Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases
Abstract
:1. Introduction
2. The Problem of the Brain
3. Matching the Vehicle to the Emitter
4. Clinical Applications
4.1. Peptide Receptor Radionuclide Therapy
4.1.1. Somatostatin Receptors
4.1.2. Neurokinin Type-1 Receptor
4.1.3. Prostate Membrane Antigen
4.2. Radioimmunotherapy
4.2.1. Epidermal Growth Factor Receptor
4.2.2. Epidermal Growth Factor Receptor Mutant Variant III
4.2.3. DNA-Histone H1 Complex
4.2.4. Tenascin
4.2.5. Fibronectin
5. Preclinical Validation
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.M.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Di Stefano, A.L.; Farina, P.; Zagonel, V.; Tabouret, E. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: An overview of the literature. Cancer Treat. Rev. 2014, 40, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Lu-Emerson, C.; Eichler, A.F. Brain Metastases. Contin. Lifelong Learn. Neurol. 2012, 18, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Lloyd, N.; Wong, R.K.S.; Chow, E.; Rakovitch, E.; Laperriere, N.; Xu, W.; Sahgal, A. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2012, 2012, CD003869. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Menard, C.; Polgar, C.; Lindegaard, J.C.; Kirisits, C.; Potter, R. Advancements in brachytherapy. Adv. Drug Deliv. Rev. 2017, 109, 15–25. [Google Scholar] [CrossRef]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. BioMed Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef]
- Newlands, E.S.; Stevenst, M.F.G.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E., 2nd; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Lesniak, M.S. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006, 8, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn-Pirio, A.M.; Vlahovic, G. Immunotherapy approaches in the treatment of malignant brain tumors. Cancer 2017, 123, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Kabraji, S.; Ni, J.; Lin, N.U.; Xie, S.; Winer, E.P.; Zhao, J.J. Drug Resistance in HER2-Positive Breast Cancer Brain Metastases: Blame the Barrier or the Brain? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1795–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.N.; Bhatt, R.; Rotow, J.; Rohrberg, J.; Olivas, V.; Wang, V.E.; Hemmati, G.; Martins, M.M.; Maynard, A.; Kuhn, J.; et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat. Med. 2019, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Shih, J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Su, Y.; Bidlingmaier, S.; Liu, B. Manipulation of cell-type selective antibody internalization by a guide-effector bispecific design. Mol. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Bezak, E.; Borysenko, A.; Brown, M.P. Targeted alpha-therapy using 227Th-APOMAB and cross-fire antitumour effects: Preliminary in-vivo evaluation. Nucl. Med. Commun. 2014, 35, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, G.L. Concepts in radioimmunotherapy and immunotherapy: Radioimmunotherapy from a Lym-1 perspective. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2005; Volume 32, pp. 27–35. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Chacko, A.M.; Li, C.; Pryma, D.A.; Brem, S.; Coukos, G.; Muzykantov, V. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: Crossing the blood-brain barrier divide. Expert Opin. Drug Deliv. 2013, 10, 907–926. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Schumacher, T.; Hofer, S.; Eichhorn, K.; Wasner, M.; Zimmerer, S.; Freitag, P.; Probst, A.; Gratzl, O.; Reubi, J.C.; Maecke, R.; et al. Local injection of the 90Y-labelled peptidic vector DOTATOC to control gliomas of WHO grades II and III: An extended pilot study. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Casaco, A.; Lopez, G.; Garcia, I.; Rodriguez, J.A.; Fernandez, R.; Figueredo, J.; Torres, L.; Perera, A.; Batista, J.; Leyva, R.; et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol. Ther. 2008, 7, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kawakami, M.; Kioi, M.; Husain, S.R.; Puri, R.K. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J. Neurosurg. 2004, 101, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Howell, R.W.; Zalutsky, M.R. A model for optimizing delivery of targeted radionuclide therapies into resection cavity margins for the treatment of primary brain cancers. Biomed. Phys. Eng. Express 2017, 3. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery 1998, 42, 1083–1099. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Wang, A.; Han, R. The effect of mannitol on intraoperative brain relaxation in patients undergoing supratentorial tumor surgery: Study protocol for a randomized controlled trial. Trials 2014, 15, 165. [Google Scholar] [CrossRef]
- Packer, R.J.; Krailo, M.; Mehta, M.; Warren, K.; Allen, J.; Jakacki, R.; Villablanca, J.G.; Chiba, A.; Reaman, G. A Phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer 2005, 104, 1968–1974. [Google Scholar] [CrossRef]
- Thomas, H.D.; Lind, M.J.; Ford, J.; Bleehen, N.; Calvert, A.H.; Boddy, A.V. Pharmacokinetics of carboplatin administered in combination with the bradykinin agonist Cereport (RMP-7) for the treatment of brain tumours. Cancer Chemother. Pharmacol. 2000, 45, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mitran, B.; Guler, R.; Roche, F.P.; Lindstrom, E.; Selvaraju, R.K.; Fleetwood, F.; Rinne, S.S.; Claesson-Welsh, L.; Tolmachev, V.; Stahl, S.; et al. Radionuclide imaging of VEGFR2 in glioma vasculature using biparatopic affibody conjugate: Proof-of-principle in a murine model. Theranostics 2018, 8, 4462–4476. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Ishikawa, E.; Yamamoto, T.; Hatano, K.; Joraku, A.; Iizumi, Y.; Masuda, Y.; Nishiyama, H.; Matsumura, A. Potential use of prostate specific membrane antigen (PSMA) for detecting the tumor neovasculature of brain tumors by PET imaging with (89)Zr-Df-IAB2M anti-PSMA minibody. J. Neurooncol. 2018, 138, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, R.; Novak, N.; Curin Serbec, V.; Rajcevic, U. Targeting Malignant Brain Tumors with Antibodies. Front. Immunol. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, M.; Valkema, R.; Jamar, F.; Kvols, L.K.; Kwekkeboom, D.J.; Breeman, W.A.P.; Krenning, E.P. Somatostatin receptor-targeted radionuclide therapy of tumors: Preclinical and clinical findings. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2002; Volume 32, pp. 133–140. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, S.; Kvols, L.K.; Barone, R.; Jamar, F.; Bakker, W.H.; Kwekkeboom, D.J.; Bouterfa, H.; Krenning, E.P. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3] octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2006; Volume 36, pp. 147–156. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef]
- Heute, D.; Kostron, H.; von Guggenberg, E.; Ingorokva, S.; Gabriel, M.; Dobrozemsky, G.; Stockhammer, G.; Virgolini, I.J. Response of recurrent high-grade glioma to treatment with (90)Y-DOTATOC. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010, 51, 397–400. [Google Scholar] [CrossRef]
- Merlo, A.; Hausmann, O.; Wasner, M.; Steiner, P.; Otte, A.; Jermann, E.; Freitag, P.; Reubi, J.C.; Müller-Brand, J.; Gratzl, O.; et al. Locoregional Regulatory Peptide Receptor Targeting with the Diffusible Somatostatin Analogue 90Y-Labeled DOTA0-D-Phe1-Tyr3-octreotide (DOTATOC): A Pilot Study in Human Gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 1025–1033. [Google Scholar]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid-substance p. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef]
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Muller-Brand, J.; Macke, H.; Reubi, J.C.; Merlo, A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-substance P: A pilot trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Krolicki, B.; Jakucinski, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with (213)Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Merlo, A. Long-Term Results of Targeted Low-Grade Glioma Treatment with 213Bi-DOTA-[Thi8,Met(O2)11]-Substance P. Cancer Biother. Radiopharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2016; Volume 46, pp. 243–249. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Morgenstern, A.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Rola, R.; et al. Safety and Therapeutic Efficacy of 225Ac-DOTA-Substance P for Therapy of Brain Tumors. JMIRS 2019, 50, S22. [Google Scholar] [CrossRef]
- Dureja, S.; Thakral, P.; Pant, V.; Sen, I. Rare Sites of Metastases in Prostate Cancer Detected on Ga-68 PSMA PET/CT Scan-A Case Series. Indian J. Nucl. Med. 2017, 32, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.S.; Kumar, R.; Tripathi, M.; Das, C.J.; Bal, C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin. Nucl. Med. 2015, 40, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Ho, B.; Chan, L.; Emmett, L. Asymptomatic Prostate Cancer Brain Metastases on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2019, 44, e382–e384. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Schlenkhoff, C.; Schwarz, B.; Essler, M.; Ahmadzadehfar, H. Combination of 177Lu-PSMA-617 and External Radiotherapy for the Treatment of Cerebral Metastases in Patients With Castration-Resistant Metastatic Prostate Cancer. Clin. Nucl. Med. 2017, 42, 704–706. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Saffar, H.; Noohi, M.; Tavangar, S.M.; Saffar, H.; Azimi, S. Expression of Prostate-Specific Membrane Antigen (PSMA) in Brain Glioma and its Correlation with Tumor Grade. Iran. J. Pathol. 2018, 13, 45–53. [Google Scholar] [PubMed] [Green Version]
- Wernicke, A.G.; Varma, S.; Greenwood, E.A.; Christos, P.J.; Chao, K.S.C.; Liu, H.; Bander, N.H.; Shin, S.J. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 2014, 122, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Macklis, R.M. How and why does radioimmunotherapy work? Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Merdes, A.; Stumm, G.; Albert, F.K.; Forsting, M.; Hynes, N.; Kiessling, M. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int. J. Cancer 1994, 56, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Ren, Z.P.; Wester, K.; Sundberg, A.L.; Heldin, N.E.; Hesselager, G.; Persson, M.; Gedda, L.; Tolmachev, V.; Lundqvist, H.; et al. Planning for intracavitary anti-EGFR radionuclide therapy of gliomas. Literature review and data on EGFR expression. J. Neurooncol. 2006, 77, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Emrich, J.G.; Brady, L.W.; Quang, T.S.; Class, R.; Miyamoto, C.; Black, P.; Rodeck, U. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: Ten-year synopsis of a novel treatment. Am. J. Clin. Oncol. 2002, 25, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Quang, T.S.; Gracely, E.J.; Kim, J.H.; Emrich, J.G.; Yaeger, T.E.; Jenrette, J.M.; Cohen, S.C.; Black, P.; Brady, L.W. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010, 113, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.S.; Brady, L.W. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 972–975. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef]
- Reist, C.J.; Foulon, C.F.; Alston, K.; Bigner, D.D.; Zalutsky, M.R. Astatine-211 labeling of internalizing anti-EGFRvIII monoclonal antibody using N-succinimidyl 5-[211At]astato-3-pyridinecarboxylate. Nucl. Med. Biol. 1999, 26, 405–411. [Google Scholar] [CrossRef]
- Ohman, L.; Gedda, L.; Hesselager, G.; Larsson, R.; Nister, M.; Stigbrand, T.; Wester, K.; Carlsson, J. A new antibody recognizing the vIII mutation of human epidermal growth factor receptor. Tumour Biol. 2002, 23, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Barth, R.F.; Wu, G.; Kawabata, S.; Sferra, T.J.; Bandyopadhyaya, A.K.; Tjarks, W.; Ferketich, A.K.; Moeschberger, M.L.; Binns, P.J.; et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Shapiro, W.R.; Laske, D.W.; Jensen, R.L.; Asher, A.L.; Wessels, B.W.; Carpenter, S.P.; Shan, J.S. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: Initial experience in 51 patients. Neurosurgery 2005, 56, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Hdeib, A.; Sloan, A. Targeted radioimmunotherapy: The role of ¹³¹I-chTNT-1/B mAb (Cotara) for treatment of high-grade gliomas. Future Oncol. 2012, 8, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, W.; Carpenter, S.; Roberts, K.; Shan, J. 131I-chTNT-1/B mAb: Tumour necrosis therapy for malignant astrocytic glioma. Expert Opin. Biol. Ther. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, J.; Wikstrand, C.; Ostrowski, L.; Bourdon, M.; Lightner, V.; Bigner, D. Tenascin expression in human glioma cell lines and normal tissues. J. Neuroimmunol. 1992, 36, 41–55. [Google Scholar] [CrossRef]
- Riva, P.; Arista, A.; Franceschi, G.; Frattarelli, M.; Sturiale, C.; Riva, N.; Casi, M.; Rossitti, R. Local treatment of malignant gliomas by direct infusion of specific monoclonal antibodies labeled with 131I: Comparison of the results obtained in recurrent and newly diagnosed tumors. Cancer Res. 1995, 55, 5952–5956. [Google Scholar]
- Reardon, D.A.; Zalutsky, M.R.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Herndon, J.E., II; McLendon, R.E.; Pegram, C.N.; Quinn, J.A.; Rich, J.N.; et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 2008, 10, 182–189. [Google Scholar] [CrossRef]
- Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Herndon, J.E., II; McLendon, R.E.; Pegram, C.N.; Provenzale, J.M.; Quinn, J.A.; et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: Phase II study results. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 115–122. [Google Scholar] [CrossRef]
- Reardon, D.; Akabani, G.; Coleman, R.; Friedman, A.; Friedman, H.; Herndon, J.; Cokgor, I.; McLendon, R.; Pegram, C.; Provenzale, J.; et al. Phase II trial of murine (131)I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Cokgor, I.; Akabani, G.; Kuan, C.; Friedman, H.; Friedman, A.; Coleman, R.; McLendon, R.; Bigner, S.; Zhao, X.; Garcia-Turner, A.; et al. Phase I trial results of iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Mazzetta, C.; Handkiewicz-Junak, D.; Bodei, L.; Rocca, P.; Grana, C.; Maira, G.; Sturiale, C.; Villa, G.; Paganelli, G. Combined treatment of glioblastoma patients with locoregional pre-targeted 90Y-biotin radioimmunotherapy and temozolomide. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 220–228. [Google Scholar] [PubMed]
- Ebbinghaus, C.; Scheuermann, J.; Neri, D.; Elia, G. Diagnostic and therapeutic applications of recombinant antibodies: Targeting the extra-domain B of fibronectin, a marker of tumor angiogenesis. Curr. Pharm. Des. 2004, 10, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Borsi, L.; Balza, E.; Bestagno, M.; Castellani, P.; Carnemolla, B.; Biro, A.; Leprini, A.; Sepulveda, J.; Burrone, O.; Neri, D.; et al. Selective targeting of tumoral vasculature: Comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int. J. Cancer 2002, 102, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Erba, P.A.; Petrini, M.; Menrad, A.; Giovannoni, L.; Grana, C.; Hirsch, B.; Zardi, L.; Paganelli, G.; Mariani, G.; et al. Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in Hodgkin lymphoma patients. Blood 2009, 113, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.; Sollini, M.; Boni, R.; Claudio Traino, A.; Giovannoni, L.; Neri, D.; Menssen, H.; Mariani, G. Results of a phase I/II dose-finding and efficacy study of the tumor-targeting 131I-L19SIP human recombinant mini-antibody in patients (pts) with cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010, 51, 1153. [Google Scholar]
- Poli, G.L.; Bianchi, C.; Virotta, G.; Bettini, A.; Moretti, R.; Trachsel, E.; Elia, G.; Giovannoni, L.; Neri, D.; Bruno, A. Radretumab radioimmunotherapy in patients with brain metastasis: A 124I-L19SIP dosimetric PET study. Cancer Immunol. Res. 2013, 1, 134–143. [Google Scholar] [CrossRef]
- Virotta, G.; Poli, G.L.; Bettini, A.; Bianchi, C.; Giovannoni, L.; Gerali, A.; Quadri, A.; Tondini, C.; Bruno, A. Radioimmunotherapy with 131I-L19SIP (Radretumab) in metastatic solid tumors: Preliminary results. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 498. [Google Scholar]
- Mahesparan, R.; Read, T.A.; Lund-Johansen, M.; Skaftnesmo, K.O.; Bjerkvig, R.; Engebraaten, O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003, 105, 49–57. [Google Scholar] [CrossRef]
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Glioblastoma Treatments: An Account of Recent Industrial Developments. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra48. [Google Scholar] [CrossRef] [PubMed]
- Boskovitz, A.; McLendon, R.E.; Okamura, T.; Sampson, J.H.; Bigner, D.D.; Zalutsky, M.R. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of alpha-particle-emitting (211)At-labeled trastuzumab. Nucl. Med. Biol. 2009, 36, 659–669. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. 131I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [PubMed]
- Puttemans, J.; D’Huyvetter, M.; Windhorst, B.; Lahoutte, T.; Devoogdt, N. CAM-H2 effectively targets and treats HER2 positive brain lesions: A comparative preclinical study with trastuzumab. Ann. Oncol. 2019, 30. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68Ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Keyaerts, M.; Vos, J.D.; Duhoux, F.P.; Caveliers, V.; Fontaine, C.; Vanhoeij, M.; D’Huyvetter, M.; Everaert, H.; Ghykiere, P.; Devoogdt, N.; et al. Phase I results of CAM-H2: Safety profile and tumor targeting in patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, e13017. [Google Scholar] [CrossRef]
- Sofou, S. Radionuclide carriers for targeting of cancer. Int. J. Nanomed. 2008, 3, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Rampling, R.; Cruickshank, G.; Lewis, A.D.; Fitzsimmons, S.A.; Workman, P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 427–431. [Google Scholar] [CrossRef]
- Behling, K.; Maguire, W.F.; López Puebla, J.C.; Sprinkle, S.R.; Ruggiero, A.; O’Donoghue, J.; Gutin, P.H.; Scheinberg, D.A.; McDevitt, M.R. Vascular Targeted Radioimmunotherapy for the Treatment of Glioblastoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1576–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behling, K.; Maguire, W.F.; Di Gialleonardo, V.; Heeb, L.E.; Hassan, I.F.; Veach, D.R.; Keshari, K.R.; Gutin, P.H.; Scheinberg, D.A.; McDevitt, M.R. Remodeling the Vascular Microenvironment of Glioblastoma with α-Particles. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, R.; Khaket, T.P.; Dutta, C.; Chakraborty, B.; Mukherjee, T.K. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell. Oncol. 2017, 40, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Falzone, N.; Ackerman, N.L.; Rosales, L.F.; Bernal, M.A.; Liu, X.; Peeters, S.G.; Soto, M.S.; Corroyer-Dulmont, A.; Bernaudin, M.; Grimoin, E.; et al. Dosimetric evaluation of radionuclides for VCAM-1-targeted radionuclide therapy of early brain metastases. Theranostics 2018, 8, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corada, M.; Zanetta, L.; Orsenigo, F.; Breviario, F.; Lampugnani, M.G.; Bernasconi, S.; Liao, F.; Hicklin, D.J.; Bohlen, P.; Dejana, E. A monoclonal antibody to vascular endothelial–cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood 2002, 100, 905–911. [Google Scholar] [CrossRef]
- Pommier, Y.; O’Connor, M.J.; de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016, 8, 362ps17. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1-Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef]
- Desai, R.; Suryadevara, C.M.; Batich, K.A.; Farber, S.H.; Sanchez-Perez, L.; Sampson, J.H. Emerging immunotherapies for glioblastoma. Expert Opin. Emerg. Drugs 2016, 21, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Radionuclide | Abbreviation | Emission | Half-Life | Energymax (keV) | Travel Distance | Characteristics of Radiation Class |
|---|---|---|---|---|---|---|
| Actinium-225 | 225Ac | Alpha/beta−/gamma | 9.92 days | 7.069 | 50–100 μm | + Short range, high energy |
| Astatine-211 | 211At | Alpha | 7.20 h | 5.867 | 50–100 μm | + Double stranded DNA breakage |
| Bismuth-213 | 213Bi | Alpha/gamma | 46 min | 6.051 | 50–100 μm | + Oxygen independent |
| Lead-212 | 212Pb | Alpha/beta−/gamma | 10.64 h | 8.785 | 50–100 μm | − No crossfire |
| Iodine-131 | 131I | Beta−/gamma | 8.02 days | 606 | 200 µm–1 mm | + Crossfire effect |
| Lutetium-177 | 177Lu | Beta−/gamma | 6.68 days | 498 | 230 µm | − Oxygen dependent |
| Rhenium-188 | 188Re | Beta−/gamma | 16.98 h | 2.110 | 11 mm | − Long range, low energy |
| Yttrium-90 | 90Y | Beta− | 2.67 days | 2.280 | 12 mm | − Single stranded DNA breakage |
| Indium-111 | 111In | Auger/gamma | 2.8 days | 245 | 4 nm | + Very short range |
| Iodine-125 | 125I | Auger/gamma | 59.49 days | 35 | 2 nm | − Necessary to be internalized |
| Disease | Target | Compound | Administration Route | Testing Phase | Results | Reference/Clinical Trial Identifier: |
|---|---|---|---|---|---|---|
| Neuroblastoma, meningioma, glioma, GBM | Somatostatin receptors | [90Y]-DOTATOC | Directly injected or via subcutaneous reservoir system into resection cavity | Phase I/II | + Partial or complete remission. − Well tolerated, with minimal neurological toxicity. | [23,38,39,40,41,42] NCT03273712, NCT00006368, NCT02441088, NCT00006368 |
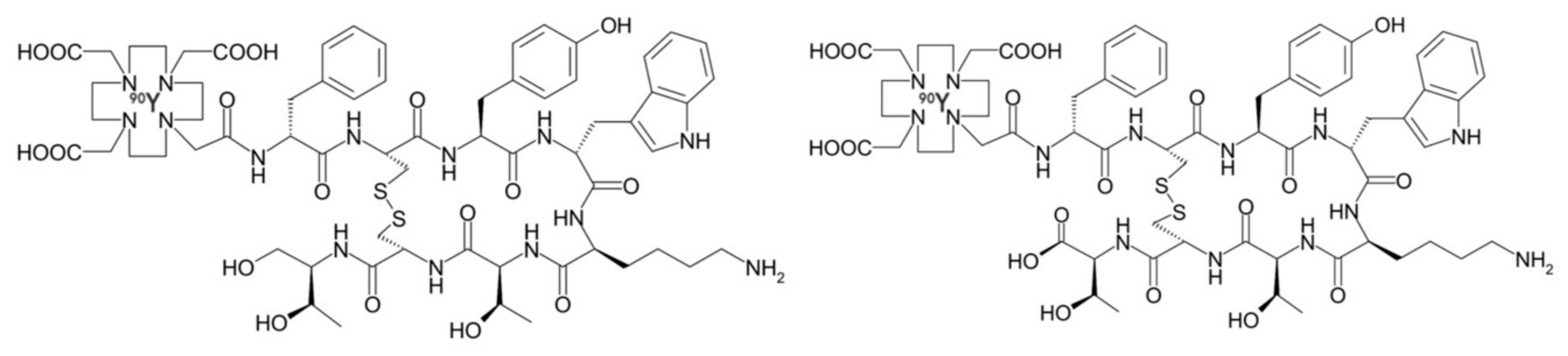
| Grade II–IV gliomas | Neurokinin type-1 receptor | [90Y]-DOTAGA-Substance P | Intratumorally via trans-cerebellar catheter | Phase I | + Disease stabilization and/or improved neurologic status. − No significant local or systemic toxicity | [43] |
| [225Ac]-DOTA-Substance P | Intratumorally or into the post-surgical cavity | Phase I/II | + OS prolongation up to 32 months − Well tolerated, with mild, transient edema, aphasia or epileptic seizures. | [48] | ||
| [213Bi]-DOTA-Substance P | Trans-cerebellar catheter | Phase I/II | + Partial or complete remission. Disease stabilization and/or improved neurologic status. − No significant local or systemic toxicity | [43,44,45,46,47] | ||
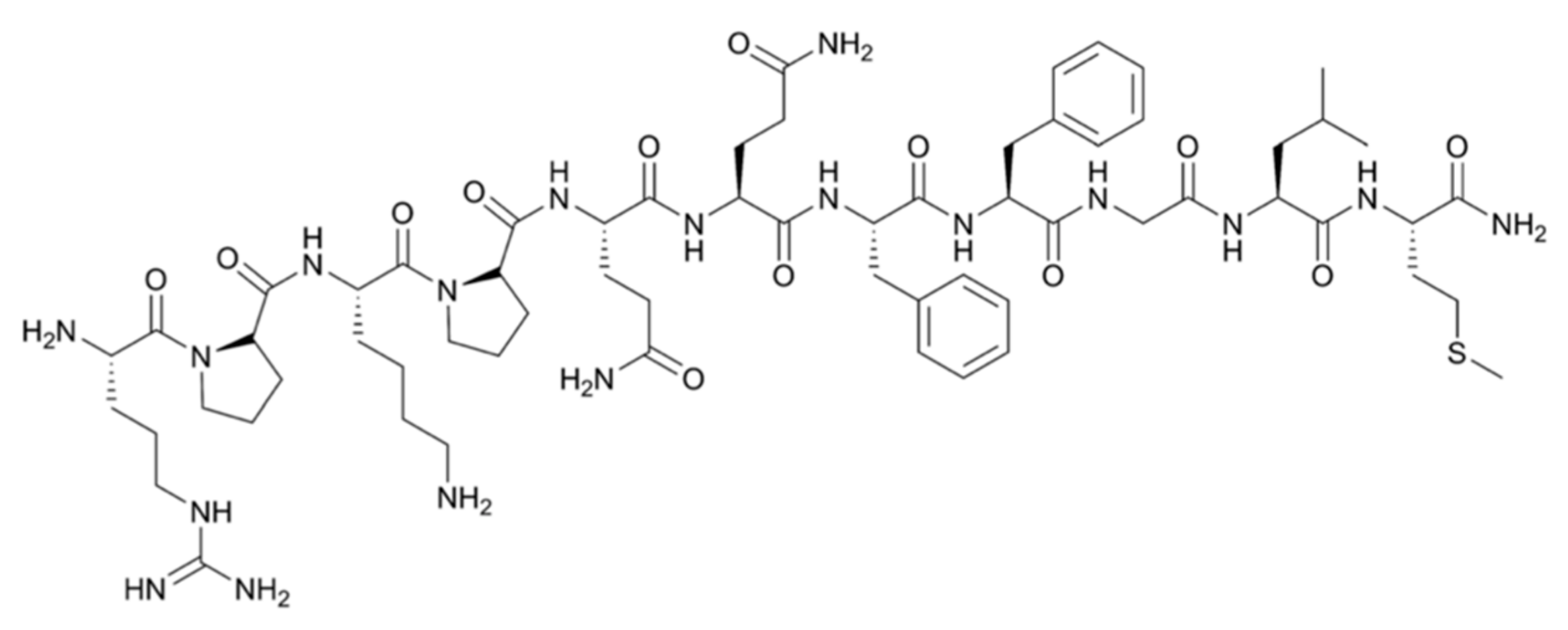
| Grade II–IV gliomas, anaplastic astrocytoma | Epidermal growth factor receptor (mutant variant III) | [125I]-mAb 425 | Intravenous | Phase II | + Median survival benefit of 20.4 months − Mild skin irritation at injection site | [60,61,62] |
| [188Re]-labeled Nimotuzumab | Directly injected into resection cavity | Phase I | + 1/11 partial response, 2/11 complete response after 3 years. − Dose-dependent neurotoxicity was observed in some patients | [24] | ||
| Grade II–IV gliomas, anaplastic astrocytoma | DNA-histone H1 complex | [131I]-chTNT-1/B MAb | Intratumorally via convection-enhanced delivery | Phase I/II | + Clinical efficacy not definitively established due to low patient number. Median survival time was noted as 37.9. weeks for subset of patients. − Edema, hemiparesis and headache | [68,69,70] NCT00677716, NCT00509301, NCT00128635, NCT00004017 |
| Grade I–IV gliomas | Tenascin | [131I]-BC-2 mAb or [131I]-BC-4 mAb | Intratumorally | Phase I/II | + Partial or complete remission. Response rate of 40% − No systemic or cerebral adverse effects, HAMA response did not affect tumor-targeting | [72] |
| 3-step pretargeting strategy with biotin-coupled BC-4 + Avidin + [90Y]-Biotin | Trans-cerebellar catheter | Phase I/II | + Disease stabilization in 75% of patients. OS prolonged to 17.5 and 25 months with TRNT alone or TRNT+TMZ resp. − Transient hematological toxicity, mild allergic reaction | [77] | ||
| [131I]- or [211At]-labeled 81C6 mAb | Directly injected into resection cavity | Phase I/II | + Median survivals of up to 22 months − Reversible hematologic and neurologic events. No adverse effects related to HAMA response | [22,73,74,75,76] NCT00003461, NCT00003484, NCT00002752, NCT00003478, NCT00002753 | ||
| Brain metastasis from breast carcinoma and NSCLC | Fibronectin | [131I]-L19SIP | Intravenous | Phase I/II | + Intra- and extracranial lesions showed reduced [18F]-FDG-uptake during 6-month follow-up − No adverse events reported | [81,82,83] NCT01125085 |
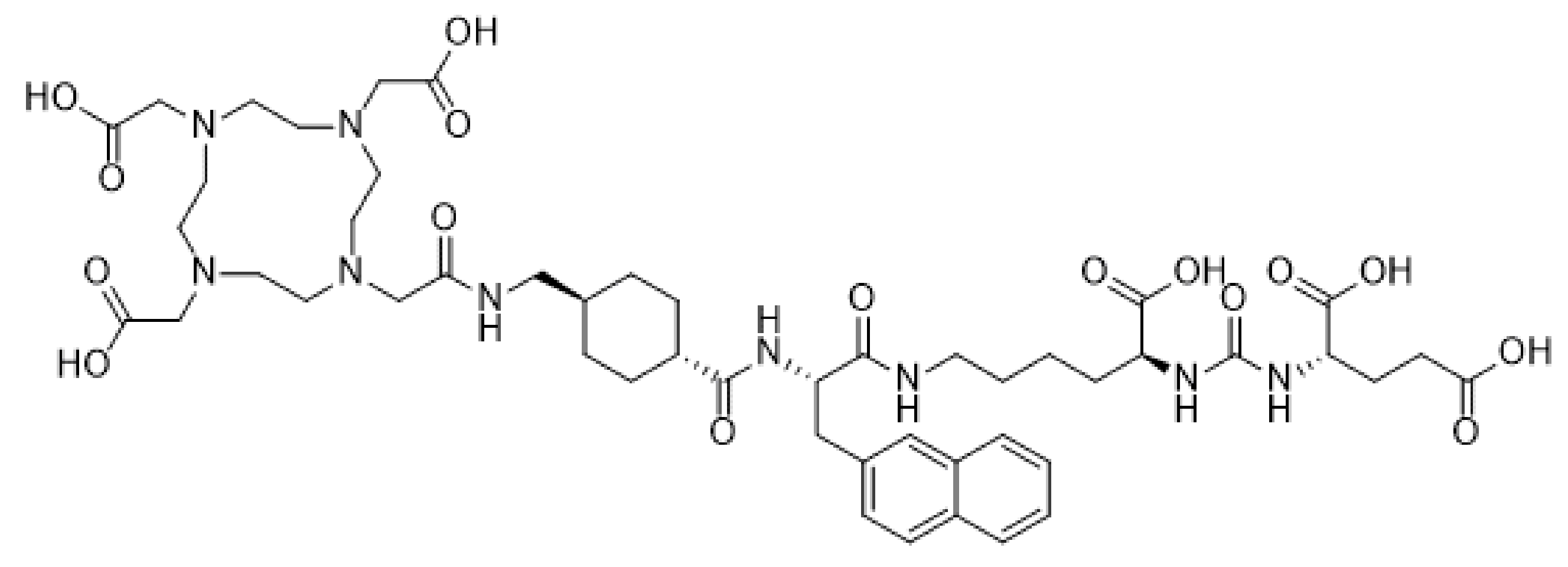
| Brain metastasis from prostate cancer | Prostate membrane antigen | [177Lu]-PSMA-617 | Intravenous | Phase I | + Significant decrease in size and PSMA expression − Minimal toxicity to salivary glands | [52] NCT03511664 |
| [225Ac]-PSMA-617 | Intravenous | Phase I/II | + More potent effect than [177Lu]-PSMA-617 − Severe xerostomia | [53,54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttemans, J.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019, 11, 376. https://doi.org/10.3390/pharmaceutics11080376
Puttemans J, Lahoutte T, D’Huyvetter M, Devoogdt N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics. 2019; 11(8):376. https://doi.org/10.3390/pharmaceutics11080376
Chicago/Turabian StylePuttemans, Janik, Tony Lahoutte, Matthias D’Huyvetter, and Nick Devoogdt. 2019. "Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases" Pharmaceutics 11, no. 8: 376. https://doi.org/10.3390/pharmaceutics11080376
APA StylePuttemans, J., Lahoutte, T., D’Huyvetter, M., & Devoogdt, N. (2019). Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics, 11(8), 376. https://doi.org/10.3390/pharmaceutics11080376






