Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Solid Dispersions
2.2.2. Characterization
2.2.3. Determination of Curcumin Concentration
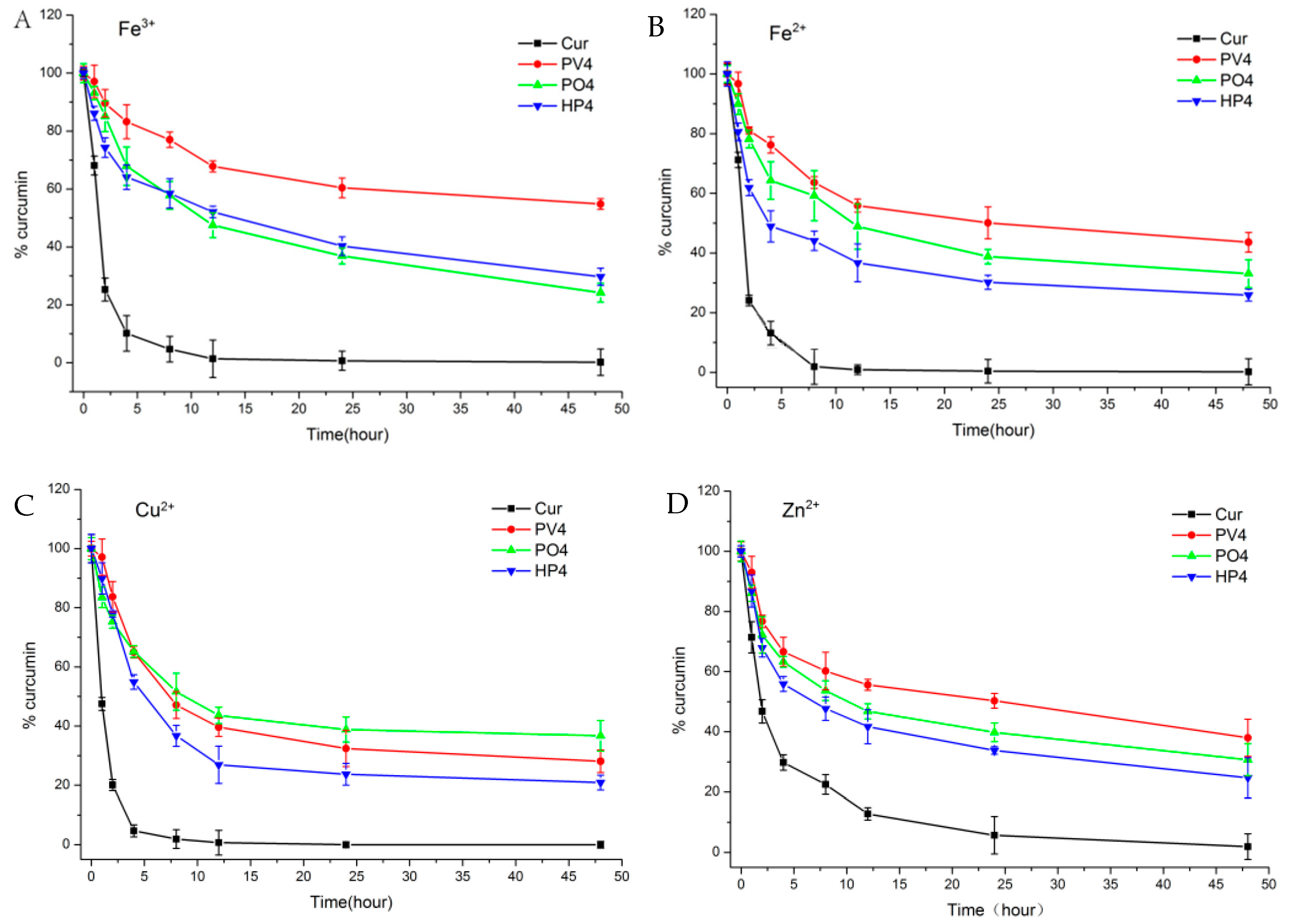
2.2.4. Solubility, Dissolution, and Stability Studies
2.2.5. In Vivo Bioavailability Studies in SD Rats
2.2.6. Mouse Ear Edema Model and Elisa Assay
2.2.7. Molecular Dynamics Simulation Details
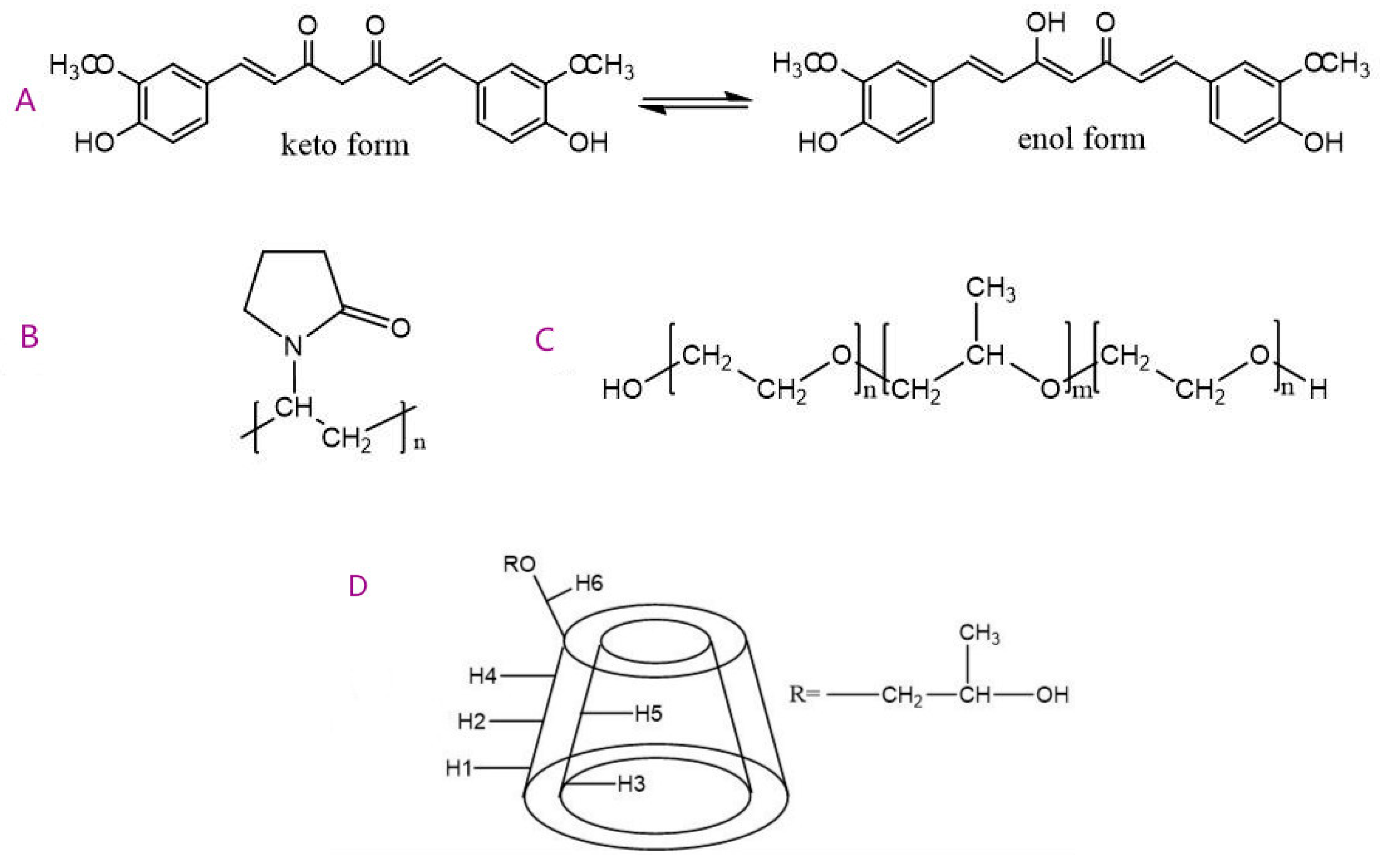
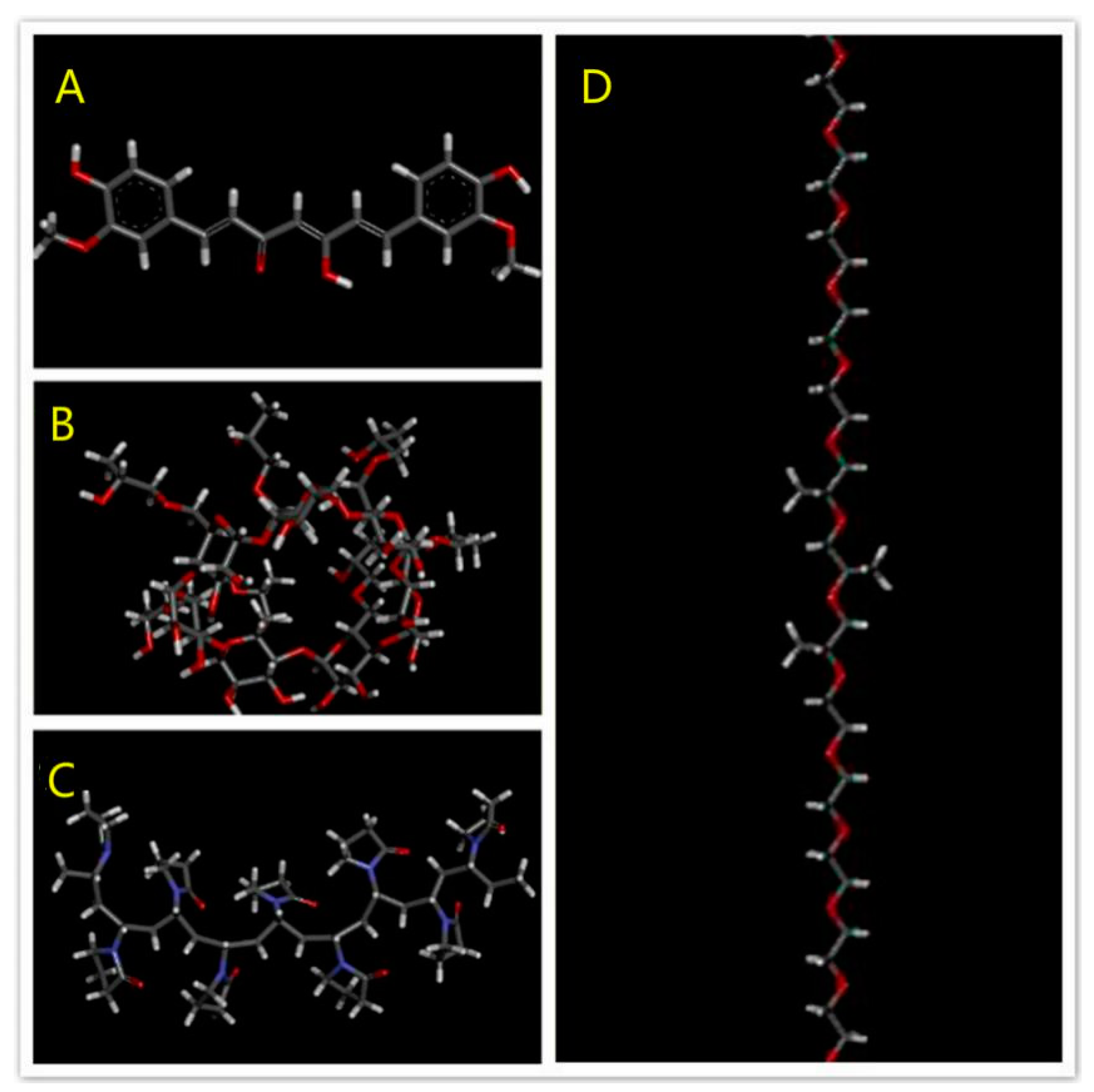
Model Building of Drug and Polymer Segments
The Molecular Dynamics (MD) Simulation
3. Results and Discussion
3.1. Physicochemical Characterization
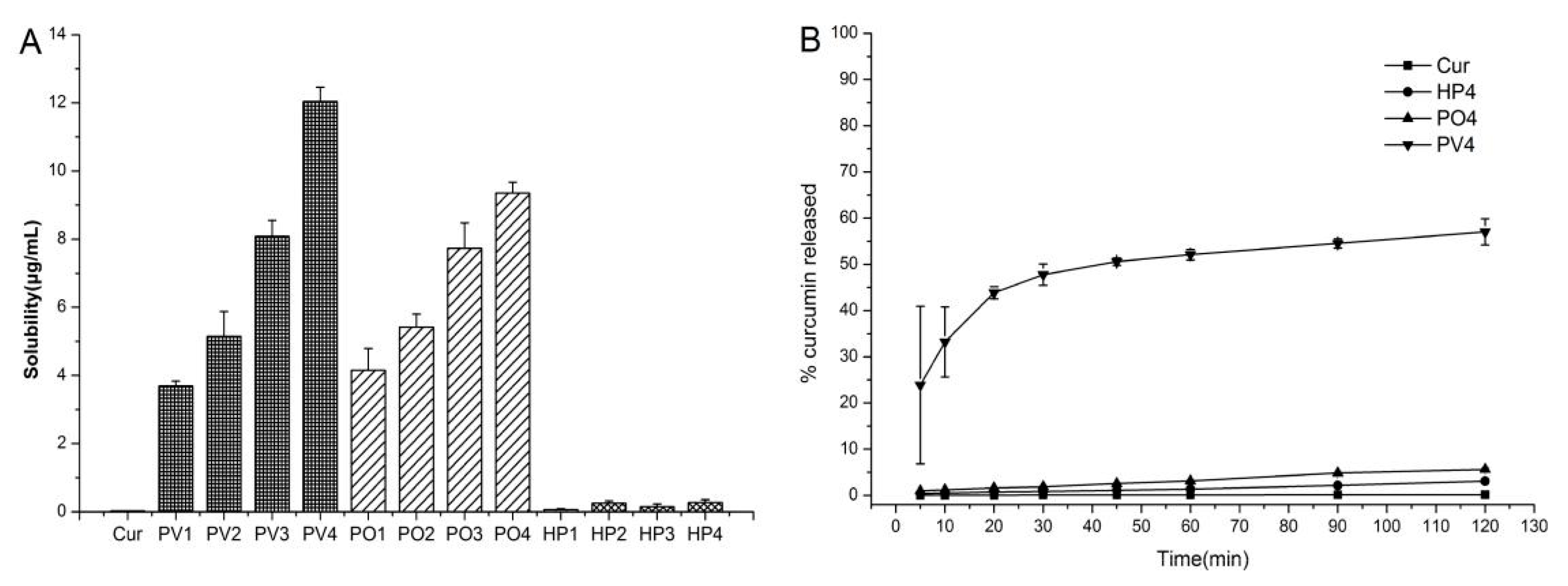
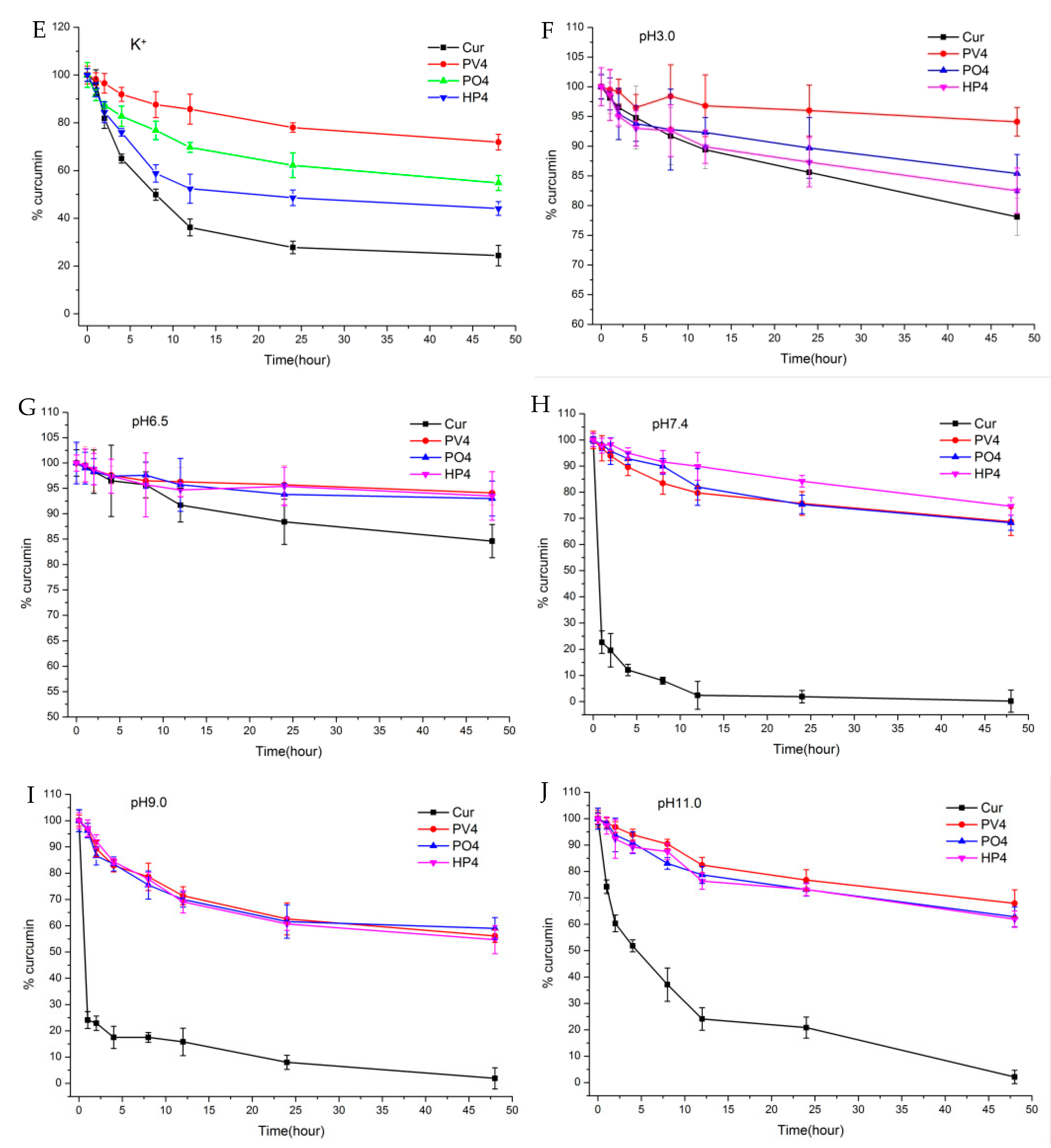
3.1.1. Solubility, Dissolution, and Stability
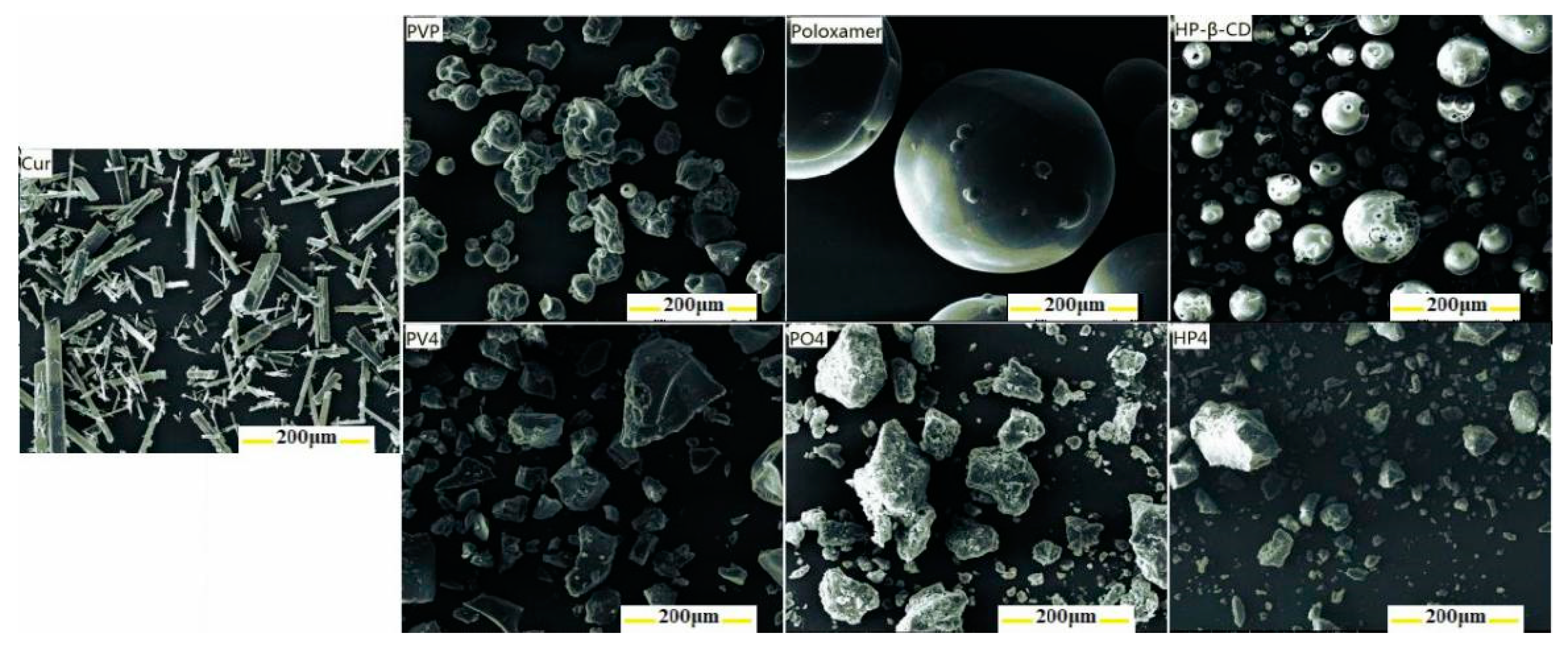
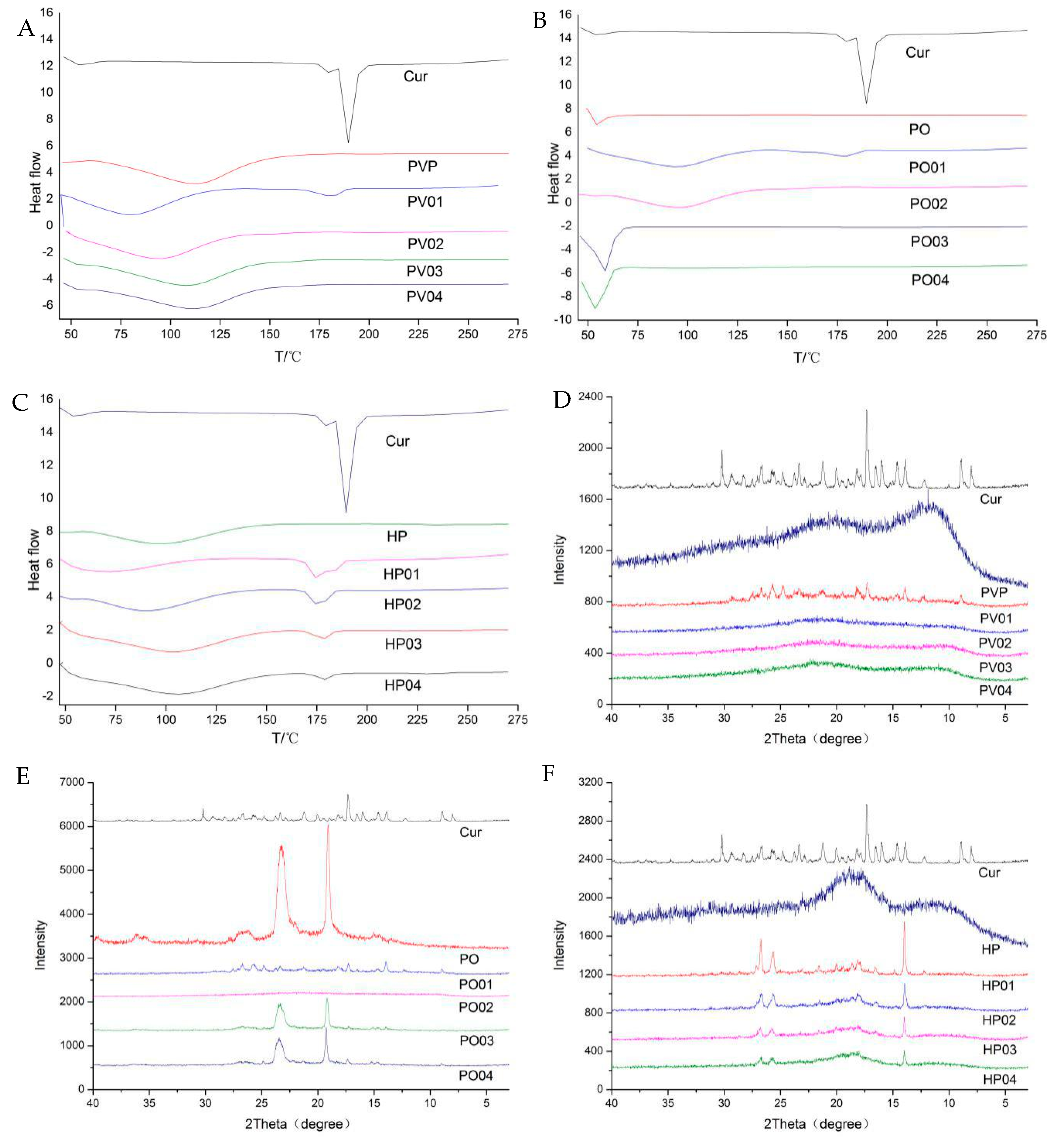
3.1.2. Other Characterizations
3.1.3. 1H-NMR Analysis
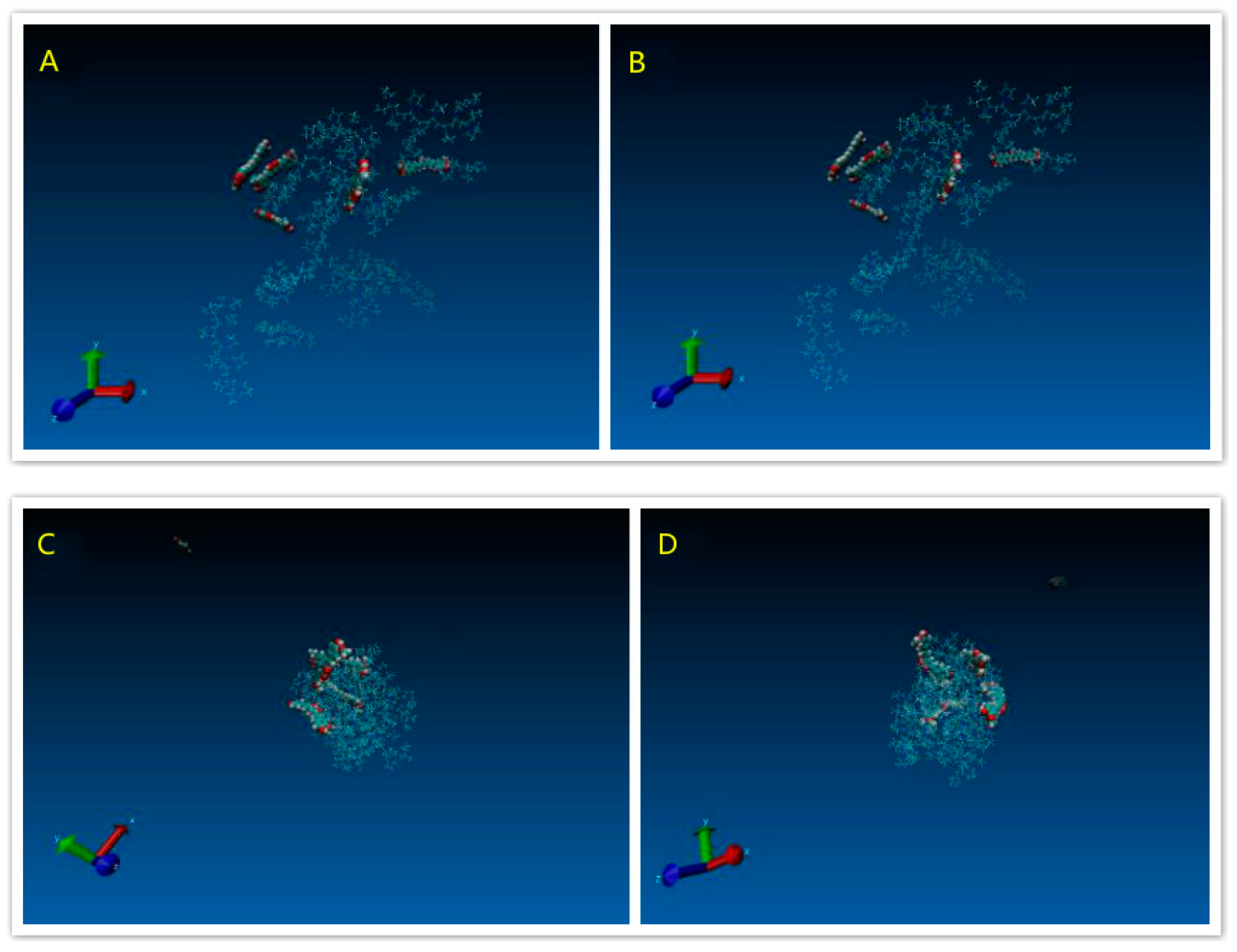
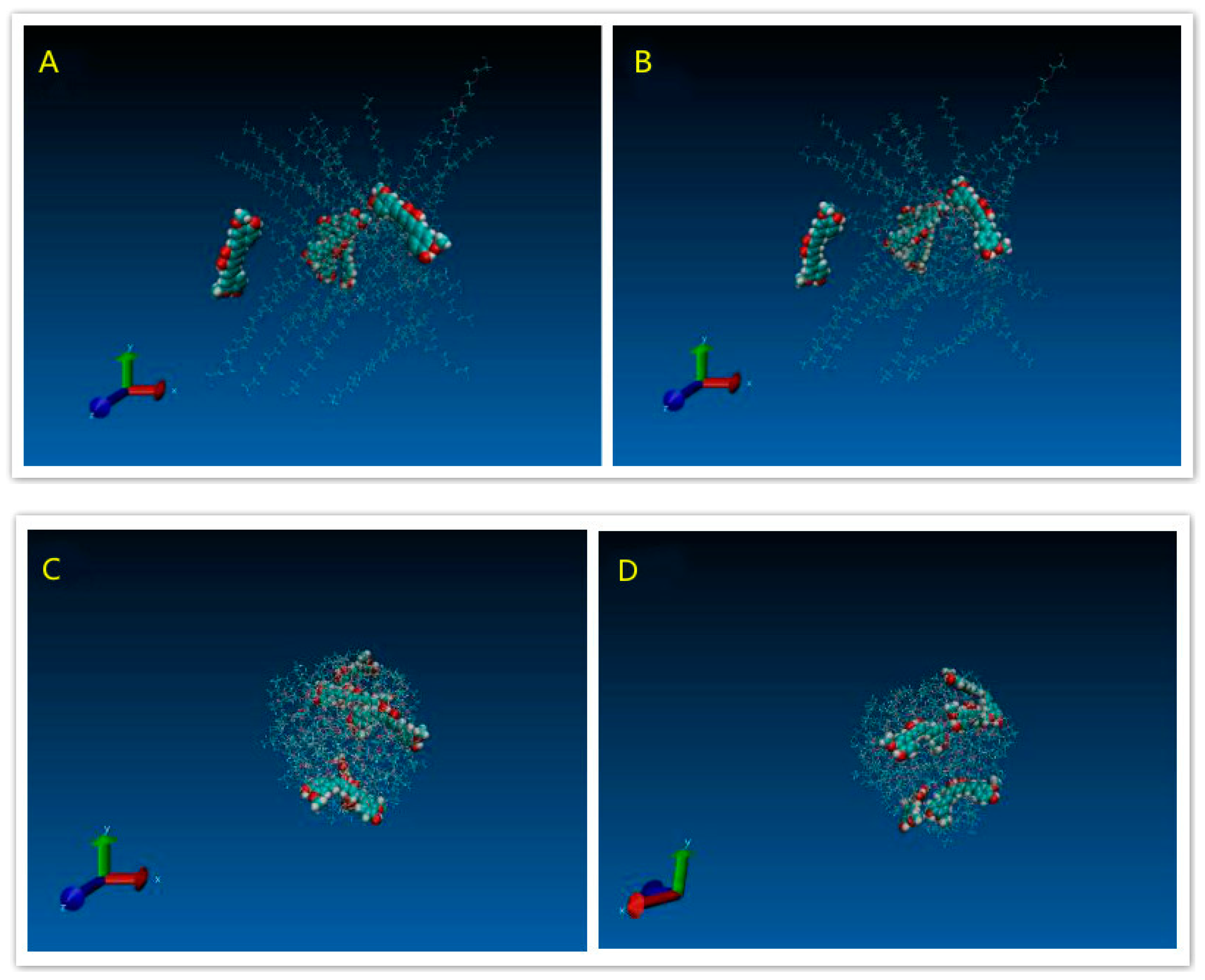
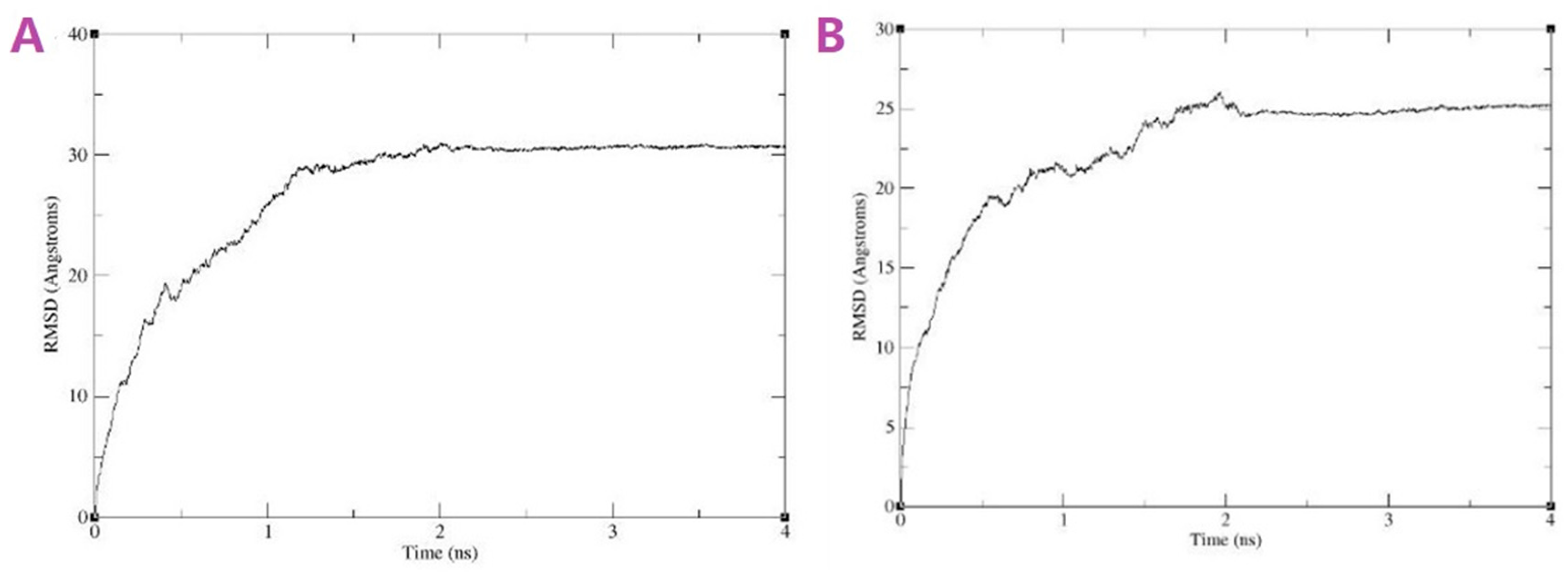
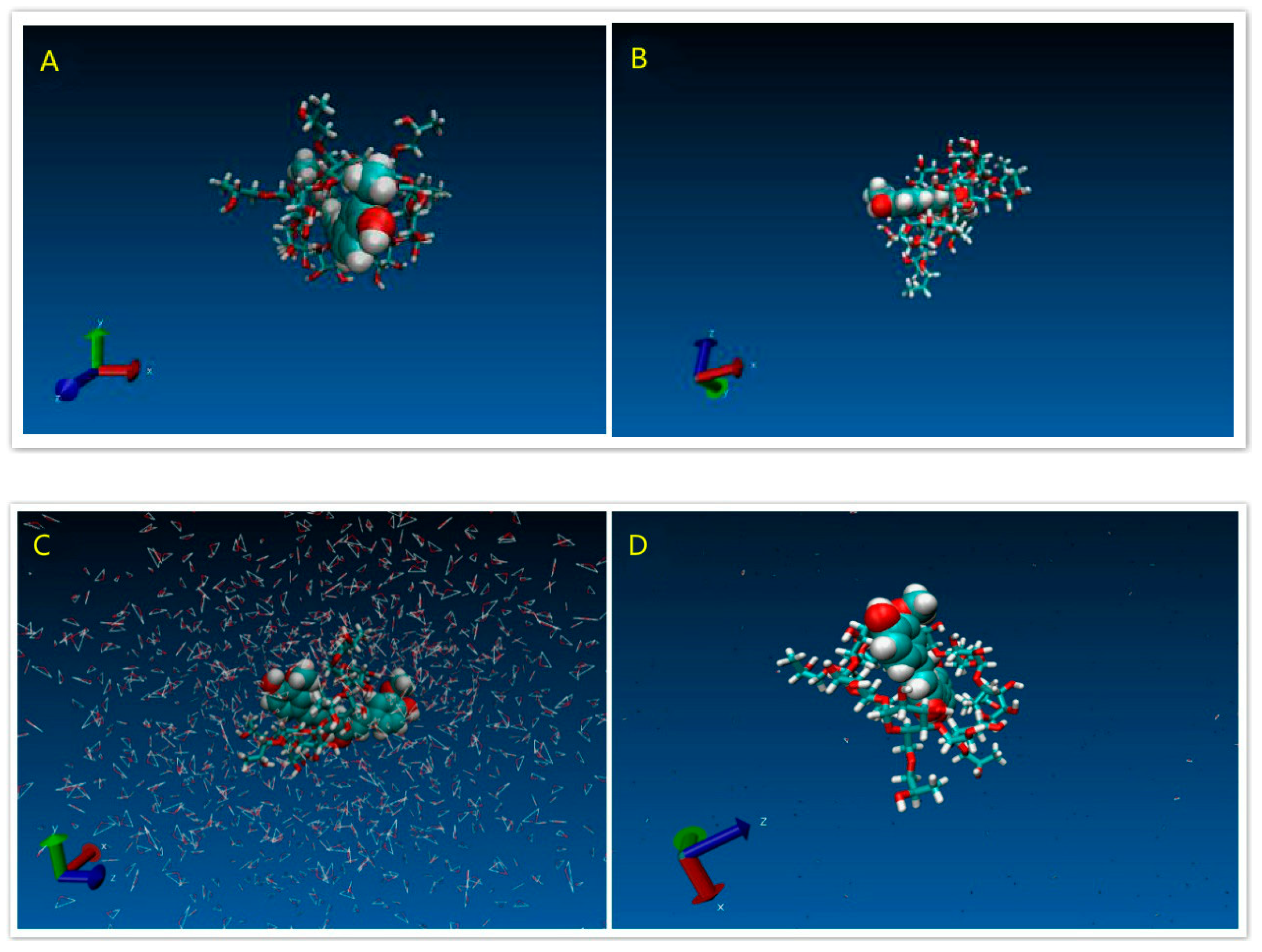
3.1.4. Modeling Results
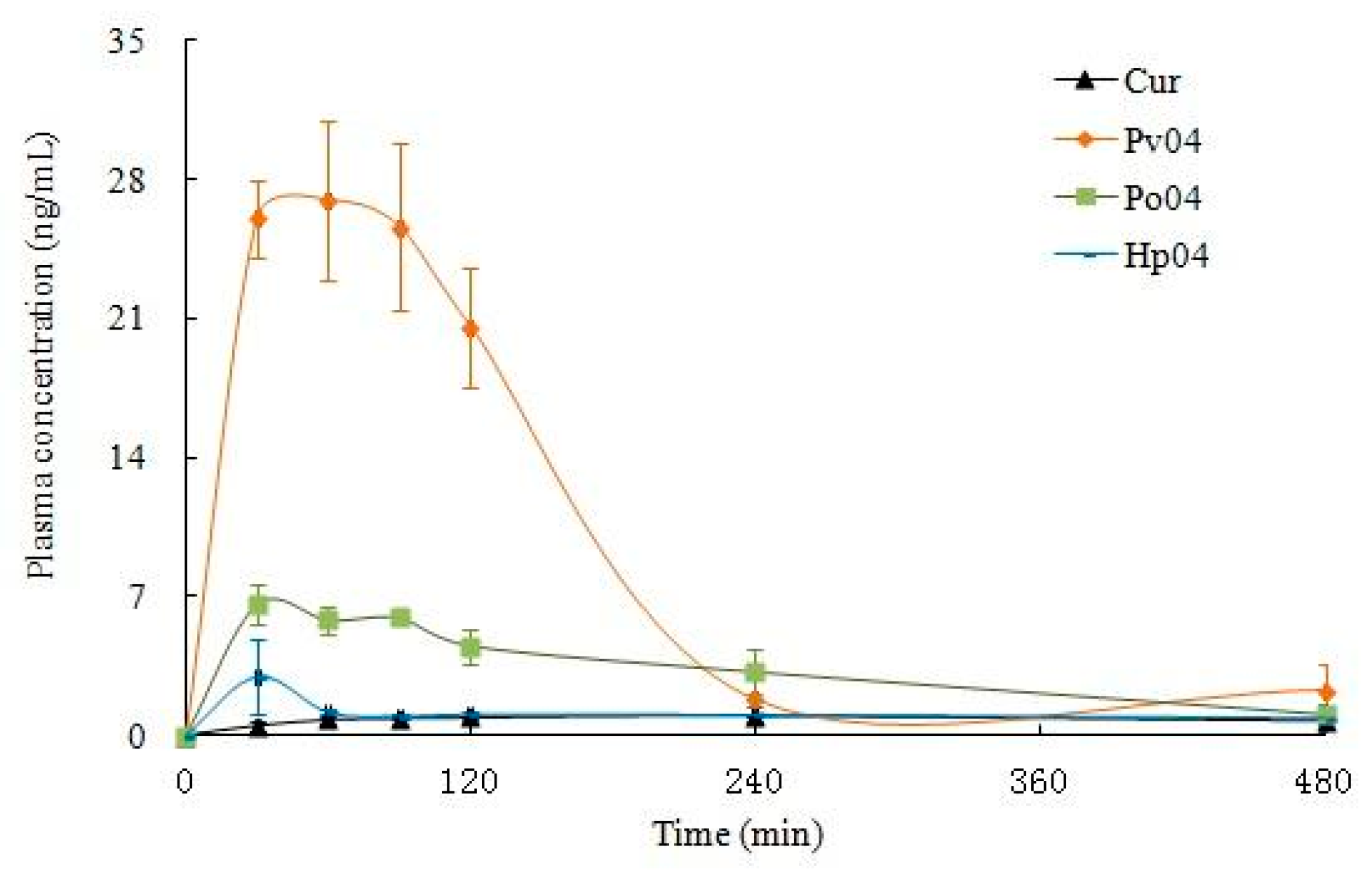
3.2. Oral Bioavailability In Vitro
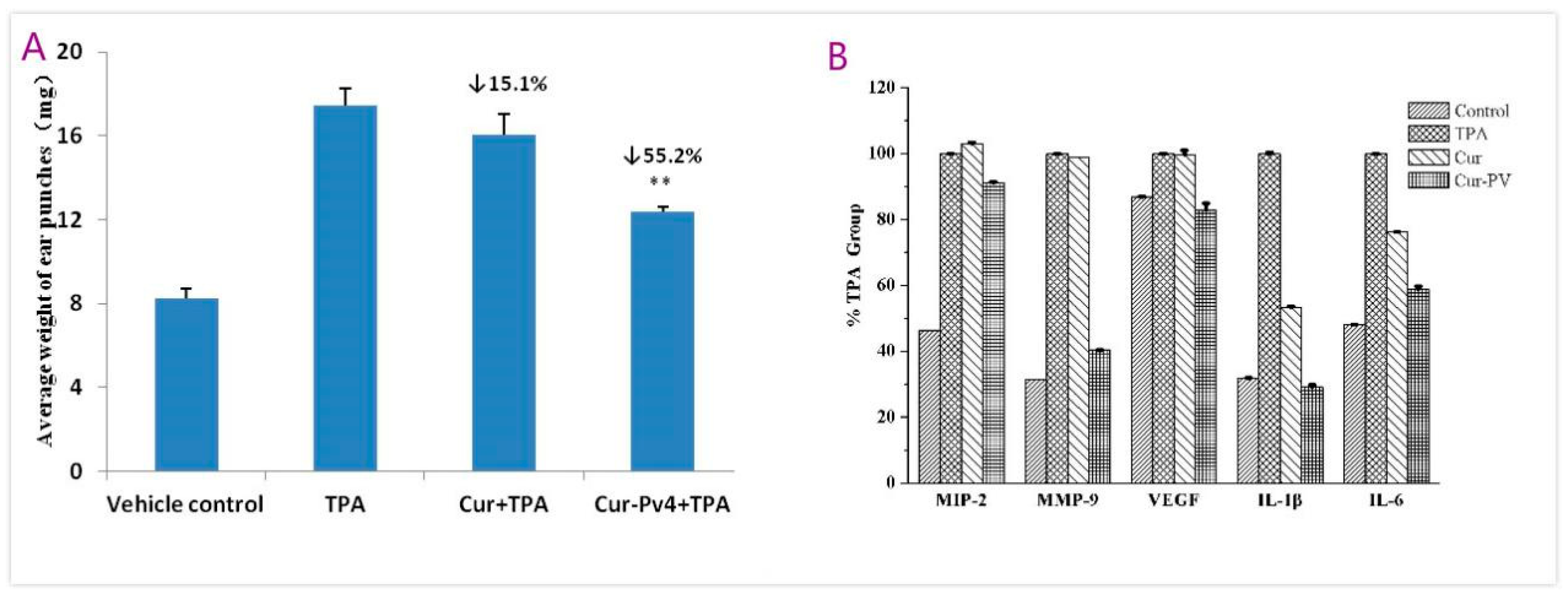
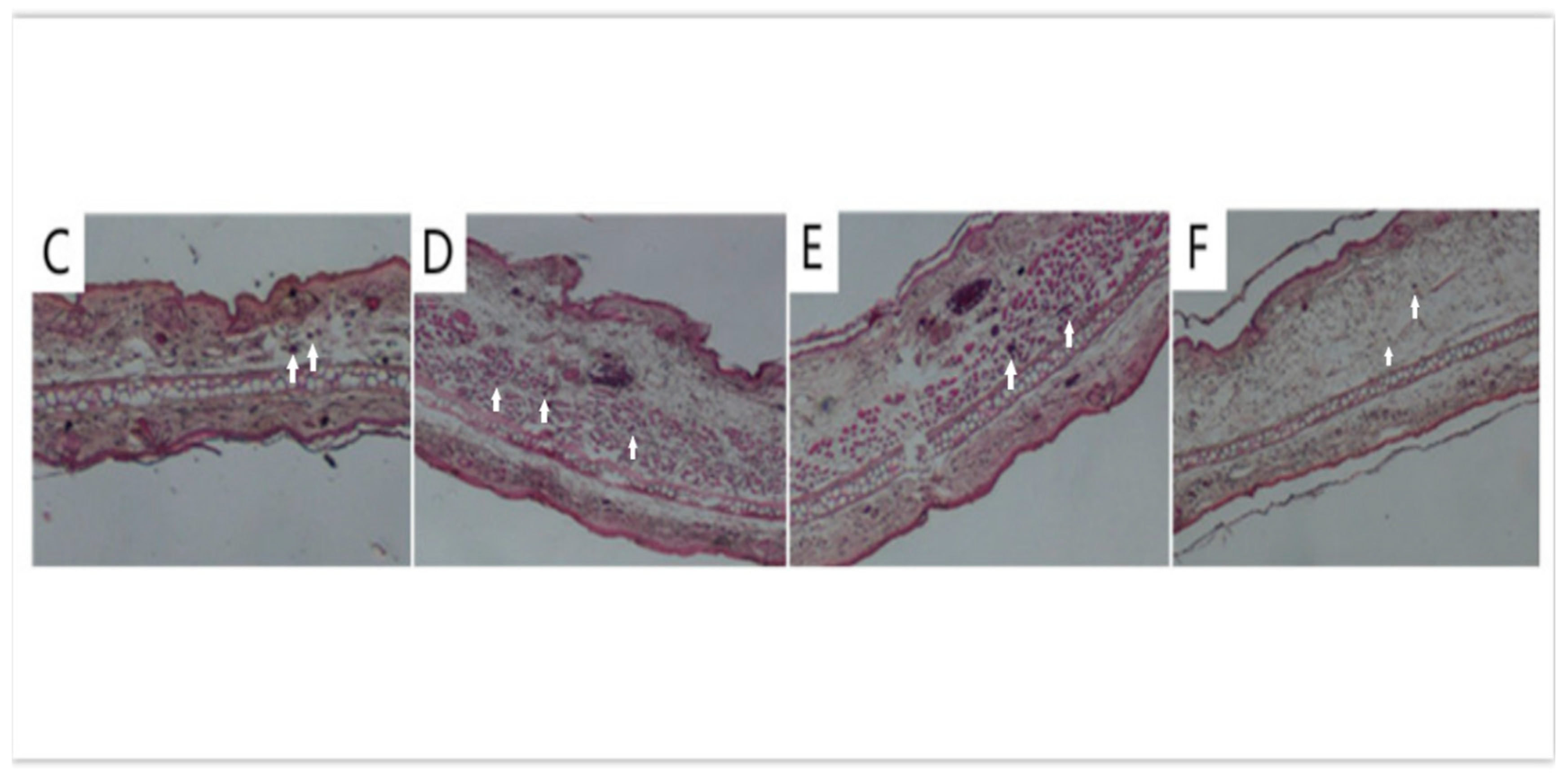
3.3. Anti-Inflammatory Efficacy on TPA-Induced Mouse Ear Edema
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, H.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation and chronic diseases, how are they linked. Molecules 2015, 20, 9183–9213. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin, Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation, Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Shah, N.J.; Farhat, S.; Siriki, R.; Mittimanj, K.; Atluri, S.; Rahaman, M. Curcumin, anti-oxidant, and pioglitazone therapy with inclusion of vitamine in non-alcoholic fatty liver disease-a randomized open label placebo controlled clinical prospective trial (captive). J. Clin. Exp. Hepatol. 2013, 3, S26–S27. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar ABharti, A.C. Anticancer potential of Curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Li, W.; Wu, M.; Tang, L.; Pan, Y.; Liu, Z.; Zeng, C. Novel Curcumin analogue 14p protects against myocardial ischemia reperfusion injury through Nrf2-activating anti-oxidative activity. Toxicol. Appl. Pharm. 2015, 282, 175–183. [Google Scholar] [CrossRef]
- Pae, H.O.; Jeong, G.S.; Jeong, S.O.; Kim, H.S.; Kim, S.A.; Kim, Y.C.; Yoo, S.J.; Kim, H.D.; Chung, H.T. Roles of heme oxygenase-1 in Curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 2007, 39, 267–277. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (Curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Epelbaum, R.; Sehaffer, M. Curcumin as an anti-cancer agent, review of the gap between basic and clinical applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention, molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Romano, B.; Izzo, A.A.; Borrelli, F. The clinical efficacy of curcumin-containing nutraceuticals: An overview of systematic reviews. Pharmacol Res. 2018, 134, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of Curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral Curcumin, biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.F.; Reddy, K.; Pinnapureddy, J.; Kumar, A. Evaluation of the concomitant use of methotrexate and Curcumin on Freund’s complete adjuvant-induced arthritis and hematological indices in rats. J. Pharmacol. 2011, 43, 546. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.C. Synthesis and Antitumor Activity Curcumin Analogs. Ph.D. Thesis, Guangdong University of Technology, Guangzhou, China, 2011. [Google Scholar]
- Ghosh, M.; Singh, A.T.; Xu, W.; Sulchek, T.; Gordon, L.I.; Ryan, R.O. Curcumin nanodisks, formulation and characterization. Nanomedicine 2011, 7, 162–167. [Google Scholar] [CrossRef]
- Song, L.; Shen, Y.; Hou, J.; Lei, L.; Guo, S.; Qian, C. Polymeric micelles for parenteral delivery of Curcumin, preparation, characterization and in vitro evaluation, Colloids and Surfaces A. Physicochem. Eng. Asp. 2011, 390, 25–32. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing Curcumin, enhanced in vitro anti-cancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef]
- Bansal, S.S.; Kausar, H.; Vadhanam, M.V.; Ravoori, S.; Gupta, R.C. Controlled systemic delivery by polymeric implants enhances and plasma Curcumin levels compared with oral administration. Eur. J. Pharm. Biopharm. 2012, 80, 571–577. [Google Scholar] [CrossRef]
- Li, M.; Xin, M.; Guo, C.; Lin, G.; Wu, X. New nanomicelle Curcumin formulation for ocular delivery: Improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev. Ind. Pharm. 2017, 43, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Gold nanoparticles generated and stabilized by water soluble Curcumin-polymer conjugate, blood compatibility evaluation and targeted drug delivery onto cancer cells. J. Colloid Interface Sci. 2012, 368, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Michel, D.; Chitanda, J.M.; Balogh, R.; Yang, P.; Singh, J.; Das, U.; El-Aneed, A.; Dimmock, J.; Verrall, R.; Badea, I. Design and evaluation of cyclodextrin-based delivery systems to incorporate poorly soluble Curcumin analogs for the treatment of melanoma. Eur. J. Pharm. Biopharm. 2012, 81, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Bornali, B.; Palash, S.; Robin, D. Binding and stabilization of Curcumin by mixed chitosan-surfactant systems, a spectroscopic study. J. Photochem. Photobiol. A 2012, 245, 18–27. [Google Scholar]
- Al-Obaidi, H.; Lawrence, M.J.; Shah, S.; Moghul, H.; Al-Saden, N.; Bari, F. Effect of drug–polymer interactions on the aqueous solubility of milled solid dispersions. Int. J. Pharm. 2013, 446, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bunker, M.; Parker, A.; Madden-Smith, C.E.; Patel, N.; Roberts, C.J. The stability of solid dispersions of felodipine in polyvinylpyrrolidone characterized by nanothermal analysis. Int. J. Pharm. 2011, 414, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pathak, K. Hydrogen bond replacement—Unearthing a novel molecular mechanism of surface solid dispersion for enhanced solubility of a drug for veterinary use. Int. J. Pharm. 2013, 441, 99–110. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Morgan, D.J.; Mendham, A.P.; Pang, J.; Snowden, M.J.; Douroumis, D. Drug-polymer intermolecular interactions in hot-melt extruded solid dispersions. Int. J. Pharm. 2013, 443, 199–208. [Google Scholar] [CrossRef]
- Rawlinson, C.F.; Williams, A.C.; Timmins, P.; Grimsey, I. Polymer-mediated disruption of drug crystallinity. Int. J. Pharm. 2007, 336, 42–48. [Google Scholar] [CrossRef]
- Manna, L.; Banchero, M.; Sola, D.; Ferri, A.; Ronchetti, S.; Sicardi, S. Impregnation of PVP microparticles with ketoprofen in the presence of supercritical CO2. J. Supercrit. Fluids 2007, 42, 378–384. [Google Scholar] [CrossRef]
- Ali, W.; Williams, A.C.; Rawlinson, C.F. Stochiometrically governed molecular interactions in drug, Poloxamer solid dispersions. Int. J. Pharm. 2010, 391, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fang, Z.; Yao, L.; Dahmani, F.Z.; Yin, L.F.; Zhou, J.P.; Yao, J. A micelle-like structure of poloxamer-methotrexate conjugates as nanocarrier for methotrexate delivery. Int. J. Pharm. 2015, 487, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.A.; Martini, M.F.; Pickholz, M.; Yokaichiya, F.; Franco, M.K.D.; Cabeça, L.F.; Guilherme, V.A.; Silva, C.M.G.; Limia, C.E.G.; Paula, E. Clonidine complexation with hydroxypropyl-beta-cyclodextrin: From physico-chemical characterization to in vivo adjuvant effect in local anesthesia. J. Pharm. Biomed. Anal. 2016, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
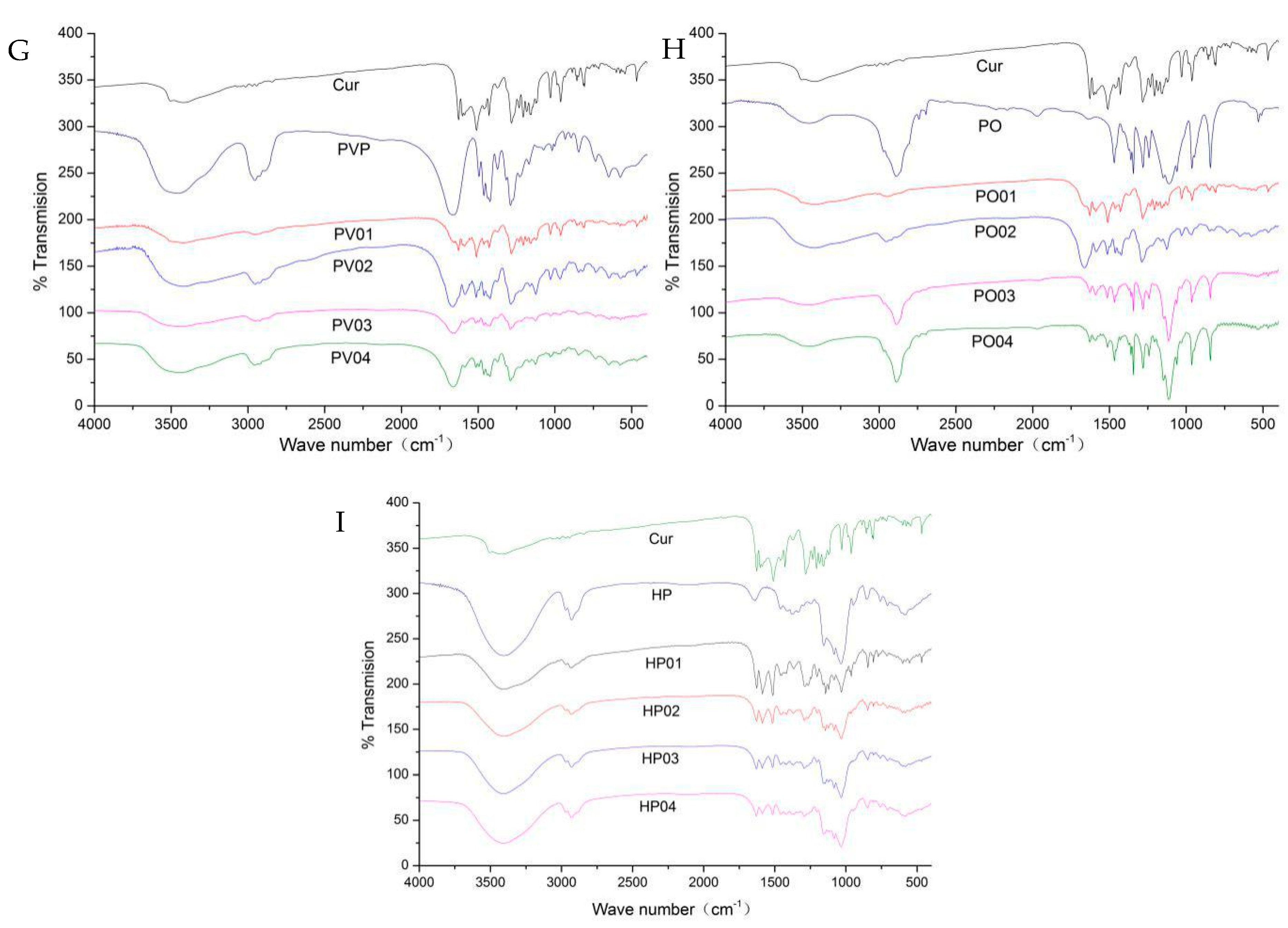
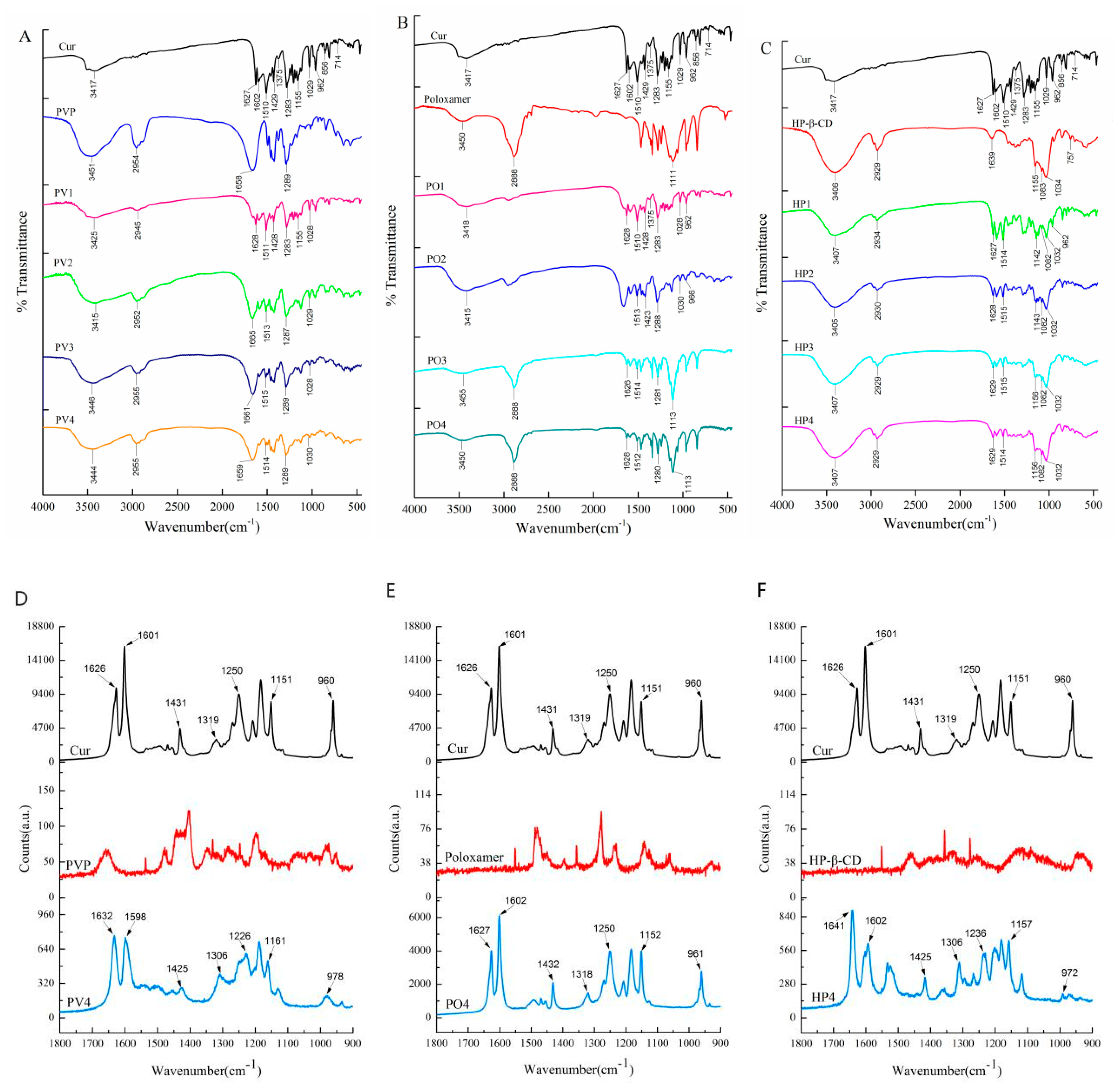
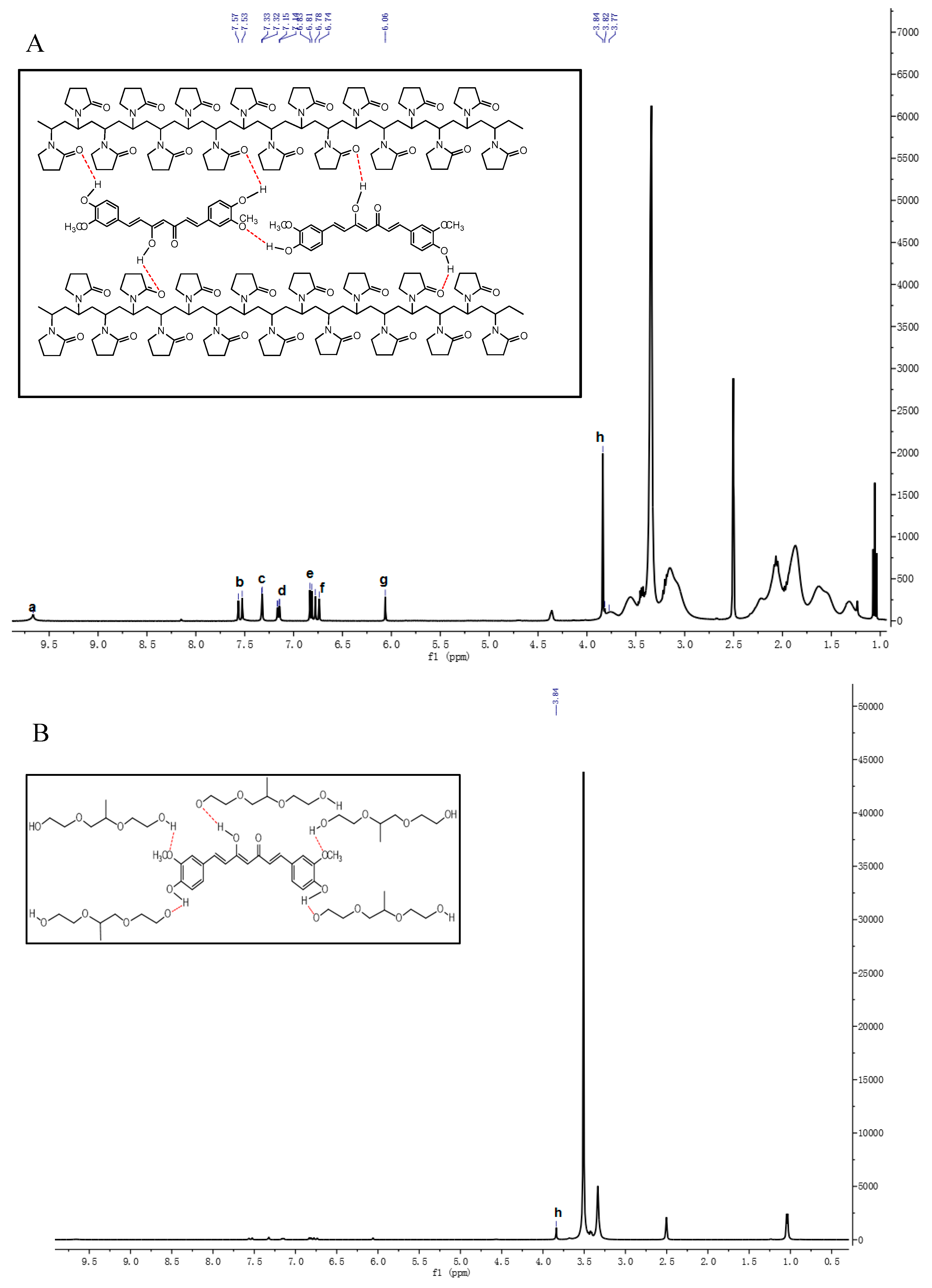
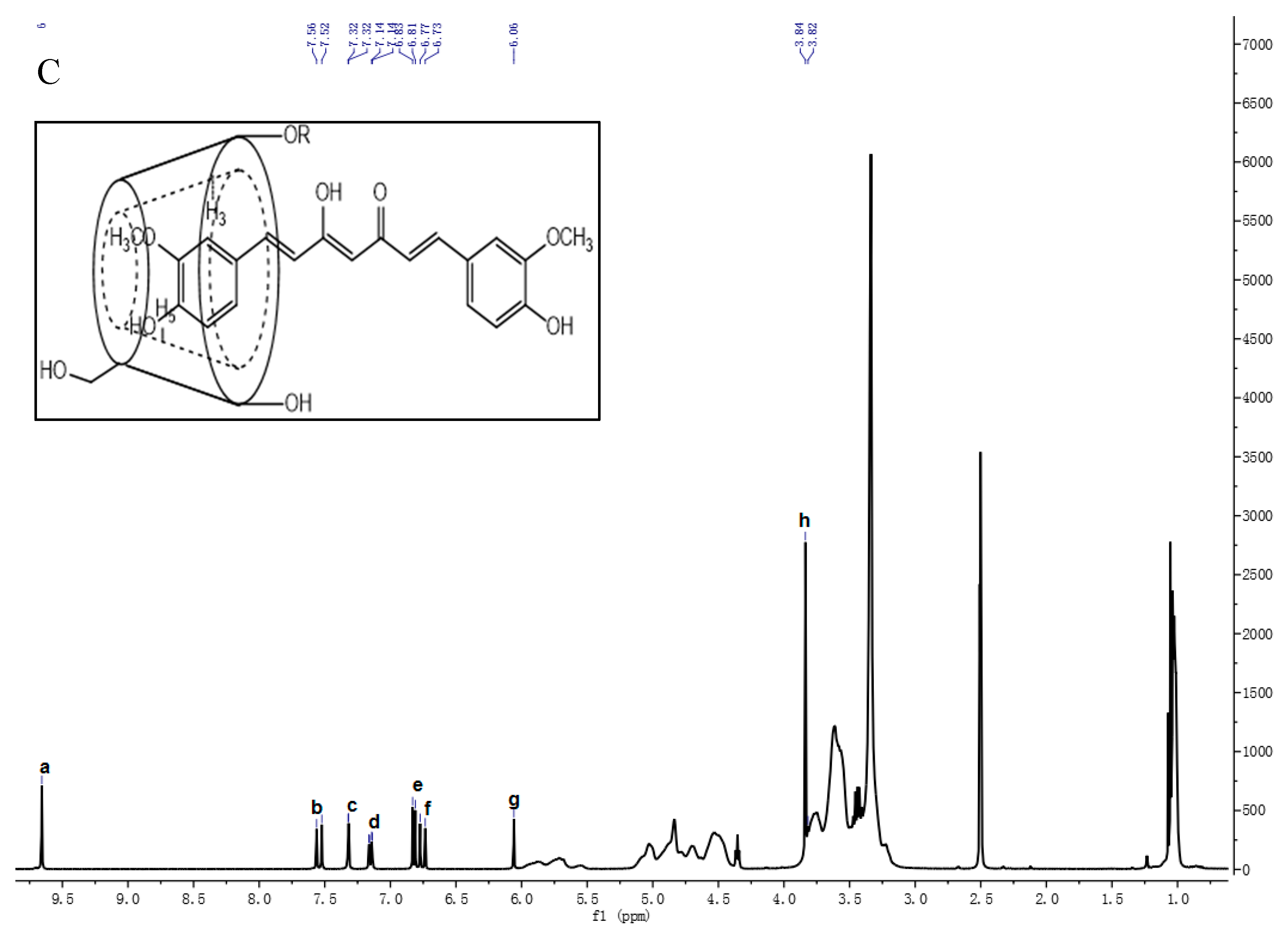
| Cur (cm−1) | PV4 (cm−1) | PO4 (cm−1) | HP4 (cm−1) | Peak Assignment |
|---|---|---|---|---|
| 1626 | 1632 | 1627 | 1641 | Expansion vibration of C=C and C=O on enol structure |
| 1601 | 1598 | 1602 | 1602 | Telescopic vibration of C=C on the benzene ring |
| 1431 | 1425 | 1432 | 1425 | Planar vibration of C–C, C–CH, and C–OH on the benzene ring |
| 1319 | 1306 | 1318 | 1306 | C–CH planar vibration of C10 and C11 on the enenol structure |
| 1250 | 1229 | 1250 | 1236 | Deformation vibration of CCH on the benzene ring and C–OH of enenol |
| 1151 | 1161 | 1152 | 1157 | Deformation and vibration of C–CH on the benzene ring, C–OH, and C=CH |
| 960 | 978 | 961 | 972 | Planar vibration of CCH on the benzene ring |
| Parameters | ||||
|---|---|---|---|---|
| Tmax (h) | 1.33 ± 0.00 | 1.17 ± 0.58 | 1.00 ± 0.50 | 0.50 ± 0.00 |
| Cmax (ng/ mL) | 1.07 ± 0.18 | 28.63 ± 5.50 | 6.64 ± 1.00 | 2.98 ± 1.89 |
| AUC0–t (ng/mL·h) | 6.72 ± 1.25 | 74.76 ± 21.61 | 26.46 ± 1.53 | 8.73 ± 2.72 |
| T1/2 (h) | 10.17 ± 0.00 | 1.17 ± 0.21 | 2.80 ± 0.21 | 4.50 ± 3.12 |
| F | 11.13 | 3.94 | 1.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. https://doi.org/10.3390/pharmaceutics11090442
He Y, Liu H, Bian W, Liu Y, Liu X, Ma S, Zheng X, Du Z, Zhang K, Ouyang D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics. 2019; 11(9):442. https://doi.org/10.3390/pharmaceutics11090442
Chicago/Turabian StyleHe, Yan, Hongfei Liu, Wangqing Bian, Yue Liu, Xinyang Liu, Shijing Ma, Xi Zheng, Zhiyun Du, Kun Zhang, and Defang Ouyang. 2019. "Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects" Pharmaceutics 11, no. 9: 442. https://doi.org/10.3390/pharmaceutics11090442





