (PLGA) fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2018, 18, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Gunnella, F.; Kunisch, E.; Bungartz, M.; Maenz, S.; Horbert, V.; Xin, L.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. Low-dose BMP-2 is sufficient to enhance the bone formation induced by an injectable, PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Chohayeb, A.; Shulman, A. Apatite calcium phosphates: Possible dental restorative materials. J. Dent. Res. 1982, 61, 343. [Google Scholar]
- Brown, W.E.; Chow, L.C. A new calcium-phosphate setting cement. J. Dent. Res. 1983, 62, 672. [Google Scholar]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Xin, L.; Bungartz, M.; Maenz, S.; Horbert, V.; Hennig, M.; Illerhaus, B.; Günster, J.; Bossert, J.; Bischoff, S.; Borowski, J.; et al. Decreased extrusion of calcium phosphate cement versus high viscosity PMMA cement into spongious bone marrow—An ex vivo and in vivo study in sheep vertebrae. Spine J. 2016, 16, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.H.; Marom, E.M.; Ahrar, K.; Truong, M.T.; Madewell, J.E. Pulmonary Embolism of Polymethyl Methacrylate During Percutaneous Vertebroplasty and Kyphoplasty. Am. J. Roentgenol. 2004, 183, 1097–1102. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Traykova, T.; Planell, J. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Kalteis, T.; Luring, C.; Gugler, G.; Zysk, S.; Caro, W.; Handel, M.; Grifka, J. Acute tissue toxicity of PMMA bone cements. Z. Orthop. Grenzgeb. 2004, 142, 666–672. [Google Scholar] [CrossRef]
- Cho, A.-R.; Kim, H.-K.; Kwon, J.-Y.; Kim, T.-K.; Choi, Y.-M.; Kim, K.-H. The incorporation of platelet-rich plasma into calcium phosphate cement enhances bone regeneration in osteoporosis. Pain Physician 2014, 17, 737–745. [Google Scholar]
- Lee, J.H.; Lee, D.-O.; Lee, J.-H.; Lee, H.-S. Comparison of radiological and clinical results of balloon kyphoplasty according to anterior height loss in the osteoporotic vertebral fracture. Spine J. 2014, 14, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Maestretti, G.; Sutter, P.; Monnard, E.; Ciarpaglini, R.; Wahl, P.; Hoogewoud, H.; Gautier, E. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur. Spine J. 2014, 23, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Hirano, N.; Zukawa, M.; Suzuki, K.; Hirose, J.; Kimura, T.; Kawaguchi, Y. Vertebroplasty Using Calcium Phosphate Cement for Osteoporotic Vertebral Fractures: Study of Outcomes at a Minimum Follow-up of Two Years. Asian Spine J. 2012, 6, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zaryanov, A.V.; Park, D.K.; Khalil, J.G.; Baker, K.C.; Fischgrund, J.S. Cement augmentation in vertebral burst fractures. Neurosurg. Focus 2014, 37, E5. [Google Scholar] [CrossRef] [PubMed]
- Blattert, T.R.; Jestaedt, L.; Weckbach, A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: A controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine 2009, 34, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Maenz, S.; Kunisch, E.; Mühlstädt, M.; Böhm, A.; Kopsch, V.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Enhanced mechanical properties of a novel, injectable, fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2014, 39, 328–338. [Google Scholar] [CrossRef]
- Maenz, S.; Hennig, M.; Mühlstädt, M.; Kunisch, E.; Bungartz, M.; Brinkmann, O.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Effects of oxygen plasma treatment on interfacial shear strength and post-peak residual strength of a PLGA fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2016, 57, 347–358. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Kwiatkowski, W.; Gray, P.C.; Choe, S. Engineering TGF-beta superfamily ligands for clinical applications. Trends Pharmacol. Sci. 2014, 35, 648–657. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Reddi, A.H.; Huggins, C. Biochemical Sequences in the Transformation of Normal Fibroblasts in Adolescent Rats. Proc. Natl. Acad. Sci. USA 1972, 69, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.; Rosen, V.; Celeste, A.; Mitsock, L.; Whitters, M.; Kriz, R.; Hewick, R.; Wang, E. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V.; Wozney, J.M.; Wang, E.A.; Cordes, P.; Celeste, A.; McQuaid, D.; Kurtzberg, L. Purification and molecular cloning of a novel group of BMPs and localization of BMP mRNA in developing bone. Connect. Tissue Res. 1989, 20, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Scarfì, S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J. Stem Cells 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, G.; Zilberman, Y.; Zhou, S.; Kelly, P.; Moutsatsos, I.K.; Kharode, Y.P.; Borella, L.E.; Bex, F.J.; Komm, B.S.; Bodine, P.V.; et al. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J. Cell. Biochem. 2002, 86, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Sarban, S.; Şenköylü, A.; Isikan, U.E.; Korkusuz, P.; Korkusuz, F. Can rhBMP-2 Containing Collagen Sponges Enhance Bone Repair in Ovariectomized Rats?: A Preliminary Study. Clin. Orthop. Relat. Res. 2009, 467, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, X.; Ge, B. Calcium Phosphate Cement with BMP-2-loaded Gelatin Microspheres Enhances Bone Healing in Osteoporosis: A Pilot Study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef]
- Egermann, M.; Baltzer, A.; Adamaszek, S.; Evans, C.; Robbins, P.; Schneider, E.; Lill, C. Direct Adenoviral Transfer of Bone Morphogenetic Protein-2 cDNA Enhances Fracture Healing in Osteoporotic Sheep. Hum. Gene Ther. 2006, 17, 507–517. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Duda, G.N.; Giesecke, M.T.; Dienelt, A.; Schwabe, P. High-dose recombinant human bone morphogenetic protein-2 impacts histological and biomechanical properties of a cervical spine fusion segment: Results from a sheep model. J. Tissue Eng. Regen. Med. 2017, 11, 1514–1523. [Google Scholar] [CrossRef]
- Simic, P.; Culej, J.B.; Orlic, I.; Grgurevic, L.; Draca, N.; Spaventi, R.; Vukicevic, S. Systemically Administered Bone Morphogenetic Protein-6 Restores Bone in Aged Ovariectomized Rats by Increasing Bone Formation and Suppressing Bone Resorption. J. Boil. Chem. 2006, 281, 25509–25521. [Google Scholar] [CrossRef]
- Hustedt, J.W.; Blizzard, D.J. The Controversy Surrounding Bone Morphogenetic Proteins in the Spine: A Review of Current Research. Yale J. Boil. Med. 2014, 87, 549–561. [Google Scholar]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Kasten, P.; Beyen, I.; Bormann, D.; Luginbuhl, R.; Ploger, F.; Richter, W. The effect of two point mutations in GDF-5 on ectopic bone formation in a beta-tricalciumphosphate scaffold. Biomaterials 2010, 31, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Ruhé, P.Q.; Boerman, O.C.; Russel, F.G.M.; Mikos, A.G.; Spauwen, P.H.M.; Jansen, J.A. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J. Mater. Sci. Mater. Electron. 2006, 17, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Van de Watering, F.C.; Molkenboer-Kuenen, J.D.; Boerman, O.C.; van den Beucken, J.J.; Jansen, J.A. Differential loading methods for BMP-2 within injectable calcium phosphate cement. J. Control. Release 2012, 164, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.; Boerman, O.C.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. Part A 2009, 91, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B. Incorporation of BMP-2 loaded collagen conjugated BCP granules in calcium phosphate cement based injectable bone substitutes for improved bone regeneration. Mater. Sci. Eng. C 2017, 77, 713–724. [Google Scholar] [CrossRef]
- Huang, B.; Wu, Z.; Ding, S.; Yuan, Y.; Liu, C. Localization and promotion of recombinant human bone morphogenetic protein-2 bioactivity on extracellular matrix mimetic chondroitin sulfate-functionalized calcium phosphate cement scaffolds. Acta Biomater. 2018, 71, 184–199. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, R.; Gong, Y.; Wang, M.; Huang, Q.; Feng, Q.; Yu, B. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics 2017, 7, 1072–1087. [Google Scholar] [CrossRef]
- Kong, X.; Wang, J.; Cao, L.; Yu, Y.; Liu, C. Enhanced osteogenesis of bone morphology protein-2 in 2- N, 6- O -sulfated chitosan immobilized PLGA scaffolds. Colloids Surf. B Biointerfaces 2014, 122, 359–367. [Google Scholar] [CrossRef]
- Lee, E.U.; Lim, H.C.; Hong, J.Y.; Lee, J.S.; Jung, U.W.; Choi, S.H. Bone regenerative efficacy of biphasic calcium phosphate collagen composite as a carrier of rhBMP-2. Clin. Oral Implants Res. 2016, 27, e91–e99. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Haku, K.; Yamanaka, T.; Dai, J.; Takayama, T.; Shohara, R.; Tachi, K.; Ishioka, M.; Hanatani, S.; Karunagaran, S.; et al. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials 2014, 35, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Ohe, J.-Y.; Lee, J.B.; Heo, D.N.; Byun, W.; Bae, H.; Kwon, Y.-D.; Kwon, I.K. Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration. Bone 2014, 59, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hortschansky, P.S.V.; Fahnert, B.; Riesenberg, D. Verfahren zur Herstellung von Dimeren, Biologisch Aktiven Knochenmorphogenetischen Proteinen. Patent Number DE19944626A1, 22 March 2001. [Google Scholar]
- Tsuji, K.; Martin, P.A.; Bussey, D.M. Automation of chromogenic substrate Limulus amebocyte lysate assay method for endotoxin by robotic system. Appl. Environ. Microbiol. 1984, 48, 550–555. [Google Scholar] [PubMed]
- Zhang, H.-X.; Zhang, X.-P.; Xiao, G.-Y.; Hou, Y.; Cheng, L.; Si, M.; Wang, S.-S.; Li, Y.-H.; Nie, L. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater. Sci. Eng. C 2016, 60, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (lactide-co-glycolide) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 47–66. [Google Scholar] [CrossRef]
- Lo, K.W.-H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of Bone Morphogenetic Protein based Surgical Repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]
- Schofer, M.D.; Fuchs-Winkelmann, S.; Gräbedünkel, C.; Wack, C.; Dersch, R.; Rudisile, M.; Wendorff, J.H.; Greiner, A.; Paletta, J.R.J.; Boudriot, U. Influence of Poly(L-Lactic Acid) Nanofibers and BMP-2–Containing Poly(L-Lactic Acid) Nanofibers on Growth and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. World J. 2008, 8, 1269–1279. [Google Scholar] [CrossRef]
- Huber, E.; Pobloth, A.-M.; Bormann, N.; Kolarczik, N.; Schmidt-Bleek, K.; Schell, H.; Schwabe, P.; Duda, G.N.; Wildemann, B. Demineralized Bone Matrix as a Carrier for Bone Morphogenetic Protein-2: Burst Release Combined with Long-Term Binding and Osteoinductive Activity Evaluated In Vitro and In Vivo. Tissue Eng. Part A 2017, 23, 1321–1330. [Google Scholar] [CrossRef]
- Ziegler, J.; Mayr-Wohlfart, U.; Kessler, S.; Breitig, D.; Günther, K.-P. Adsorption and release properties of growth factors from biodegradable implants. J. Biomed. Mater. Res. 2001, 59, 422–428. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Dow, M.; Gomez, J.; Soulvie, M.; Tsiagalis, G. The in vitro elution of BMP-7 from demineralized bone matrix. Cell Tissue Bank. 2012, 13, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Zhou, Y.; Wu, G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng. Part B Rev. 2014, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Anger, D.; Krummenauer, F.; Breitig, D.; Fickert, S.; Guenther, K.-P.; Guenther, K. Biological activity of recombinant human growth factors released from biocompatible bone implants. J. Biomed. Mater. Res. Part A 2008, 86, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Autefage, H.; Briand-Mesange, F.; Cazalbou, S.; Drouet, C.; Fourmy, D.; Gonçalvès, S.; Salles, J.P.; Combes, C.; Swider, P.; Rey, C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 706–715. [Google Scholar] [CrossRef]
- Li, R.H.; Bouxsein, M.L.; Blake, C.A.; D’Augusta, D.; Kim, H.; Li, X.J.; Wozney, J.M.; Seeherman, H.J. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J. Orthop. Res. 2003, 21, 997–1004. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Nickel, J.; Kotzsch, A.; Sebald, W.; Mueller, T.D. A Single Residue of GDF-5 Defines Binding Specificity to BMP Receptor IB. J. Mol. Boil. 2005, 349, 933–947. [Google Scholar] [CrossRef]
- Kotzsch, A.; Nickel, J.; Seher, A.; Sebald, W.; Müller, T.D. Crystal structure analysis reveals a spring-loaded latch as molecular mechanism for GDF-5–type I receptor specificity. EMBO J. 2009, 28, 937–947. [Google Scholar] [CrossRef]
- Kissling, S.; Seidenstuecker, M.; Pilz, I.H.; Suedkamp, N.P.; Mayr, H.O.; Bernstein, A. Sustained release of rhBMP-2 from microporous tricalciumphosphate using hydrogels as a carrier. BMC Biotechnol. 2016, 16, 98. [Google Scholar] [CrossRef]
- Nahar, N.N.; Missana, L.R.; Garimella, R.; Tague, S.E.; Anderson, H.C. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J. Bone Miner. Metab. 2008, 26, 514–519. [Google Scholar] [CrossRef]
- Bonucci, E. Calcification in Biological System; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Mundy, G.R.; Martin, T.J. Physiology and Pharmacology of Bone; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Vallejo, L.F.; Rinas, U. Optimized procedure for renaturation of recombinant human bone morphogenetic protein-2 at high protein concentration. Biotechnol. Bioeng. 2004, 85, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, R.; Hoffmann, E.; Sebald, W. Human Bone Morphogenetic Protein 2 Contains a Heparin-Binding Site which Modifies Its Biological Activity. JBIC J. Boil. Inorg. Chem. 1996, 237, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M.; Akiyama, S.; Katagiri, T.; Suzuki, A.; Ueno, N.; Yamaji, N.; Rosen, V.; Wozney, J.M.; Suda, T. A Kinase Domain-truncated Type I Receptor Blocks Bone Morphogenetic Protein-2-induced Signal Transduction in C2C12 Myoblasts. J. Boil. Chem. 1997, 272, 22046–22052. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, T.; Tada, K.; Kitajima, I.; Tojo, K.; Sampath, T.K.; Kawabata, M.; Miyazono, K.; Imamura, T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999, 112 Pt 20, 3519–3527. [Google Scholar]
- Akiyama, H.; Shukunami, C.; Nakamura, T.; Hiraki, Y. Differential Expressions of BMP Family Genes during Chondrogenic Differentiation of Mouse ATDC5 Cells. Cell Struct. Funct. 2000, 25, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Seemann, P.; Schwappacher, R.; Kjaer, K.W.; Krakow, D.; Lehmann, K.; Dawson, K.; Stricker, S.; Pohl, J.; Plöger, F.; Staub, E.; et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J. Clin. Investig. 2005, 115, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, A.M.; Diefenderfer, D.L.; Bhargave, G.; Leboy, P.S. Different Effects of BMP-2 on Marrow Stromal Cells from Human and Rat Bone. Cells Tissues Organs 2004, 176, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Graninger, W.; Bobacz, K.; Watzek, G.; Erlacher, L. BMP-6-induced osteogenic differentiation of mesenchymal cell lines is not modulated by sex steroids and resveratrol. Cytokine 2003, 23, 133–137. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Boil. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Plöger, F.; Mundlos, S.; Seemann, P.; Kegler, M.S.-V.; Lehmann, K.; Seidel, J.; Kjaer, K.W.; Pohl, J. Brachydactyly type A2 associated with a defect in proGDF5 processing. Hum. Mol. Genet. 2008, 17, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; Heuvel, S.V.D. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H. Identification of Type I and Type II Serine/Threonine Kinase Receptors for Growth/Differentiation Factor-5. J. Boil. Chem. 1996, 271, 21345–21352. [Google Scholar]
- Sonomoto, K.; Yamaoka, K.; Kaneko, H.; Yamagata, K.; Sakata, K.; Zhang, X.; Kondo, M.; Zenke, Y.; Sabanai, K.; Nakayamada, S.; et al. Spontaneous Differentiation of Human Mesenchymal Stem Cells on Poly-Lactic-Co-Glycolic Acid Nano-Fiber Scaffold. PLoS ONE 2016, 11, e0153231. [Google Scholar] [CrossRef]
- Klammert, U.; Mueller, T.D.; Hellmann, T.V.; Wuerzler, K.K.; Kotzsch, A.; Schliermann, A.; Schmitz, W.; Kuebler, A.C.; Sebald, W.; Nickel, J. GDF-5 can act as a context-dependent BMP-2 antagonist. BMC Boil. 2015, 13, 115. [Google Scholar] [CrossRef] [PubMed]









| Gene | Forward/Reverse Primers | Tannealing °C | Tmelting °C | Accession Number |
|---|---|---|---|---|
| Runx2 Runt-related transcription factor 2 | FW: gccttcaaggtggtagccc Rev: cgttacccgccatgacagta | 60 | 79 | NM_001024630 |
| Osterix | FW: tgcttgaggaggaagttcac Rev: aggtcactgcccacagagta | 60 | 78 | NM_001173467 |
| Alkaline Phosphatase | FW: gcacctgccttactaactc Rev: agacacccatcccatctc | 60 | 79 | NM_000478 |
| Collagen I | FW: tggagcaagaggcgagag Rev: caccagcatcacccttagc | 60 | 85 | NM_000088 |
| Osteopontin (OPN) | FW: ttgcagtgatttgcttttgc Rev: gaacacgcatctgggtattt | 60 | 82 | NM_001040058 |
| Osteocalcin | FW: gcaaaggtgcagcctttgtg Rev: ggctcccagccattgatacag | 60 | 79 | NM_199173 |
| Collagen II | FW: tagggccggtctgcttcttgtaaa Rev: acatcaggtcaggtcagccattca | 60 | 78 | NM_001844 |
| Aggrecan | FW: tctgtcaggcaaatctgggatggt Rev: atgccacttggtaggccact | 60 | 78 | NM_001135 |
| Aldolase | FW: tcatcctcttccatgagacagtct Rev: attctgctggcagatactggcataa | 58 | 82 | NM_000034 |
| GDF5 | BB-1 | BMP-2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point (days) | 1 h | 1 | 3 | 7 | 14 | 1 h | 1 | 3 | 7 | 14 | 1 h | 1 | 3 | 7 | 14 | |
| Release (%) | Pure CPC | 9.68 | 14.14 | 16.39 | 17.13 | 17.64 | 5.35# | 9.19 | 10.54# | 10.94# | 11.29# | 5.42# | 13.84#§ | 21.09#§ | 24.36#§ | 25.71#§ |
| CPC + 5% fibers | 11.57 | 16.45 | 17.88 | 18.24 | 18.54 | 5.30# | 8.99 | 10.12 | 10.44 | 10.71 | 4.78# | 15.94#§ | 25.73#§ | 31.63#§ | 33.92#§ | |
| CPC + 10% fibers | 12.93 | 19.99 | 21.88 | 22.32 | 22.59 | 6.21# | 10.85# | 12.58 | 12.96 | 13.18# | 6.26# | 26.06#§ | 36.23#§ | 41.34#§ | 43.72#§ | |
| GDF5 | BB-1 | BMP-2 | ||||||||||||||
| Time point (days) | 1 h | 1 | 3 | 7 | 14 | 1 h | 1 | 3 | 7 | 14 | 1 h | 1 | 3 | 7 | 14 | |
| Release (ng/mL) | Pure CPC | 968.21 | 445.87 | 225.50 | 73.90 | 50.84 | 535.58# | 383.70 | 135.57# | 39.17# | 35.67# | 542.95# | 841.53#§ | 724.80#§ | 326.84#§ | 135.20#§ |
| CPC + 5% fibers | 1157.43 | 488.07 | 142.64 | 36.64 | 29.30 | 530.47# | 369.27 | 112.37 | 32.16 | 26.89 | 478.83# | 1115.30#§ | 979.73#§ | 590.00#§ | 228.97#§ | |
| CPC + 10% fibers | 1293.16 | 705.91 | 189.64 | 43.64 | 26.90 | 621.76# | 464.14# | 172.40 | 38.41 | 21.42# | 626.51# | 1979.53#§ | 1017.47#§ | 511.34#§ | 237.37#§ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunnella, F.; Kunisch, E.; Horbert, V.; Maenz, S.; Bossert, J.; Jandt, K.D.; Plöger, F.; Kinne, R.W. In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement. Pharmaceutics 2019, 11, 455. https://doi.org/10.3390/pharmaceutics11090455
Gunnella F, Kunisch E, Horbert V, Maenz S, Bossert J, Jandt KD, Plöger F, Kinne RW. In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement. Pharmaceutics. 2019; 11(9):455. https://doi.org/10.3390/pharmaceutics11090455
Chicago/Turabian StyleGunnella, Francesca, Elke Kunisch, Victoria Horbert, Stefan Maenz, Jörg Bossert, Klaus D. Jandt, Frank Plöger, and Raimund W. Kinne. 2019. "In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement" Pharmaceutics 11, no. 9: 455. https://doi.org/10.3390/pharmaceutics11090455
APA StyleGunnella, F., Kunisch, E., Horbert, V., Maenz, S., Bossert, J., Jandt, K. D., Plöger, F., & Kinne, R. W. (2019). In Vitro Release of Bioactive Bone Morphogenetic Proteins (GDF5, BB-1, and BMP-2) from a PLGA Fiber-Reinforced, Brushite-Forming Calcium Phosphate Cement. Pharmaceutics, 11(9), 455. https://doi.org/10.3390/pharmaceutics11090455