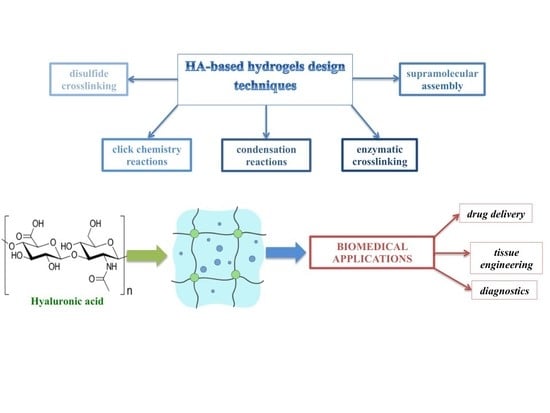
Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery
Abstract
:1. Introduction
2. Physical and Chemical Hydrogels
2.1. Chemical Hydrogels
2.1.1. Diels Alder Reaction (Click Chemistry)
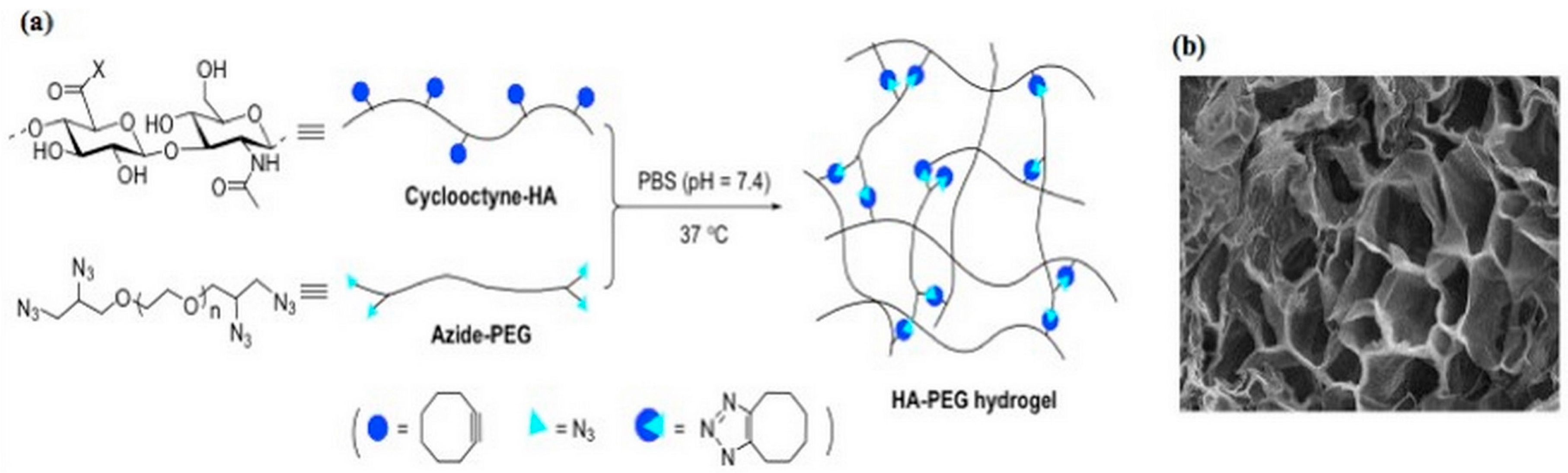
2.1.2. Azide-Alkyn Huisgen Cycloaddition (Click Chemistry)
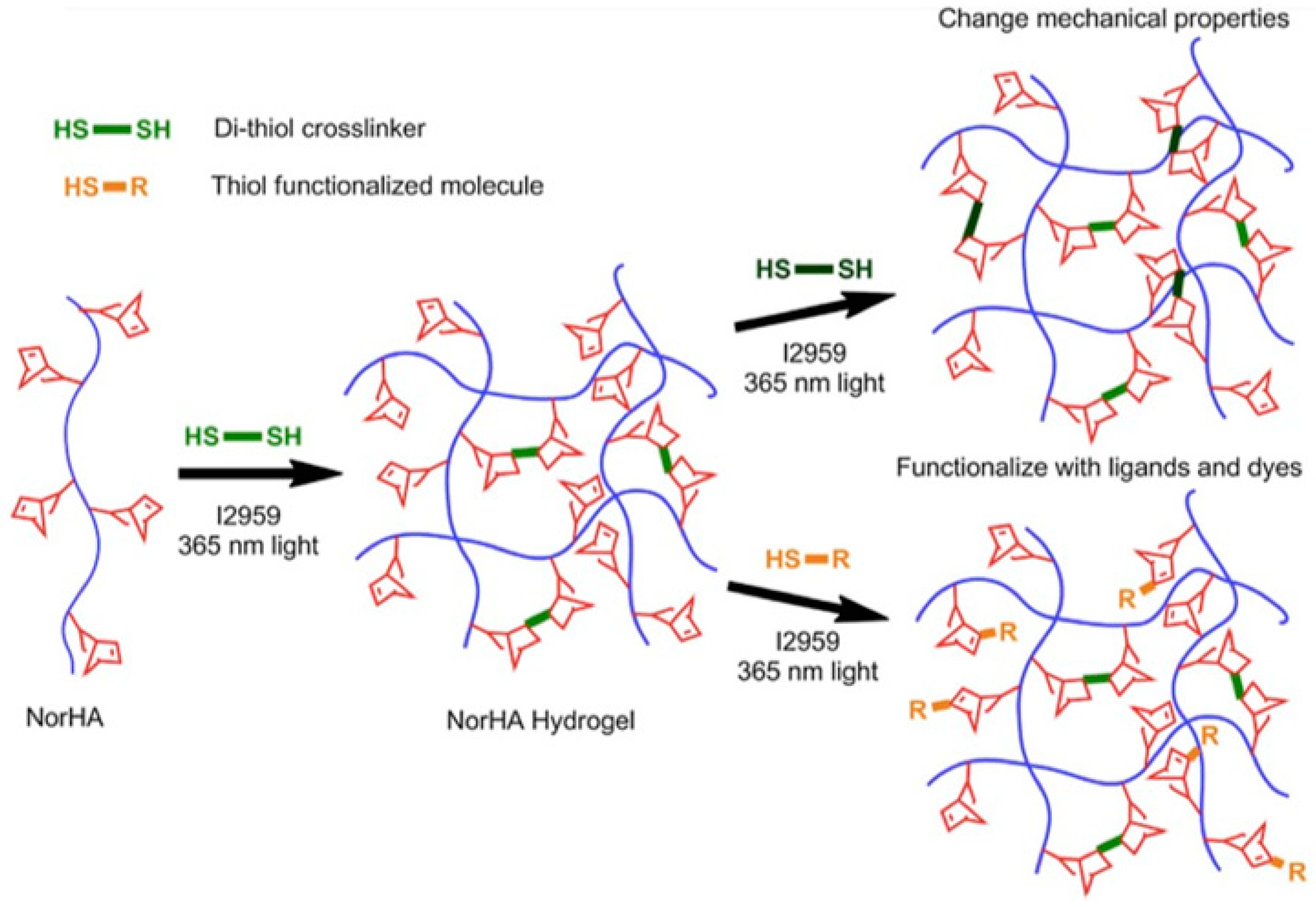
2.1.3. Thiol-ene Photocoupling (Click Chemistry)
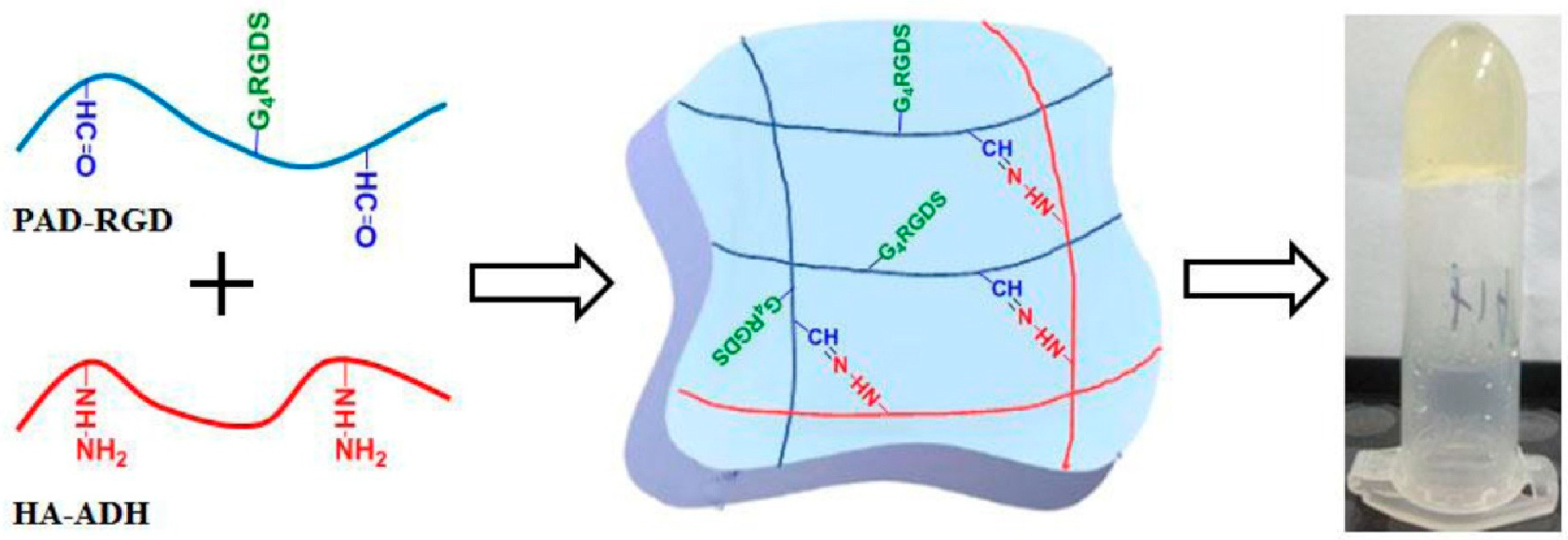
2.1.4. Aldehyde-Hydrazide Coupling (Click Chemistry)
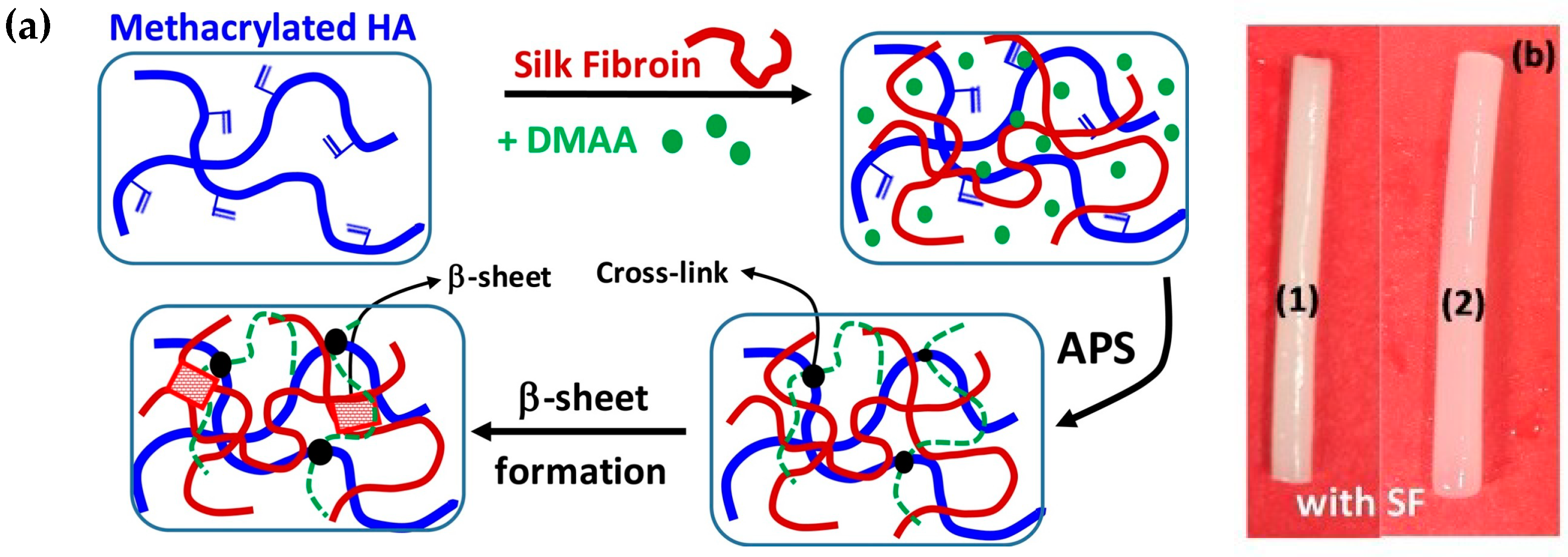
2.1.5. Enzymatic Crosslinking
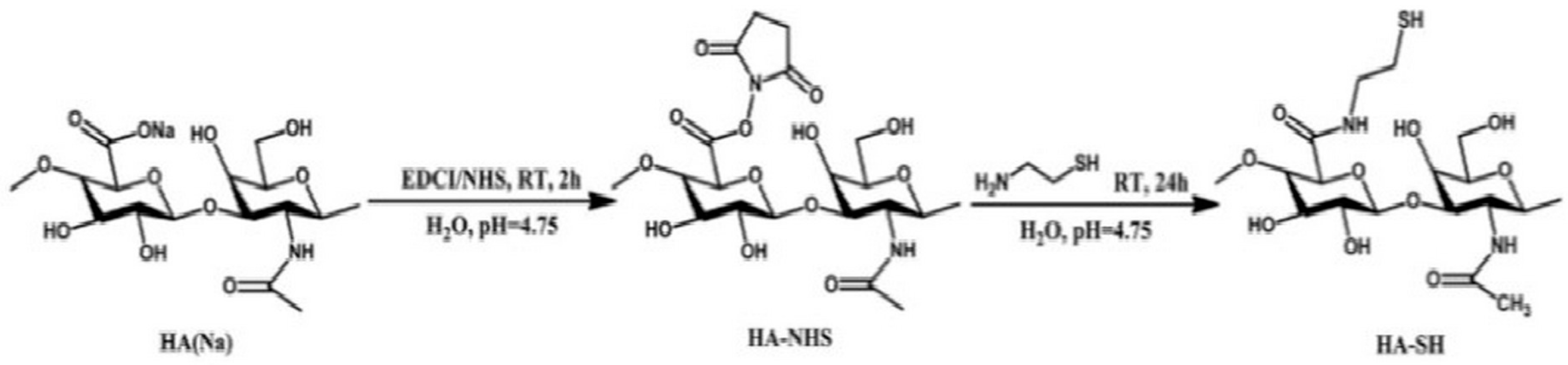
2.1.6. Disulfide Crosslinking
2.1.7. Crosslinking by Radical Polymerization
2.1.8. Crosslinking by Condensation Reactions
2.2. Physical Hydrogels
3. HA-Based Hydrogels for Biomedical Applications
3.1. Drug Delivery
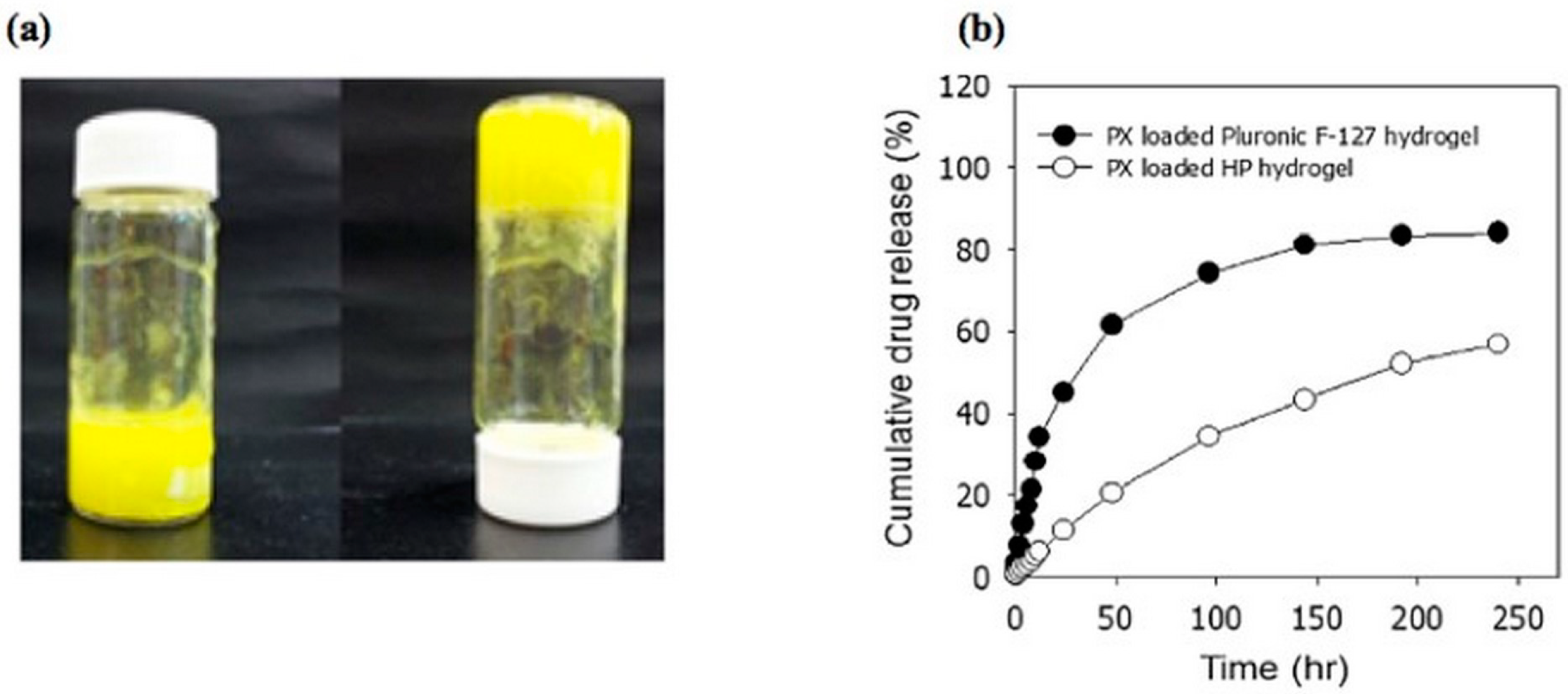
3.1.1. Stimuli-Responsive Hydrogels
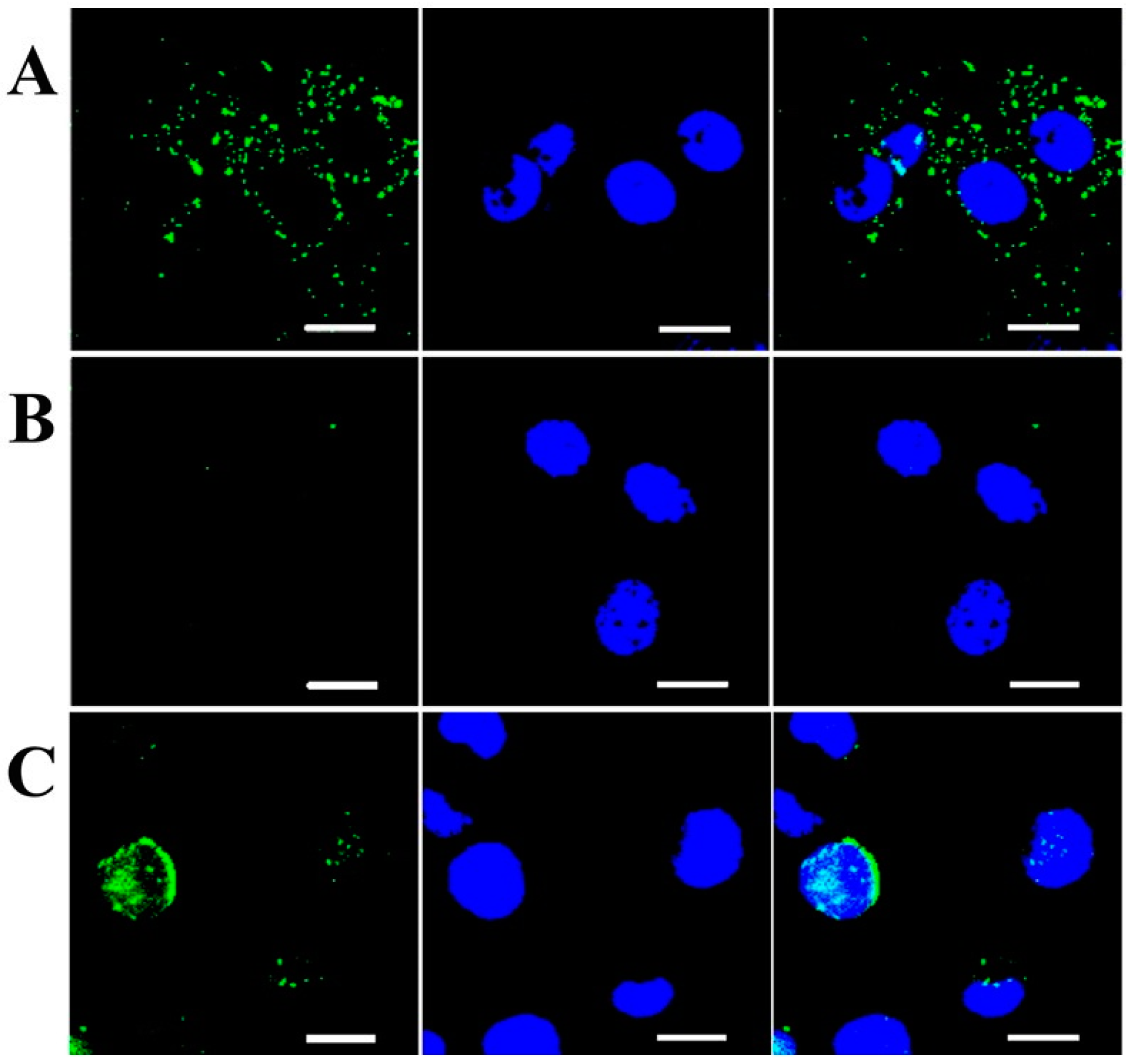
3.1.2. HA-Based Hydrogels for Targeted Cancer Treatment
3.1.3. HA-Based Hydrogels for Osteoarthritis Treatment
4. Conclusions
Funding
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- ConvaTec Website. Available online: https://www.convatec.it/ (accessed on 12 June 2019).
- Covidien Website. Available online: https://www.covidien.com/ (accessed on 12 June 2019).
- Blanchard, C.R.; Timmons, S.F.; Smith, R.A. Keratin-Based Hydrogel for Biomedical Applications and Method of Production. U.S. Patent 6,379,690 B2, 30 April 2002. [Google Scholar]
- Kumar, A. Hydrogel scaffolds for tissue engineering. U.S. Patent 2013/0236971 A1, 12 September 2013. [Google Scholar]
- Ocular Therapeutix Website. Available online: https://www.ocutx.com/products/dextenza/ (accessed on 12 June 2019).
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Prehm, P. Hyaluronate is synthesized at plasma membranes. Biochem. J. 1984, 220, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Kang, Q.K.; Ramamurthi, A. The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels. J. Biomed. Mater. Res. Part A 2010, 94, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, V.; Francescangeli, A.; Taglienti, A. New gelatin-based hydrogels via enzymatic networking. Biomacromolecules 2002, 3, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.W.; Swann, D.A.; Prestwich, G.D. Chemical modification of hyaluronic acid by carbodiimides. Bioconj. Chem. 1991, 2, 232–241. [Google Scholar] [CrossRef]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Fisher, S.A.; Anandakumaran, P.N.; Owen, S.C.; Shoichet, M.S. Tuning the microenvironment: Click-crosslinked hyaluronic acid-based hydrogels provide a platform for studying breast cancer cell invasion. Adv. Funct. Mater. 2015, 25, 7163–7172. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Li, Y.; Chen, X. Diels–Alder click-based hydrogels for direct spatiotemporal postpatterning via photoclick chemistry. ACS Macro Lett. 2015, 4, 289–292. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Pahimanolis, N.; Sorvari, A.; Luong, N.D.; Seppälä, J. Thermoresponsive xylan hydrogels via copper-catalyzed azide-alkyne cycloaddition. Carbohydr. Polym. 2014, 102, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Adzima, B.J.; Baker, N.H.; Bowman, C.N. Photopolymerization reactions using the photoinitiated Copper (I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) reaction. Adv. Mater. 2013, 25, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Manzi, G.; Zoratto, N.; Matano, S.; Sabia, R.; Villani, C.; Coviello, T.; Matricardi, P.; Di Meo, C. “Click” hyaluronan based nanohydrogels as multifunctionalizable carriers for hydrophobic drugs. Carbohydr. Polym. 2017, 174, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.T.; Baskin, J.M.; Amacher, S.L.; Bertozzi, C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 2008, 320, 664–667. [Google Scholar] [CrossRef]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly (ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Sawicki, L.A.; Kloxin, A.M. Design of thiol–ene photoclick hydrogels using facile techniques for cell culture applications. Biomater.Sci. 2014, 2, 1612–1626. [Google Scholar] [CrossRef]
- Yang, C.; Mariner, P.D.; Nahreini, J.N.; Anseth, K.S. Cell-mediated delivery of glucocorticoids from thiol-ene hydrogels. J. Controll. Release 2012, 162, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Halevi, A.E.; Nuttelman, C.R.; Bowman, C.N.; Anseth, K.S. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv. Mater. 2009, 21, 5005–5010. [Google Scholar] [CrossRef]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef]
- Gramlich, W.M.; Kim, I.L.; Burdick, J.A. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 2013, 34, 9803–9811. [Google Scholar] [CrossRef] [Green Version]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly (L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, C.; Hou, S.; Yu, X.; Cui, F.; Xu, Q.; Sheng, S.; Cui, H.; Li, H. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: In vitro. J. Controll. Release 2005, 102, 13–22. [Google Scholar] [CrossRef]
- Ito, T.; Fraser, I.P.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Anti–inflammatory function of an in situ cross–linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials 2007, 28, 1778–1786. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self–crosslinking and injectable hyaluronic acid/RGD–functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently adaptable elastin-like protein–hyaluronic acid (ELP–HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 2017, 27, 1605609. [Google Scholar] [CrossRef]
- Kurisawa, M.; Lee, F.; Wang, L.S.; Chung, J.E. Injectable enzymatically crosslinked hydrogel system with independent tuning of mechanical strength and gelation rate for drug delivery and tissue engineering. J. Mater. Chem. 2010, 20, 5371–5375. [Google Scholar] [CrossRef]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.M.; Park, K.D. Supramolecular hydrogels exhibiting fast in situ gel forming and adjustable degradation properties. Biomacromolecules 2010, 11, 617–625. [Google Scholar] [CrossRef]
- Roberts, J.J.; Naudiyal, P.; Lim, K.S.; Poole–Warren, L.A.; Martens, P.J. A comparative study of enzyme initiators for crosslinking phenol–functionalized hydrogels for cell encapsulation. Biomater. Res. 2016, 20, 30. [Google Scholar] [CrossRef]
- Rizzuto, F.; Spikes, J.D. The eosin-sensitized photooxidation of substituted phenylalanines and tyrosines. Photochem. Photobiol. 1977, 25, 465–476. [Google Scholar] [CrossRef]
- Xu, K.; Narayanan, K.; Lee, F.; Bae, K.H.; Gao, S.; Kurisawa, M. Enzyme–mediated hyaluronic acid–tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015, 24, 159–171. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk–hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Su, J. Thiol–mediated chemoselective strategies for in situ formation of hydrogels. Gels 2018, 4, 72. [Google Scholar] [CrossRef]
- Choh, S.-Y.; Cross, D.; Wang, C. Facile synthesis and characterization of disulfide–cross–linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules 2011, 12, 1126–1136. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self–crosslinking smart hyaluronic acid hydrogels as injectable three–dimensional scaffolds for cells culture. Coll. Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Azémar, A.; Oommen, O.P.; Hilborn, J.N.; Varghese, O.P. Modulating Thiol pKa promotes disulfide formation at physiological pH: An elegant strategy to design disulfide cross–linked hyaluronic acid hydrogels. Biomacromolecules 2019, 20, 1412–1420. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Ibrahim, S.; Kothapalli, C.; Kang, Q.; Ramamurthi, A. Characterization of glycidyl methacrylate–crosslinked hyaluronan hydrogel scaffolds incorporating elastogenic hyaluronan oligomers. Acta Biomater. 2011, 7, 653–665. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.; Öner, F.C.; Dhert, W.J.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PloS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef]
- Tavsanli, B.; Okay, O. Mechanically robust and stretchable silk/hyaluronic acid hydrogels. Carbohydr. Polym. 2019, 208, 413–420. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent–free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, J.; Wang, H.; Huang, J.; Yu, Y.; Zhang, Q.; Cheng, Y. Dynamic softening or stiffening a supramolecular hydrogel by ultraviolet or near–infrared light. ACS Appl. Mater. Interfaces 2017, 9, 24511–24517. [Google Scholar] [CrossRef]
- Rombouts, W.H.; de Kort, D.W.; Pham, T.T.; van Mierlo, C.P.; Werten, M.W.; de Wolf, F.A.; van der Gucht, J. Reversible temperature–switching of hydrogel stiffness of coassembled, silk–collagen–like hydrogels. Biomacromolecules 2015, 16, 2506–2513. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin–based inclusion complexation bridging supramolecular chemistry and macromolecular self–assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Rodell, C.B.; Kaminski, A.L.; Burdick, J.A. Rational design of network properties in guest–host assembled and shear–thinning hyaluronic acid hydrogels. Biomacromolecules 2013, 14, 4125–4134. [Google Scholar] [CrossRef]
- Rosales, A.M.; Rodell, C.B.; Chen, M.H.; Morrow, M.G.; Anseth, K.S.; Burdick, J.A. Reversible control of network properties in azobenzene–containing hyaluronic acid–based hydrogels. Bioconj. Chem. 2018, 29, 905–913. [Google Scholar] [CrossRef]
- Rowland, M.J.; Atgie, M.; Hoogland, D.; Scherman, O.A. Preparation and supramolecular recognition of multivalent peptide–polysaccharide conjugates by cucurbit [8] uril in hydrogel formation. Biomacromolecules 2015, 16, 2436–2443. [Google Scholar] [CrossRef]
- Montanari, E.; D’arrigo, G.; Di Meo, C.; Virga, A.; Coviello, T.; Passariello, C.; Matricardi, P. Chasing bacteria within the cells using levofloxacin–loaded hyaluronic acid nanohydrogels. Eur. J. Pharm. Biopharm. 2014, 87, 518–523. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo–sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F–127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the future: Toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv. Mater. 2018, 30, 1705388. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Highley, C.B.; Kim, M.; Lee, D.; Burdick, J.A. Near–infrared light triggered release of molecules from supramolecular hydrogel–nanorod composites. Nanomedicine 2016, 11, 1579–1590. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, Y.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 2012, 3, 603. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH–sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Rao, N.V.; Yoon, H.Y.; Han, H.S.; Ko, H.; Son, S.; Lee, M.; Lee, H.; Jo, D.-G.; Kang, Y.M.; Park, J.H. Recent developments in hyaluronic acid–based nanomedicine for targeted cancer treatment. Expert Opin. Drug Deliv. 2016, 13, 239–252. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic acid–based activatable nanomaterials for stimuli-responsive imaging and therapeutics: Beyond CD44-mediated drug delivery. Adv. Mater. 2019, 1803549. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic acid–based biopharmaceutical delivery and tumor–targeted drug delivery system. J. Controll. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Fu, C.; Li, H.; Li, N.; Miao, X.; Xie, M.; Du, W.; Zhang, L.-M. Conjugating an anticancer drug onto thiolated hyaluronic acid by acid liable hydrazone linkage for its gelation and dual stimuli–response release. Carbohydr. Polym. 2015, 128, 163–170. [Google Scholar] [CrossRef]
- Bajaj, G.; Kim, M.R.; Mohammed, S.I.; Yeo, Y. Hyaluronic acid–based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Controll. Release 2012, 158, 386–392. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic acid nanogels with enzyme–sensitive cross–linking group for drug delivery. J. Controll. Release 2015, 205, 206–217. [Google Scholar] [CrossRef]
- Jhan, H.-J.; Liu, J.-J.; Chen, Y.-C.; Liu, D.-Z.; Sheu, M.-T.; Ho, H.-O. Novel injectable thermosensitive hydrogels for delivering hyaluronic acid–doxorubicin nanocomplexes to locally treat tumors. Nanomedicine 2015, 10, 1263–1274. [Google Scholar] [CrossRef]
- Chen, J.; He, H.; Deng, C.; Yin, L.; Zhong, Z. Saporin–loaded CD44 and EGFR dual–targeted nanogels for potent inhibition of metastatic breast cancer in vivo. Int. J. Pharm. 2019, 560, 57–64. [Google Scholar] [CrossRef]
- Dakwar, G.R.; Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Nanomedicine–based intraperitoneal therapy for the treatment of peritoneal carcinomatosis—Mission possible? Adv. Drug Deliv. Rev. 2017, 108, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Sun, B.; Doh, K.-O.; Wilson, E.M.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal delivery of platinum with in–situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 2015, 37, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment–sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Wei, C.-X.; Wu, C.-W. Onion–structure bionic hydrogel capsules based on chitosan for regulating doxorubicin release. Carbohydr. Polym. 2019, 209, 152–160. [Google Scholar] [CrossRef]
- Goldring, M.B.; Berenbaum, F. Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 2015, 22, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougados, M. Sodium hyaluronate therapy in osteoarthritis: Arguments for a potential beneficial structural effect. Semin. Arthr. Rheum. 2000, 30, 19–25. [Google Scholar] [CrossRef]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Mineral Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef]
- Cai, Y.; López-Ruiz, E.; Wengel, J.; Creemers, L.B.; Howard, K.A. A hyaluronic acid–based hydrogel enabling CD44–mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J. Controll. Release 2017, 253, 153–159. [Google Scholar] [CrossRef]
- Garcia, J.P.; Stein, J.; Cai, Y.; Riemers, F.; Wexselblatt, E.; Wengel, J.; Tryfonidou, M.; Yayon, A.; Howard, K.A.; Creemers, L.B. Fibrin–hyaluronic acid hydrogel–based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co–delivered and resident joint cells in osteoarthritis. J. Controll. Release 2019, 294, 247–258. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. https://doi.org/10.3390/pharmaceutics11080407
Trombino S, Servidio C, Curcio F, Cassano R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics. 2019; 11(8):407. https://doi.org/10.3390/pharmaceutics11080407
Chicago/Turabian StyleTrombino, Sonia, Camilla Servidio, Federica Curcio, and Roberta Cassano. 2019. "Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery" Pharmaceutics 11, no. 8: 407. https://doi.org/10.3390/pharmaceutics11080407