Therapeutic Applications of Pretargeting
Abstract
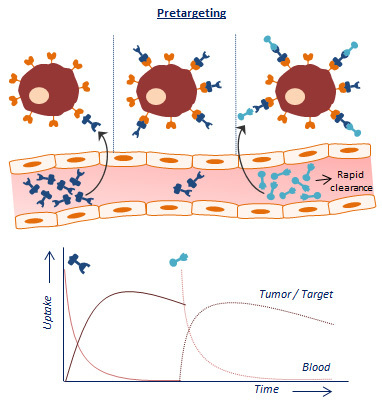
:1. Introduction
2. Pretargeted Radioimmunotherapy with Beta-Emitting Radionuclides
2.1. Hematologic Cancers
2.1.1. CD20 Antigen
2.1.2. Other Hematological Tumor Targets
2.2. Solid Cancers
2.2.1. Carcinoembryonic Antigen (CEA)
2.2.2. Epithelial Cell Adhesion Molecule
2.2.3. TAG-72
2.2.4. Tenascin-C
2.2.5. Other Solid Tumor Targets
3. Pretargeted Alpha-Particle Therapy
4. Combination Therapy
5. Novel Applications of Pretargeting: Beyond Antibodies as Targeting Vector
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Wittrup, K.D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 2009, 8, 2861–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardan, D.T.; Meares, C.F.; Goodwin, D.A.; McTigue, M.; David, G.S.; Stone, M.R.; Leung, J.P.; Bartholomew, R.M.; Frincke, J.M. Antibodies against metal chelates. Nature 1985, 316, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.A.; Mears, C.F.; McTigue, M.; David, G.S. Monoclonal antibody hapten radiopharmaceutical delivery. Nucl. Med. Commun. 1986, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Pimm, M.V.; Fells, H.F.; Perkins, A.C.; Baldwin, R.W. Iodine-131 and indium-111 labelled avidin and streptavidin for pre-targetted immunoscintigraphy with biotinylated anti-tumour monoclonal antibody. Nucl. Med. Commun. 1988, 9, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Stickney, D.R.; Anderson, L.D.; Slater, J.B.; Ahlem, C.N.; Kirk, G.A.; Schweighardt, S.A.; Frincke, J.M. Bifunctional antibody: A binary radiopharmaceutical delivery system for imaging colorectal carcinoma. Cancer Res. 1991, 51, 6650–6655. [Google Scholar] [PubMed]
- Le Doussal, J.M.; Martin, M.; Gautherot, E.; Delaage, M.; Barbet, J. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: Enhanced divalent hapten affinity for cell-bound antibody conjugate. J. Nucl. Med. 1989, 30, 1358–1366. [Google Scholar]
- Kuijpers, W.H.; Bos, E.S.; Kaspersen, F.M.; Veeneman, G.H.; van Boeckel, C.A. Specific recognition of antibody-oligonucleotide conjugates by radiolabeled antisense nucleotides: A novel approach for two-step radioimmunotherapy of cancer. Bioconjug. Chem. 1993, 4, 94–102. [Google Scholar] [CrossRef]
- Liu, G.; Mang’era, K.; Liu, N.; Gupta, S.; Rusckowski, M.; Hnatowich, D.J. Tumor pretargeting in mice using (99m)tc-labeled morpholino, a DNA analog. J. Nucl. Med. 2002, 43, 384–391. [Google Scholar]
- Rossin, R.; Verkerk, P.R.; Van Den Bosch, S.M.; Vulders, R.C.M.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Patra, M.; Zarschler, K.; Pietzsch, H.J.; Stephan, H.; Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 2016, 45, 6415–6431. [Google Scholar] [CrossRef] [Green Version]
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef]
- Knight, J.C.; Cornelissen, B. Bioorthogonal chemistry: Implications for pretargeted nuclear (pet/spect) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 96–113. [Google Scholar]
- Liu, G. A revisit to the pretargeting concept-a target conversion review. Front. Pharmacol. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Botto, B.; Rohatiner, A.Z.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.; et al. 90yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-hodgkin lymphoma: Updated results after a median follow-up of 7.3 years from the international, randomized, phase iii first-lineindolent trial. J. Clin. Oncol. 2013, 31, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Vitolo, U.; Soubeyran, P.; Tilly, H.; Huijgens, P.C.; Kolstad, A.; d’Amore, F.; Gonzalez Diaz, M.; et al. Phase iii trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol. 2008, 26, 5156–5164. [Google Scholar] [CrossRef] [PubMed]
- Press, O.W.; Unger, J.M.; Rimsza, L.M.; Friedberg, J.W.; LeBlanc, M.; Czuczman, M.S.; Kaminski, M.; Braziel, R.M.; Spier, C.; Gopal, A.K.; et al. Phase iii randomized intergroup trial of chop plus rituximab compared with chop chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-hodgkin lymphoma: Swog s0016. J. Clin. Oncol. 2013, 31, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Press, O.W.; Corcoran, M.; Subbiah, K.; Hamlin, D.K.; Scott Wilbur, D.; Johnson, T.; Theodore, L.; Yau, E.; Mallett, R.; Meyer, D.L.; et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of cd20-expressing lymphoma xenografts. Blood 2001, 98, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, K.; Hamlin, D.K.; Pagel, J.M.; Wilbur, D.S.; Meyer, D.L.; Axworthy, D.B.; Mallett, R.W.; Theodore, L.J.; Stayton, P.S.; Press, O.W. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in cd20-expressing human lymphoma xenografts. J. Nucl. Med. 2003, 44, 437–445. [Google Scholar]
- Weiden, P.L.; Breitz, H.B.; Press, O. Pretargeted radioimmunotherapy (prit™) for treatment of non-hodgkin’s lymphoma (nhl): Initial phase i/ii study results. Cancer Biother Radiopharm 2000, 15, 15–29. [Google Scholar] [CrossRef]
- Weiden, P.L.; Breitz, H.B. Pretargeted radioimmunotherapy (prit™) for treatment of non-hodgkin’s lymphoma (nhl). Crit. Rev. Oncol. Hematol. 2001, 40, 37–51. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Karacay, H.; Chang, C.H.; McBride, W.J.; Horak, I.D.; Goldenberg, D.M. Improved therapy of non-hodgkin’s lymphoma xenografts using radionuclides pretargeted with a new anti-cd20 bispecific antibody. Leukemia 2005, 19, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Karacay, H.; Litwin, S.; Rossi, E.A.; McBride, W.J.; Chang, C.H.; Goldenberg, D.M. Improved therapeutic results by pretargeted radioimmunotherapy of non-hodgkin’s lymphoma with a new recombinant, trivalent, anti-cd20, bispecific antibody. Cancer Res. 2008, 68, 5282–5290. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Lin, Y.; Sanderson, J.; Zuo, Y.; Stone, D.; Mallett, R.; Wilbert, S.; Axworthy, D. A tetravalent single-chain antibody-streptavidin fusion protein for pretargeted lymphoma therapy. Cancer Res. 2000, 60, 6663–6669. [Google Scholar] [PubMed]
- Weiden, P.L. Pretargeted radioimmmunotherapy (prit™) using an antibody-streptavidin fusion protein in non-hodgkin’s lymphoma. Leuk. Lymphoma 2002, 43, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Forero, A.; Weiden, P.L.; Vose, J.M.; Knox, S.J.; LoBuglio, A.F.; Hankins, J.; Goris, M.L.; Picozzi, V.J.; Axworthy, D.B.; Breitz, H.B.; et al. Phase 1 trial of a novel anti-cd20 fusion protein in pretargeted radioimmunotherapy for b-cell non-hodgkin lymphoma. Blood 2004, 104, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.M.; Lin, Y.; Hedin, N.; Pantelias, A.; Axworthy, D.; Stone, D.; Hamlin, D.K.; Wilbur, D.S.; Press, O.W. Comparison of a tetravalent single-chain antibody-streptavidin fusion protein and an antibody-streptavidin chemical conjugate for pretargeted anti-cd20 radioimmunotherapy of b-cell lymphomas. Blood 2006, 108, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Frayo, S.L.; Lin, Y.; Hamlin, D.K.; Fisher, D.R.; Frost, S.H.L.; Kenoyer, A.L.; Hylarides, M.D.; Gopal, A.K.; Gooley, T.A.; et al. Comparative analysis of bispecific antibody and streptavidin-targeted radioimmunotherapy for b-cell cancers. Cancer Res. 2016, 76, 6669–6679. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Press, O.W.; Meyer, D.L.; Hamlin, D.K.; Axworthy, D.; Wilbur, D.S.; Stayton, P.S. Role of biotin-binding affinity in streptavidin-based pretargeted radioimmunotherapy of lymphoma. Bioconjugate Chem. 2005, 16, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Shenoi, J.; Frayo, S.M.; Hamlin, D.K.; Lin, Y.; Wilbur, D.S.; Stayton, P.S.; Orgun, N.; Hylarides, M.; Buchegger, F.; et al. Pretargeted radioimmunotherapy using genetically engineered antibody-streptavidin fusion proteins for treatment of non-hodgkin lymphoma. Clin. Cancer Res. 2011, 17, 7373–7382. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.H.L.; Frayo, S.L.; Miller, B.W.; Orozco, J.J.; Booth, G.C.; Hylarides, M.D.; Lin, Y.K.; Green, D.J.; Gopal, A.K.; Pagel, J.M.; et al. Comparative efficacy of lu-177 and y-90 for anti-cd20 pretargeted radioimmunotherapy in murine lymphoma xenograft models. PLoS ONE 2015, 10, e0120561. [Google Scholar] [CrossRef] [PubMed]
- de Boer, C.J.; van Krieken, J.H.; Schuuring, E.; Kluin, P.M. Bcl-1/cyclin d1 in malignant lymphoma. Ann. Oncol. 1997, 8 (Suppl. 2), 109–117. [Google Scholar] [CrossRef]
- de Boisferon, M.H.; Manetti, C.; Raguin, O.; Gautherot, E.; Rostene, W.; Barbet, J.; Gruaz-Guyon, A. Pretargeted radioimmunotherapy using i-131-labelled bivalent hapten-bearing peptides. Lett. Pept. Sci. 1997, 4, 331–339. [Google Scholar] [CrossRef]
- Pagel, J.M.; Orgun, N.; Hamlin, D.K.; Wilbur, D.S.; Gooley, T.A.; Gopal, A.K.; Park, S.I.; Green, D.J.; Lin, Y.; Press, O.W. A comparative analysis of conventional and pretargeted radioimmunotherapy of b-cell lymphomas by targeting cd20, cd22, and hla-dr singly and in combinations. Blood 2009, 113, 4903–4913. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Grana, C.M.; Cocca, F.; De Santis, R.; Del Principe, D.; Baio, S.M.; Mei, R.; Paganelli, G. Pretargeted antibody-guided radioimmunotherapy in a child affected by resistant anaplastic large cell lymphoma. Eur. J. Haematol. 2007, 79, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Pagel, J.M.; Axworthy, D.; Pantelias, A.; Hedin, N.; Press, O.W. A genetically engineered anti-cd45 single-chain antibody-streptavidin fusion protein for pretargeted radioimmunotherapy of hematologic malignancies. Cancer Res. 2006, 66, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.M.; Hedin, N.; Drouet, L.; Wood, B.L.; Pantelias, A.; Lin, Y.; Hamlin, D.K.; Wilbur, D.S.; Gopal, A.K.; Green, D.; et al. Eradication of disseminated leukemia in a syngeneic murine leukemia model using pretargeted anti-cd45 radioimmunotherapy. Blood 2008, 111, 2261–2268. [Google Scholar] [CrossRef]
- Pagel, J.M.; Matthews, D.C.; Kenoyer, A.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Gopal, A.K.; Lin, Y.; Saganic, L.; Appelbaum, F.R.; et al. Pretargeted radioimmunotherapy using anti-cd45 monoclonal antibodies to deliver radiation to murine hematolymphoid tissues and human myeloid leukemia. Cancer Res. 2009, 69, 185–192. [Google Scholar] [CrossRef]
- Green, D.J.; Pagel, J.M.; Nemecek, E.R.; Lin, Y.; Kenoyer, A.; Pantelias, A.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Rajendran, J.G.; et al. Pretargeting cd45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood 2009, 114, 1226–1335. [Google Scholar] [CrossRef]
- Pagel, J.M.; Hedin, N.; Subbiah, K.; Meyer, D.; Mallet, R.; Axworthy, D.; Theodore, L.J.; Wilbur, D.S.; Matthews, D.C.; Press, O.W. Comparison of anti-cd20 and anti-cd45 antibodies for conventional and pretargeted radioimmunotherapy of b-cell lymphomas. Blood 2003, 101, 2340–2348. [Google Scholar] [CrossRef]
- Green, D.J.; Orgun, N.N.; Jones, J.C.; Hylarides, M.D.; Pagel, J.M.; Hamlin, D.K.; Wilbur, D.S.; Lin, Y.; Fisher, D.R.; Kenoyer, A.L.; et al. A preclinical model of cd38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Res. 2014, 74, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; O’Steen, S.; Lin, Y.; Comstock, M.L.; Kenoyer, A.L.; Hamlin, D.K.; Scott Wilbur, D.; Fisher, D.R.; Nartea, M.; Hylarides, M.D.; et al. Cd38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other b-cell malignancies. Blood 2018, 131, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Bartholoma, M.D. Radioimmunotherapy of solid tumors: Approaches on the verge of clinical application. J. Labelled Comp. Radiopharm. 2018, 61, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Gautherot, E.; Bouhou, J.; Le Doussal, J.M.; Manetti, C.; Martin, M.; Rouvier, E.; Barbet, J. Therapy for colon carcinoma xenografts with bispecific antibody- targeted, iodine-131-labeled bivalent hapten. Cancer 1997, 80, 2618–2623. [Google Scholar] [CrossRef]
- Karacay, H.; McBride, W.J.; Griffiths, G.L.; Sharkey, R.M.; Barbet, J.; Hansen, H.J.; Goldenberg, D.M. Experimental pretargeting studies of cancer with a humanized anti-cea × murine anti-[in-dtpa] bispecific antibody construct and a (99m)tc-/188re-labeled peptide. Bioconjugate Chem. 2000, 11, 842–854. [Google Scholar] [CrossRef]
- Yazaki, P.J.; Lee, B.; Channappa, D.; Cheung, C.W.; Crow, D.; Chea, J.; Poku, E.; Li, L.; Andersen, J.T.; Sandlie, I.; et al. A series of anti-cea/anti-dota bispecific antibody formats evaluated for pre-targeting: Comparison of tumor uptake and blood clearance. Protein Eng. Des. Sel. 2013, 26, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Karacay, H.; Brard, P.Y.; Sharkey, R.M.; Chang, C.H.; Rossi, E.A.; McBride, W.J.; Ragland, D.R.; Horak, I.D.; Goldenberg, D.M. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin. Cancer Res. 2005, 11, 7879–7885. [Google Scholar] [CrossRef]
- Gautherot, E.; Rouvier, E.; Daniel, L.; Loucif, E.; Bouhou, J.; Manetti, C.; Martin, M.; Le Doussal, J.M.; Barbet, J. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131i-labeled bivalent hapten. J. Nucl. Med. 2000, 41, 480–487. [Google Scholar]
- Hosono, M.; Hosono, M.N.; Kraeber-Bodéré, F.; Devys, A.; Thédrez, P.; Fiche, M.; Gautherot, E.; Barbet, J.; Chatal, J.F. Biodistribution and dosimetric study in medullary thyroid cancer xenograft using bispecific antibody and iodine-125-labeled bivalent hapten. J. Nucl. Med. 1998, 39, 1608–1613. [Google Scholar]
- Kraeber-Bodere, F.; Faivre-Chauvet, A.; Sai-Maurel, C.; Gautherot, E.; Fiche, M.; Campion, L.; Le Boterff, J.; Barbet, J.; Chatal, J.F.; Thedrez, P. Bispecific antibody and bivalent hapten radioimmunotherapy in cea-producing medullary thyroid cancer xenograft. J. Nucl. Med. 1999, 40, 198–204. [Google Scholar]
- Sharkey, R.M.; Karacay, H.; Richel, H.; McBride, W.J.; Rossi, E.A.; Chang, K.; Yeldell, D.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin. Cancer Res. 2003, 9, 3897s–3913s. [Google Scholar] [PubMed]
- Mirallié, E.; Saï-Maurel, C.; Faivre-Chauvet, A.; Regenet, N.; Chang, C.H.; Goldenberg, D.M.; Chatal, J.F.; Barbet, J.; Thedrez, P. Improved pretargeted delivery of radiolabelled hapten to human tumour xenograft in mice by avidin chase of circulating bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Gautherot, E.; Kraeber-Bodéré, F.; Daniel, L.; Fiche, M.; Rouvier, É.; Saï-Maurel, C.; Thedrez, P.; Chatal, J.F.; Barbet, J. Immunohistology of carcinoembryonic antigen (cea)-expressing tumors grafted in nude mice after radioimmunotherapy with 131i-labeled bivalent hapten and anti-cea × antihapten bispecific antibody. Clin. Canc. Res. 1999, 5, 3177s–3182s. [Google Scholar]
- Kraeber-Bodéré, F.; Faivre-Chauvet, A.; Saï-Maurel, C.; Campion, L.; Fiche, M.; Gautherot, E.; Le Boterff, J.; Barbet, J.; Chatal, J.F.; Thédrez, P. Toxicity and efficacy of radioimmunotherapy in carcinoembryonic antigen- producing medullary thyroid cancer xenograft: Comparison of iodine 131- labeled f(ab’)2 and pretargeted bivalent hapten and evaluation of repeated injections. Clin. Canc. Res. 1999, 5, 3183s–3189s. [Google Scholar]
- Schoffelen, R.; Van Der Graaf, W.T.A.; Franssen, G.; Sharkey, R.M.; Goldenberg, D.M.; McBride, W.J.; Rossi, E.A.; Eek, A.; Oyen, W.J.G.; Boerman, O.C. Pretargeted 177lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice. J. Nucl. Med. 2010, 51, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Gautherot, E.; Le Doussal, J.M.; Bouhou, J.; Manetti, C.; Martin, M.; Rouvier, E.; Barbet, J. Delivery of therapeutic doses of radioiodine using bispecific antibody- targeted bivalent haptens. J. Nucl. Med. 1998, 39, 1937–1943. [Google Scholar] [PubMed]
- Gestin, J.F.; Loussouarn, A.; Bardiès, M.; Gautherot, E.; Gruaz-Guyon, A.; Saï-Maurel, C.; Barbet, J.; Curtet, C.; Chatal, J.F.; Faivre-Chauvet, A. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188re-labeled bivalent hapten: Biodistribution and dosimetry studies. J. Nucl. Med. 2001, 42, 146–153. [Google Scholar] [PubMed]
- Chinol, M.; Paganelli, G.; Sudati, F.; Meares, C.; Fazio, F. Biodistribution in tumour-bearing mice of two 90y-labelled biotins using three-step tumour targeting. Nucl. Med. Commun. 1997, 18, 176–182. [Google Scholar] [CrossRef]
- Liu, G.; Dou, S.; Mardirossian, G.; He, J.; Zhang, S.; Liu, X.; Rusckowski, M.; Hnatowich, D.J. Successful radiotherapy of tumor in pretargeted mice by 188re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin. Cancer. Res. 2006, 12, 4958–4964. [Google Scholar] [CrossRef]
- Bardies, M.; Bardet, S.; FaivreChauvet, A.; Peltier, P.; Douillard, J.Y.; Mahe, M.; Fiche, M.; Lisbona, A.; Giacalone, F.; Meyer, P.; et al. Bispecific antibody and iodine-131-labeled bivalent hapten dosimetry in patients with medullary thyroid or small-cell lung cancer. J. Nucl. Med. 1996, 37, 1853–1859. [Google Scholar]
- Chatal, J.F.; Campion, L.; Kraeber-Bodéré, F.; Bardet, S.; Vuillez, J.P.; Charbonnel, B.; Rohmer, V.; Chang, C.H.; Sharkey, R.M.; Goldenberg, D.M.; et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: A collaborative study with the french endocrine tumor group. J. Clin. Oncol. 2006, 24, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodéré, F.; Rousseau, C.; Bodet-Milin, C.; Ferrer, L.; Faivre-Chauvet, A.; Campion, L.; Vuillez, J.P.; Devillers, A.; Chang, C.H.; Goldenberg, D.M.; et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and131i-labeled bivalent hapten in a phase i optimization clinical trial. J. Nucl. Med. 2006, 47, 247–255. [Google Scholar] [PubMed]
- Salaun, P.Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase ii trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: Biomarker response and survival improvement. J. Nucl. Med. 2012, 53, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; Van Herpen, C.M.L.; Franssen, G.M.; McBride, W.J.; Chang, C.H.; Rossi, E.A.; Van Der Graaf, W.T.A.; et al. Development of an imaging-guided cea-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br. J. Cancer 2013, 109, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; Woliner-Van Der Weg, W.; Visser, E.P.; Goldenberg, D.M.; Sharkey, R.M.; McBride, W.J.; Chang, C.H.; Rossi, E.A.; Van Der Graaf, W.T.A.; Oyen, W.J.G.; et al. Predictive patient-specific dosimetry and individualized dosing of pretargeted radioimmunotherapy in patients with advanced colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Woliner-van der Weg, W.; Schoffelen, R.; Hobbs, R.F.; Gotthardt, M.; Goldenberg, D.M.; Sharkey, R.M.; Slump, C.H.; van der Graaf, W.T.; Oyen, W.J.; Boerman, O.C.; et al. Tumor and red bone marrow dosimetry: Comparison of methods for prospective treatment planning in pretargeted radioimmunotherapy. EJNMMI phys. 2014, 1, 104. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Ferrer, L.; Rauscher, A.; Masson, D.; Rbah-Vidal, L.; Faivre-Chauvet, A.; Cerato, E.; Rousseau, C.; Hureaux, J.; Couturier, O.; et al. Pharmacokinetics and dosimetry studies for optimization of pretargeted radioimmunotherapy in cea-expressing advanced lung cancer patients. Front. Med. (Lausanne) 2015, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Axworthy, D.B.; Reno, J.M.; Hylarides, M.D.; Mallett, R.W.; Theodore, L.J.; Gustavson, L.M.; Su, F.M.; Hobson, L.J.; Beaumier, P.L.; Fritzberg, A.R. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc. Natl. Acad. Sci. USA 2000, 97, 1802–1807. [Google Scholar] [CrossRef] [Green Version]
- Goshorn, S.; Sanderson, J.; Axworthy, D.; Lin, Y.; Hylarides, M.; Schultz, J. Preclinical evaluation of a humanized nr-lu-10 antibody-streptavidin fusion protein for pretargeted cancer therapy. Cancer Biother. Radiopharm. 2001, 16, 109–123. [Google Scholar] [CrossRef]
- Breitz, H.B.; Weiden, P.L.; Beaumier, P.L.; Axworthy, D.B.; Seiler, C.; Su, F.M.; Graves, S.; Bryan, K.; Reno, J.M. Clinical optimization of pretargeted radioimmunotherapy with antibody- streptavidin conjugate and 90y-dota-biotin. J. Nucl. Med. 2000, 41, 131–140. [Google Scholar]
- Breitz, H.B.; Fisher, D.R.; Goris, M.L.; Knox, S.; Ratliff, B.; Murtha, A.D.; Weiden, P.L. Radiation absorbed dose estimation for 90y-dota-biotin with pretargeted nr-lu-10/streptavidin. Cancer Biother. Radiopharm. 1999, 14, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.J.; Goris, M.L.; Tempero, M.; Weiden, P.L.; Gentner, L.; Breitz, H.; Adams, G.P.; Axworthy, D.; Gaffigan, S.; Bryan, K.; et al. Phase ii trial of yttrium-90-dota-biotin pretargeted by nr-lu-10 antibody/streptavidin in patients with metastatic colon cancer. Clin. Canc. Res. 2000, 6, 406–414. [Google Scholar]
- Lewis, M.R.; Wang, M.; Axworthy, D.B.; Theodore, L.J.; Mallet, R.W.; Fritzberg, A.R.; Welch, M.J.; Anderson, C.J. In vivo evaluation of pretargeted 64cu for tumor imaging and therapy. J. Nucl. Med. 2003, 44, 1284–1292. [Google Scholar] [PubMed]
- Domingo, R.J.; Reilly, R.M. Pre-targeted radioimmunotherapy of human colon cancer xenografts in athymic mice using streptavidin-cc49 monoclonal antibody and 90y-dota-biotin. Nucl. Med. Commun. 2000, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, D.J.; Khazaeli, M.B.; Axworthy, D.B.; Schultz, J.; Chaudhuri, T.R.; Zinn, K.R.; Carpenter, M.; LoBuglio, A.F. Intraperitoneal pretarget radioimmunotherapy with cc49 fusion protein. Clin. Cancer Res. 2005, 11, 8180–8185. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Shen, S.; Breitz, H.; Sims, R.B.; Axworthy, D.B.; Khazaeli, M.B.; Chen, K.H.; Percent, I.; Besh, S.; LoBuglio, A.F.; et al. Pretargeted radioimmunotherapy (rit) with a novel anti-tag-72 fusion protein. Cancer Biother. Radiopharm. 2005, 20, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Forero, A.; LoBuglio, A.F.; Breitz, H.; Khazaeli, M.B.; Fisher, D.R.; Wang, W.; Meredith, R.F. Patient-specific dosimetry of pretargeted radioimmunotherapy using cc49 fusion protein in patients with gastrointestinal malignancies. J. Nucl. Med. 2005, 46, 642–651. [Google Scholar] [PubMed]
- Liu, G.; Dou, S.; Baker, S.; Akalin, A.; Cheng, D.; Chen, L.; Rusckowski, M.; Hnatowich, D.J. A preclinical 188re tumor therapeutic investigation using morf/cmorf pretargeting and an antitag-72 antibody cc49. Cancer Biol. Ther. 2010, 10, 767–774. [Google Scholar] [CrossRef]
- Liu, G.; Dou, S.; Liu, Y.; Wang, Y.; Rusckowski, M.; Hnatowich, D.J. 90y labeled phosphorodiamidate morpholino oligomer for pretargeting radiotherapy. Bioconjugate Chem. 2011, 22, 2539–2545. [Google Scholar] [CrossRef]
- Lewis, M.R.; Zhang, J.; Jia, F.; Owen, N.K.; Cutler, C.S.; Embree, M.F.; Schultz, J.; Theodore, L.J.; Ketring, A.R.; Jurisson, S.S.; et al. Biological comparison of 149pm-, 166ho-, and 177lu-dota-biotin pretargeted by cc49 scfv-streptavidin fusion protein in xenograft-bearing nude mice. Nucl. Med. Biol. 2004, 31, 213–223. [Google Scholar] [CrossRef]
- Mohsin, H.; Jia, F.; Bryan, J.N.; Sivaguru, G.; Cutler, C.S.; Ketring, A.R.; Miller, W.H.; Simón, J.; Frank, R.K.; Theodore, L.J.; et al. Comparison of pretargeted and conventional cc49 radioimmunotherapy using 149pm, 166ho, and 177lu. Bioconjugate Chem. 2011, 22, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Läppchen, T.; Van Den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels-alder reaction for tumor pretargeting: In vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, S.M.J.; Rossin, R.; Van Den Bosch, S.M.; Wheatcroft, M.P.; Hudson, P.J.; Robillard, M.S. Diabody pretargeting with click chemistry in vivo. J. Nucl. Med. 2015, 56, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, G.; Grana, C.; Chinol, M.; Cremonesi, M.; De Cicco, C.; De Braud, F.; Robertson, C.; Zurrida, S.; Casadio, C.; Zoboli, S.; et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur. J. Nucl. Med. 1999, 26, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.; Chinol, M.; Robertson, C.; Mazzetta, C.; Bartolomei, M.; De Cicco, C.; Fiorenza, M.; Gatti, M.; Caliceti, P.; Paganelli, G. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: A pilot study. Br. J. Cancer 2002, 86, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.M.; Chinol, M.; de Cicco, C.; Bartolomei, M.; Cremonesi, M.; Bodei, L.; Rocca, P.A.; Pacifici, M.; Tiberini, S.; Baio, S.M.; et al. Eleven-year experience with the avidin–biotin pretargeting system in glioblastoma: Toxicity, efficacy and survival. Open Nucl. Med. J. 2012, 4, 14–20. [Google Scholar] [CrossRef]
- Riva, P.; Franceschi, G.; Riva, N.; Casi, M.; Santimaria, M.; Adamo, M. Role of nuclear medicine in the treatment of malignant gliomas: The locoregional radioimmunotherapy approach. Eur. J. Nucl. Med. 2000, 27, 601–609. [Google Scholar] [CrossRef]
- Paganelli, G.; Bartolomei, M.; Ferrari, M.; Cremonesi, M.; Broggi, G.; Maira, G.; Sturiale, C.; Grana, C.; Prisco, G.; Gatti, M.; et al. Pre-targeted locoregional radioimmunotheraphy with 90y-biotin in glioma patients: Phase i study and preliminary therapeutic results. Cancer Biother. Radiopharm. 2001, 16, 227–235. [Google Scholar] [CrossRef]
- Urbano, N.; Papi, S.; Ginanneschi, M.; De Santis, R.; Pace, S.; Lindstedt, R.; Ferrari, L.; Choi, S.; Paganelli, G.; Chinol, M. Evaluation of a new biotin-dota conjugate for pretargeted antibody-guided radioimmunotherapy (pagrit®). Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 68–77. [Google Scholar] [CrossRef]
- Goodwin, D.A.; Meares, C.F.; Watanabe, N.; McTigue, M. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994, 54, 5937–5946. [Google Scholar]
- Lubic, S.P.; Goodwin, D.A.; Meares, C.F.; Song, C.; Osen, M.; Hays, M. Biodistribution and dosimetry of pretargeted monoclonal antibody 2d12.5 and y-janus-dota in balb/c mice with khjj mouse adenocarcinoma. J. Nucl. Med. 2001, 42, 670–678. [Google Scholar] [PubMed]
- Cremonesi, M.; Ferrari, M.; Chinol, M.; Stabin, M.G.; Grana, C.; Prisco, G.; Robertson, C.; Tosi, G.; Paganelli, G. Three-step radioimmunotherapy with yttrium-90 biotin: Dosimetry and pharmacokinetics in cancer patients. Eur. J. Nucl. Med. 1999, 26, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.; Bartolomei, M.; Handkiewicz, D.; Rocca, P.; Bodei, L.; Colombo, N.; Chinol, M.; Mangioni, C.; Malavasi, F.; Paganelli, G. Radioimmunotherapy in advanced ovarian cancer: Is there a role for pre-targeting with 90y-biotin? Gynecol. Oncol. 2004, 93, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Hosono, M.; Hosono, M.N.; Kraeber-Bodéré, F.; Devys, A.; Thédrez, P.; Faivre-Chauvet, A.; Gautherot, E.; Barbet, J.; Chatal, J.F. Two-step targeting and dosimetry for small cell lung cancer xenograft with anti-ncam/antihistamine bispecific antibody and radioiodinated bivalent hapten. J. Nucl. Med. 1999, 40, 1216–1221. [Google Scholar] [PubMed]
- Sato, N.; Hassan, R.; Axworthy, D.B.; Wong, K.J.; Yu, S.; Theodore, L.J.; Lin, Y.; Park, L.; Brechbiel, M.W.; Pastan, I.; et al. Pretargeted radioimmunotherapy of mesothelin-expressing cancer using a tetravalent single-chain fv-streptavidin fusion protein. J. Nucl. Med. 2005, 46, 1201–1209. [Google Scholar] [PubMed]
- Westwood, J.A.; Murray, W.K.; Trivett, M.; Haynes, N.M.; Solomon, B.; Mileshkin, L.; Ball, D.; Michael, M.; Burman, A.; Mayura-Guru, P.; et al. The lewis-y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected t cells despite the presence of soluble antigen in serum. J. Immunother. 2009, 32, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, M.; Axworthy, D.B.; Wong, K.J.; Garmestani, K.; Park, L.; Park, C.W.; Mallett, R.W.; Theodore, L.J.; Yau, E.K.; et al. Radioimmunotherapy of a431 xenografted mice with pretargeted b3 antibody-streptavidin and 90y-labeled 1,4,7,10-tetraazacyclododecane-n,n′,n′,n‴-tetraacetic acid (dota)-biotin. Cancer Res. 2002, 62, 5755–5760. [Google Scholar] [PubMed]
- Cheal, S.M.; Xu, H.; Guo, H.F.; Zanzonico, P.B.; Larson, S.M.; Cheung, N.K. Preclinical evaluation of multistep targeting of diasialoganglioside gd2 using an igg-scfv bispecific antibody with high affinity for gd2 and dota metal complex. Mol. Cancer Ther. 2014, 13, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Cheal, S.M.; Xu, H.; Guo, H.F.; Lee, S.G.; Punzalan, B.; Chalasani, S.; Fung, E.K.; Jungbluth, A.; Zanzonico, P.B.; Carrasquillo, J.A.; et al. Theranostic pretargeted radioimmunotherapy of colorectal cancer xenografts in mice using picomolar affinity 86y- or 177lu-dota-bn binding scfv c825/gpa33 igg bispecific immunoconjugates. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 925–937. [Google Scholar] [CrossRef]
- Cheal, S.M.; Fung, E.K.; Patel, M.; Xu, H.; Guo, H.F.; Zanzonico, P.B.; Monette, S.; Wittrup, K.D.; Cheung, N.K.V.; Larson, S.M. Curative multicycle radioimmunotherapy monitored by quantitative spect/ct-based theranostics, using bispecific antibody pretargeting strategy in colorectal cancer. J. Nucl. Med. 2017, 58, 1735–1742. [Google Scholar] [CrossRef]
- Van Rij, C.M.; Frielink, C.; Goldenberg, D.M.; Sharkey, R.M.; Lütje, S.; McBride, W.J.; Oyen, W.J.G.; Boerman, O.C. Pretargeted radioimmunotherapy of prostate cancer with an anti-trop-2×anti-hsg bispecific antibody and a 177lu-labeled peptide. Cancer Biother. Radiopharm. 2014, 29, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Lütje, S.; Frielink, C.; Sharkey, R.M.; Goldenberg, D.M.; Franssen, G.M.; McBride, W.J.; Rossi, E.A.; Oyen, W.J.G.; Boerman, O.C. Pretargeted immuno-pet and radioimmunotherapy of prostate cancer with an anti-trop-2 × anti-hsg bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Cheal, S.M.; Xu, H.; Guo, H.F.; Patel, M.; Punzalan, B.; Fung, E.K.; Lee, S.G.; Bell, M.; Singh, M.; Jungbluth, A.A.; et al. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of her2-positive breast carcinoma. Theranostics 2018, 8, 5106–5125. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, K.; Altai, M.; Mitran, B.; Konijnenberg, M.; Oroujeni, M.; Atterby, C.; De Jong, M.; Orlova, A.; Mattsson, J.; Micke, P.; et al. Radionuclide therapy of her2-expressing human xenografts using affibody-based peptide nucleic acid-mediated pretargeting: In vivo proof of principle. J. Nucl. Med. 2018, 59, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted immuno-pet of pancreatic cancer: Overcoming circulating antigen and internalized antibody to reduce radiation doses. J. Nucl. Med. 2016, 57, 453–459. [Google Scholar] [CrossRef]
- Membreno, R.; Cook, B.E.; Fung, K.; Lewis, J.S.; Zeglis, B.M. Click-mediated pretargeted radioimmunotherapy of colorectal carcinoma. Mol. Pharm. 2018, 15, 1729–1734. [Google Scholar] [CrossRef]
- Paganelli, G.; Ferrari, M.; Cremonesi, M.; De Cicco, C.; Galimberti, V.; Luini, A.; Veronesi, P.; Fiorenza, M.; Carminati, P.; Zanna, C.; et al. Iart (r): Intraoperative avidination for radionuclide treatment. A new way of partial breast irradiation. Breast 2007, 16, 17–26. [Google Scholar] [CrossRef]
- De Santis, R.; Leoni, B.; Rosi, A.; Albertoni, C.; Forni, G.; Cojoca, R.; Iezzi, M.; Musiani, P.; Paganelli, G.; Chinol, M.; et al. Avidinox™ for highly efficient tissue-pretargeted radionuclide therapy. Cancer Biother. Radiopharm. 2010, 25, 143–148. [Google Scholar] [CrossRef]
- Albertoni, C.; Leoni, B.; Rosi, A.; D’Alessio, V.; Carollo, V.; Spagnoli, L.G.; Van Echteld, C.; De Santis, R. Radionuclide therapy of unresectable tumors with avidinox and (90)Y-biotinDOTA: Tongue cancer paradigm. Cancer Biother. Radiopharm. 2015, 30, 291–298. [Google Scholar] [CrossRef]
- Park, S.I.; Shenoi, J.; Page, J.M.; Hamlin, D.K.; Wilbur, D.S.; Orgun, N.; Kenoyer, A.L.; Frayo, S.; Axtman, A.; Bäck, T.; et al. Conventional and pretargeted radioimmunotherapy using bismuth-213 to target and treat non-hodgkin lymphomas expressing cd20: A preclinical model toward optimal consolidation therapy to eradicate minimal residual disease. Blood 2010, 116, 4231–4239. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Z.; Garmestani, K.; Axworthy, D.B.; Zhang, Z.; Mallett, R.W.; Theodore, L.J.; Goldman, C.K.; Brechbiel, M.W.; Carrasquillo, J.A.; et al. Pretargeting radioimmunotherapy of a murine model of adult t-cell leukemia with the α-emitting radionuclide, bismuth 213. Blood 2002, 100, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; Garmestani, K.; Schultz, J.; Axworthy, D.B.; Goldman, C.K.; Brechbiel, M.W.; Carrasquillo, J.A.; Waldmann, T.A. Pretarget radiotherapy with an anti-cd25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc. Natl. Acad. Sci. USA 2003, 100, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.M.; Kenoyer, A.L.; Bäck, T.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Park, S.I.; Frayo, S.; Axtman, A.; Orgun, N.; et al. Anti-cd45 pretargeted radioimmunotherapy using bismuth-213: High rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood 2011, 118, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.H.L.; Bäck, T.; Chouin, N.; Jensen, H.; Hultborn, R.; Jacobsson, L.; Lindegren, S. In vivo distribution of avidin-conjugated mx35 and 211at- labeled, biotinylated poly-l-lysine for pretargeted intraperitoneal α-radioimmunotherapy. Cancer Biother. Radiopharm. 2011, 26, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.H.L.; Bäck, T.; Chouin, N.; Hultborn, R.; Jacobsson, L.; Elgqvist, J.; Jensen, H.; Albertsson, P.; Lindegren, S. Comparison of 211at-prit and 211at-rit of ovarian microtumors in a nude mouse model. Cancer Biother. Radiopharm. 2013, 28, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Frampas, E.; Maurel, C.; Saëc, P.R.L.; Mauxion, T.; Faivre-Chauvet, A.; Davodeau, F.; Goldenberg, D.M.; Bardiès, M.; Barbet, J. Pretargeted radioimmunotherapy of colorectal cancer metastases: Models and pharmacokinetics predict influence of the physical and radiochemical properties of the radionuclide. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, M.; Garmestani, K.; Axworthy, D.B.; Mallett, R.W.; Fritzberg, A.R.; Theodore, L.J.; Plascjak, P.S.; Eckelman, W.C.; Waldmann, T.A.; et al. Pretargeted α emitting radioimmunotherapy using 213bi 1,4,7,10-tetraazacyclododecane-n,n′,n″,n‴-tetraacetic acid-biotin. Clin. Cancer Res. 2004, 10, 3137–3146. [Google Scholar] [CrossRef]
- Heskamp, S.; Hernandez, R.; Molkenboer-Kuenen, J.D.M.; Essler, M.; Bruchertseifer, F.; Morgenstern, A.; Steenbergen, E.J.; Cai, W.; Seidl, C.; McBride, W.J.; et al. A-versus β-emitting radionuclides for pretargeted radioimmunotherapy of carcinoembryonic antigen-expressing human colon cancer xenografts. J. Nucl. Med. 2017, 58, 926–933. [Google Scholar] [CrossRef]
- Gustafsson-Lutz, A.; Bäck, T.; Aneheim, E.; Palm, S.; Morgenstern, A.; Bruchertseifer, F.; Albertsson, P.; Lindegren, S. Biotinylated and chelated poly-l-lysine as effector for pretargeting in cancer therapy and imaging. Int. J. Pharmcy Pharm. Sci 2017, 9, 87–93. [Google Scholar] [CrossRef]
- Su, F.M.; Beaumier, P.; Axworthy, D.; Atcher, R.; Fritzberg, A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212pb/212bi generator. Nucl. Med. Biol. 2005, 32, 741–747. [Google Scholar] [CrossRef]
- Poty, S.; Carter, L.M.; Mandleywala, K.; Membreno, R.; Abdel-Atti, D.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. Leveraging bioorthogonal click chemistry to improve 225ac-radioimmunotherapy of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2019, 25, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodéré, F.; Saï-Maurel, C.; Campion, L.; Faivre-Chauvet, A.; Mirallié, E.; Chérel, M.; Supiot, S.; Barbet, J.; Chatal, J.F.; Thédrez, P. Enhanced antitumor activity of combined pretargeted radioimmunotherapy and paclitaxel in medullary thyroid cancer xenograft. Mol. Cancer Ther. 2002, 1, 267–274. [Google Scholar] [PubMed]
- Graves, S.S.; Dearstyne, E.; Lin, Y.; Zuo, Y.; Sanderson, J.; Schultz, J.; Pantalias, A.; Gray, D.; Axworthy, D.; Jones, H.M.; et al. Combination therapy with pretarget cc49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin. Cancer Res. 2003, 9, 3712–3721. [Google Scholar] [PubMed]
- Karacay, H.; Sharkey, R.M.; Gold, D.V.; Ragland, D.R.; McBride, W.J.; Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: Tf10- 90y-imp-288 alone and combined with gemcitabine. J. Nucl. Med. 2009, 50, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Mazzetta, C.; Handkiewicz-Junak, D.; Bodei, L.; Rocca, P.; Grana, C.; Maira, G.; Sturiale, C.; Villa, G.; Paganelli, G. Combined treatment of glioblastoma patients with locoregional pre-targeted 90y-biotin radioimmunotherapy and temozolomide. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 220–228. [Google Scholar]
- Paganelli, G.; Orecchia, R.; Jereczek-Fossa, B.; Grana, C.; Cremonesi, M.; De Braud, F.; Tradati, N.; Chinol, M. Combined treatment of advanced oropharyngeal cancer with external radiotherapy and three-step radioimmunotherapy. Eur. J. Nucl. Med. 1998, 25, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Karacay, H.; Johnson, C.R.; Litwin, S.; Rossi, E.A.; Mebride, W.J.; Chang, C.H.; Goldenberg, D.M. Pretargeted versus directly targeted radioimmunotherapy combined with anti-cd20 antibody consolidation therapy of non-hodgkin lymphoma. J. Nucl. Med. 2009, 50, 444–453. [Google Scholar] [CrossRef]
- Cao, Y.; Suresh, M.R. Bispecific mab aided liposomal drug delivery. J. Drug Targeting 2000, 8, 257–266. [Google Scholar] [CrossRef]
- Loughrey, H.C.; Ferraretto, A.; Cannon, A.M.; Acerbis, G.; Sudati, F.; Bottiroli, G.; Masserini, M.; Soria, M.R. Characterisation of biotinylated liposomes for in vivo targeting applications. FEBS Lett. 1993, 332, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Longman, S.A.; Cullis, P.R.; Choi, L.; De Jong, G.; Bally, M.B. A two-step targeting approach for delivery of doxorubicin-loaded liposomes to tumour cells in vivo. Cancer Chemother. Pharmacol. 1995, 36, 91–101. [Google Scholar] [CrossRef]
- Lehtinen, J.; Raki, M.; Bergström, K.A.; Uutela, P.; Lehtinen, K.; Hiltunen, A.; Pikkarainen, J.; Liang, H.; Pitkänen, S.; Määttä, A.M.; et al. Pre-targeting and direct immunotargeting of liposomal drug carriers to ovarian carcinoma. PLoS ONE 2012, 7, e41410. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, L.; Chen, W.; Yao, M.; Lu, W. Targeting to tumor necrotic regions with biotinylated antibody and streptavidin modified liposomes. J. Control. Release 2008, 125, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Khaw, B.A.; Gada, K.S.; Patil, V.; Panwar, R.; Mandapati, S.; Hatefi, A.; Majewski, S.; Weisenberger, A. Bispecific antibody complex pre-targeting and targeted delivery of polymer drug conjugates for imaging and therapy in dual human mammary cancer xenografts: Targeted polymer drug conjugates for cancer diagnosis and therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.; Pelagi, M.; Fulci, G.; Paganelli, G.; Dellabona, P.; Casorati, G.; Siccardi, A.G.; Corti, A. Tumor cell targeting with antibody-avidin complexes and biotinylated tumor necrosis factor α. Cancer Res. 1997, 57, 1922–1928. [Google Scholar] [PubMed]
- Gasparri, A.; Moro, M.; Curnis, F.; Sacchi, A.; Pagano, S.; Veglia, F.; Casorati, G.; Siccardi, A.G.; Dellabona, P.; Corti, A. Tumor pretargeting with avidin improves the therapeutic index of biotinylated tumor necrosis factor α in mouse models. Cancer Res. 1999, 59, 2917–2923. [Google Scholar] [PubMed]
- Tarrus, M.; Van Der Sloot, A.M.; Temming, K.; Lacombe, M.; Opdam, F.; Quax, W.J.; Molema, G.; Poelstra, K.; Kok, R.J. Rgd-avidin–biotin pretargeting to αvβ3 integrin enhances the proapoptotic activity of tnfα related apoptosis inducing ligand (trail). Apoptosis 2008, 13, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.W.; Zhang, R.; Yang, J.; Chao, M.P.; Shami, P.J.; Kopeček, J. A two-step pretargeted nanotherapy for cd20 crosslinking may achieve superior anti-lymphoma efficacy to rituximab. Theranostics 2015, 5, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Wang, J.; Kopecek, J. Amplification of cd20 cross-linking in rituximab-resistant b-lymphoma cells enhances apoptosis induction by drug-free macromolecular therapeutics. ACS Nano 2018, 12, 3658–3670. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Kato, Y.; Artemov, D. Bioorthogonal two-component drug delivery in her2(+) breast cancer mouse models. Sci. Rep. 2016, 6, 24298. [Google Scholar] [CrossRef]
- Panwar, R.; Bhattarai, P.; Patil, V.; Gada, K.; Majewski, S.; Khaw, B.A. Imaging doxorubicin and polymer-drug conjugates of doxorubicin-induced cardiotoxicity with bispecific anti-myosin-anti-dtpa antibody and tc-99m-labeled polymers. J. Nucl. Cardiol. 2019, 26, 1327–1344. [Google Scholar] [CrossRef]
- Rauscher, A.; Frindel, M.; Maurel, C.; Maillasson, M.; Le Saëc, P.; Rajerison, H.; Gestin, J.F.; Barbet, J.; Faivre-Chauvet, A.; Mougin-Degraef, M. Influence of pegylation and hapten location at the surface of radiolabelled liposomes on tumour immunotargeting using bispecific antibody. Nucl. Med. Biol. 2014, 41, e66–e74. [Google Scholar] [CrossRef] [PubMed]
- Gedda, L.; Fondell, A.; Lundqvist, H.; Park, J.W.; Edwards, K. Experimental radionuclide therapy of her2-expressing xenografts using two-step targeting nuclisome particles. J. Nucl. Med. 2012, 53, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Iacono, P.; Perez-Medina, C.; Mulder, W.J.M.; Kircher, M.F.; Reiner, T. Specific binding of liposomal nanoparticles through inverse electron-demand diels-alder click chemistry. ChemistryOpen 2017, 6, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Spa, S.J.; Welling, M.M.; van Oosterom, M.N.; Rietbergen, D.D.D.; Burgmans, M.C.; Verboom, W.; Huskens, J.; Buckle, T.; van Leeuwen, F.W.B. A supramolecular approach for liver radioembolization. Theranostics 2018, 8, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Spa, S.J.; van Willigen, D.M.; Rietbergen, D.D.D.; Roestenberg, M.; Buckle, T.; van Leeuwen, F.W.B. In vivo stability of supramolecular host–guest complexes monitored by dual-isotope multiplexing in a pre-targeting model of experimental liver radioembolization. J. Control. Release 2019, 293, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Lee, S.; Na, J.H.; Kim, S.H.; Hahn, S.K.; Choi, K.; Kwon, I.C.; Jeong, S.Y.; Kim, K. Bioorthogonal copper-free click chemistry invivo for tumor-targeted delivery of nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 11836–11840. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Prabha, S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials 2016, 88, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, S.; Koo, H.; Na, J.H.; Yoon, H.Y.; Shim, M.K.; Park, J.; Kim, J.H.; Lee, S.; Pomper, M.G.; et al. Nano-sized metabolic precursors for heterogeneous tumor-targeting strategy using bioorthogonal click chemistry in vivo. Biomaterials 2017, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, L.; Liu, Y.; Dobrucka, I.T.; Dobrucki, L.W.; Yin, L.; Cheng, J. In vivo targeting of metabolically labeled cancers with ultra-small silica nanoconjugates. Theranostics 2016, 6, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.H.; Li, F.; Zhang, F.; Huang, L.L.; Zhang, L.J.; Lv, Y.L.; Wei, W.; Xie, H.Y. Amplifying nanoparticle targeting performance to tumor via diels-alder cycloaddition. Adv. Funct. Mater. 2018, 28, 1707596. [Google Scholar] [CrossRef]
- Yang, Q.; Parker, C.L.; Lin, Y.; Press, O.W.; Park, S.I.; Lai, S.K. Pretargeting with bispecific fusion proteins facilitates delivery of nanoparticles to tumor cells with distinct surface antigens. J. Control. Release 2017, 255, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeven, M.; Seimbille, Y.; Dalm, S.U. Therapeutic Applications of Pretargeting. Pharmaceutics 2019, 11, 434. https://doi.org/10.3390/pharmaceutics11090434
Verhoeven M, Seimbille Y, Dalm SU. Therapeutic Applications of Pretargeting. Pharmaceutics. 2019; 11(9):434. https://doi.org/10.3390/pharmaceutics11090434
Chicago/Turabian StyleVerhoeven, Marjolein, Yann Seimbille, and Simone U. Dalm. 2019. "Therapeutic Applications of Pretargeting" Pharmaceutics 11, no. 9: 434. https://doi.org/10.3390/pharmaceutics11090434