Pharmacokinetic Properties of Acetyl Tributyl Citrate, a Pharmaceutical Excipient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Calibration and Quality Control Standards
2.3. Analytical Method Validation
2.4. Pharmacokinetics Study in Rats
2.5. Pharmacokinetics Analysis
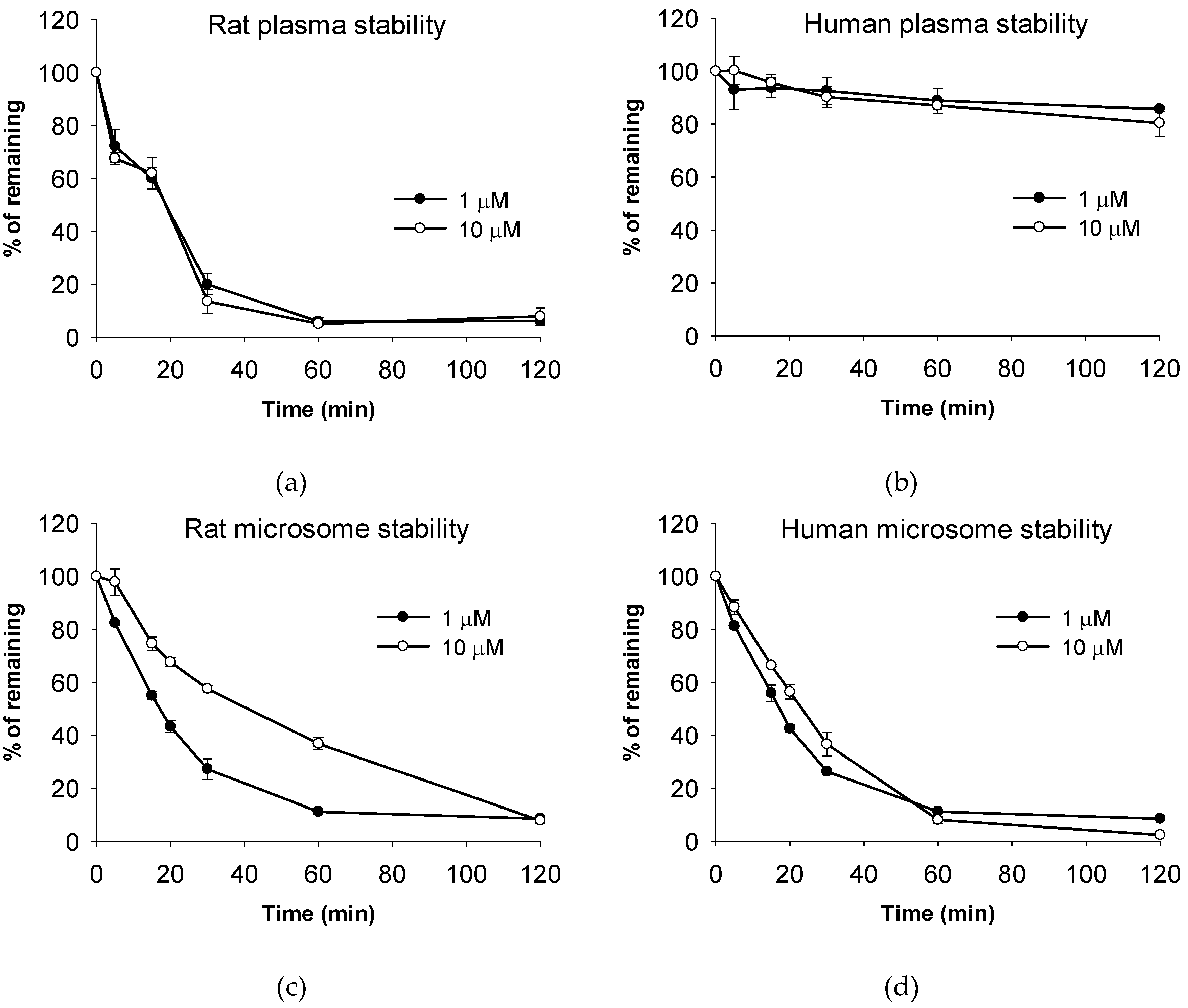
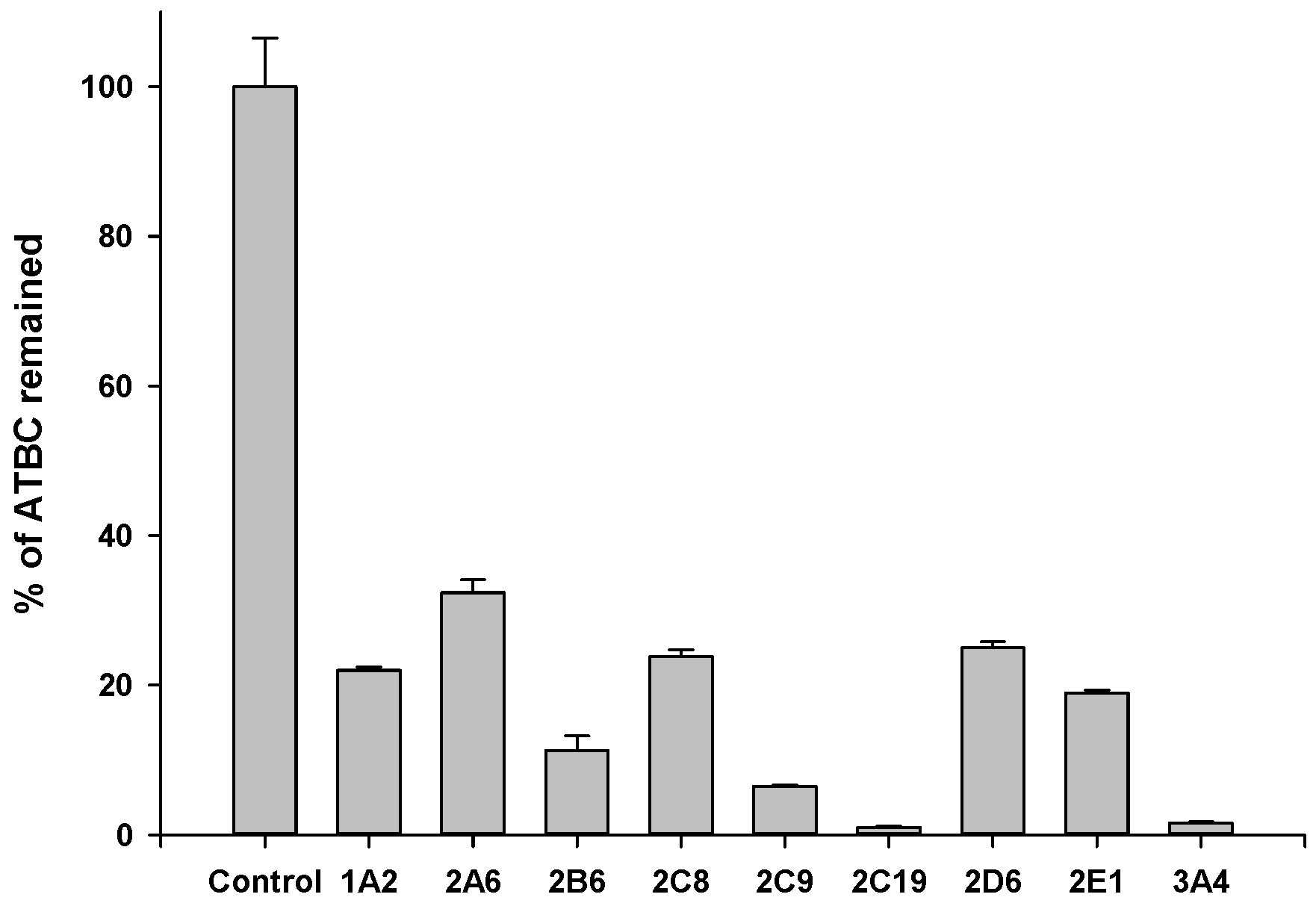
2.6. Metabolic Stability Assay
2.7. LC-MS/MS Conditions
3. Results
3.1. Analytical Method Validation
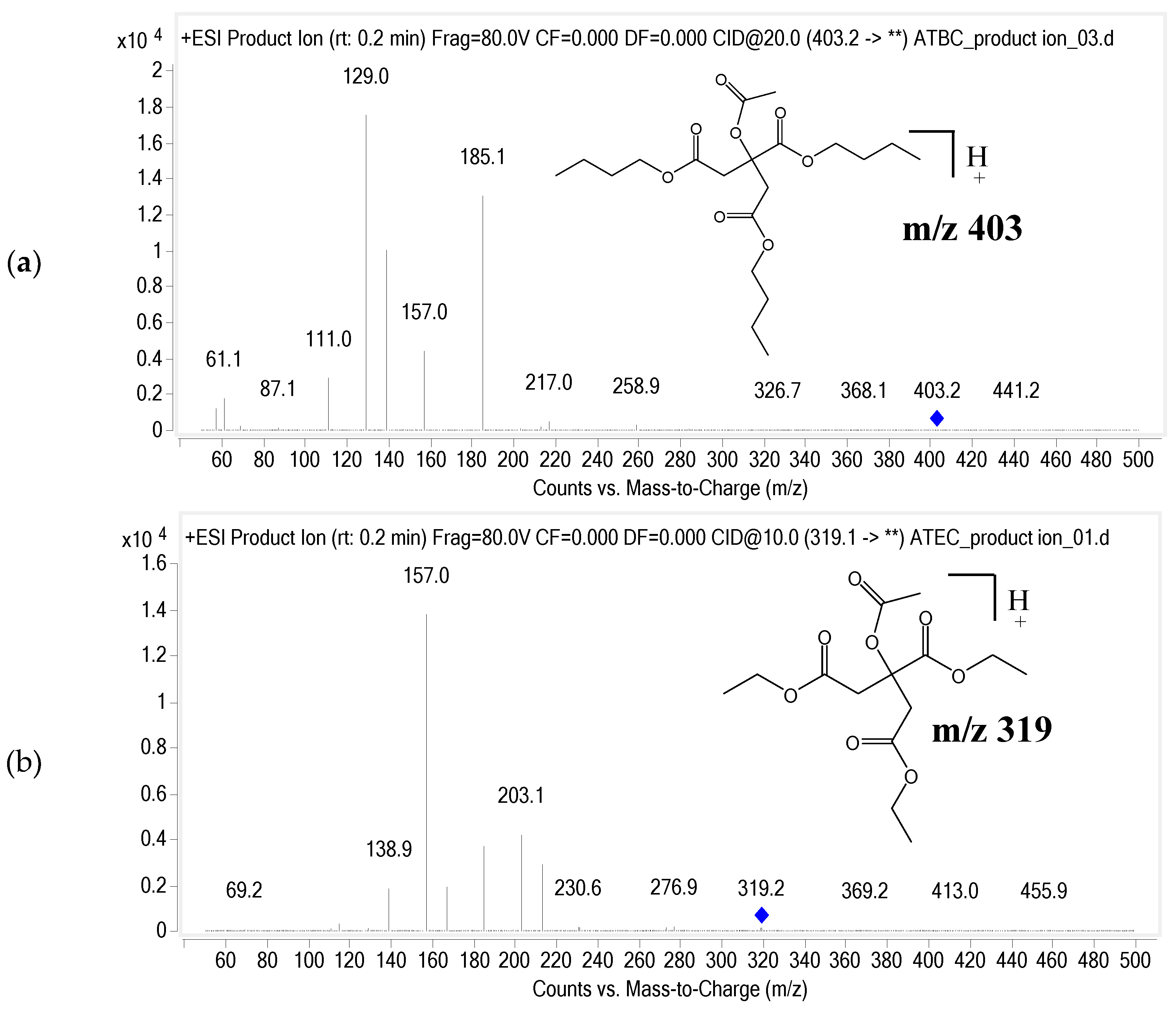
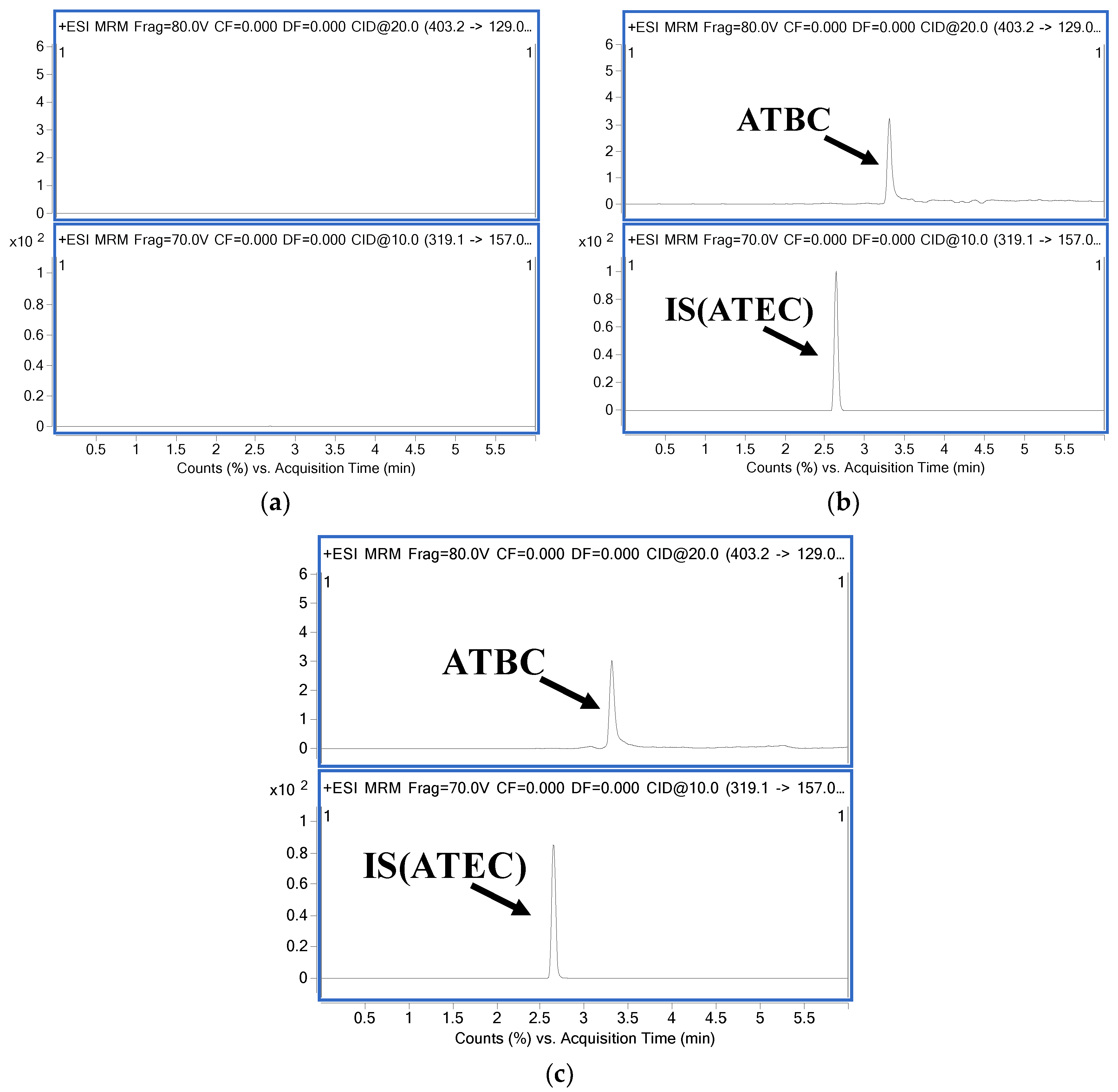
3.1.1. Selectivity
3.1.2. Linearity
3.1.3. Accuracy and Precision
3.1.4. Extraction Recovery and Matrix Effect
3.1.5. Stability
3.2. Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snejdrova, E.; Dittrich, M. Pharmaceutically used plasticizers. In Recent Advances in Plasticizers; Mohammad, L., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 45–68. [Google Scholar]
- Takeshita, A.; Igarashi-Migitaka, J.; Nishiyama, K.; Takahashi, H.; Takeuchi, Y.; Koibuchi, N. Acetyl tributyl citrate, the most widely used phthalate substitute plasticizer, induces cytochrome P450 3A through steroid and xenobiotic receptor. Toxicol. Sci. 2011, 123, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr. Final report on the safety assessment of acetyl triethyl citrate, acetyl tributyl citrate, acetyl trihexyl citrate, and acetyl trioctyl citrate. Int. J. Toxicol. 2002, 21, 1–17. [Google Scholar] [PubMed]
- Gordon, L.R. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 624–626. [Google Scholar]
- Rasmussen, L.M.; Sen, N.; Liu, X.; Craig, Z.R. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J. Appl. Toxicol. 2017, 37, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.F.; Civade, C.; Brenier, C.; Bonnet, P.A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 949–950, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Radaniel, T.; Genay, S.; Simon, N.; Feutry, F.; Quagliozzi, F.; Barthelemy, C.; Lecoeur, M.; Sautou, V.; Decaudin, B.; Odou, P. Quantification of five plasticizers used in PVC tubing through high performance liquid chromatographic-UV detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 965, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Bourdeaux, D.; Yessaad, M.; Chennell, P.; Larbre, V.; Eljezi, T.; Bernard, L.; Sautou, V. Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Bourdeaux, D.; Pereira, B.; Azaroual, N.; Barthelemy, C.; Breysse, C.; Chennell, P.; Cueff, R.; Dine, T.; Eljezi, T.; et al. Analysis of plasticizers in PVC medical devices: Performance comparison of eight analytical methods. Talanta 2017, 162, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, G.B.; Choi, M.S.; Kim, I.S.; Gye, M.C.; Yoo, H.H. Liquid chromatography-tandem mass spectrometric analysis of acetyl tributyl citrate for migration testing of food contact materials. Microchem. J. 2018, 139, 475–479. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2018. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (accessed on 10 June 2018).
- Kachingwe, B.H.; Uang, Y.S.; Huang, T.J.; Wang, L.H.; Lin, S.J. Development and validation of an LC-MS/MS method for quantification of NC-8 in rat plasma and its application to pharmacokinetic studies. J. Food. Drug Anal. 2018, 26, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Kim, I.S.; Choi, M.S.; Luo, Z.; Yao, G.; Xue, Y.; Zhang, Y.; Yoo, H.H. Evaluation of Metabolic stability of kinsenoside, an antidiabetic candidate, in rat and human liver microsomes. Mass Spectrom. Lett. 2015, 6, 48–51. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Hong, Y.; Chen, H. Development and application of high throughput plasma stability assay for drug discovery. Int. J. Pharm. 2005, 297, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Kim, I.S.; Choi, M.S.; Luo, Z.; Yao, G.; Xue, Y.; Zhang, Y.; Yoo, H.H. Development of a hydrophilic interaction liquid chromatography-tandem mass spectrometric method for the determination of kinsenoside, an antihyperlipidemic candidate, in rat plasma and its application to pharmacokinetic studies. J. Pharm. Biomed. Anal. 2016, 120, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bahar, F.G.; Ohura, K.; Ogihara, T.; Imai, T. Species difference of esterase expression and hydrolase activity in plasma. J. Pharm. Sci. 2012, 101, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.M.; Sen, N.; Vera, J.C.; Liu, X.; Craig, Z.R. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol. Reprod. 2017, 96, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.W.; Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Acetyltributyl citrate. In Handbook of Pharmaceutical Excipients; London Pharmaceutical Press: London, UK, 2006; pp. 10–11. [Google Scholar]

| Nominal Conc. (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||
|---|---|---|---|---|---|---|
| Measured Conc. (ng/mL) | Accuracy (%) | Precision (%) | Measured Conc. (ng/mL) | Accuracy (%) | Precision (%) | |
| 10 | 11.8 | 117.6 | 6.8 | 10.3 | 103.4 | 11.4 |
| 30 | 31.2 | 103.8 | 5.4 | 31.2 | 104.1 | 5.0 |
| 400 | 405.1 | 101.3 | 4.9 | 411.7 | 102.9 | 4.8 |
| 800 | 725.9 | 90.7 | 11.4 | 801.3 | 100.2 | 6.2 |
| Stability | Recovery (% Remained) | |
|---|---|---|
| Concentration (ng/mL) | ||
| 30 | 800 | |
| Short-term (Room temperature for 8 h) | 94.1 ± 7.6 | 94.9 ± 8.9 |
| Long-term (−20 °C for 14 days) | 100.6 ± 11.5 | 99.9 ± 14.5 |
| Freeze-thaw (−20 °C, 3 cycles) | 103.8 ± 10.7 | 104.2 ± 8.2 |
| Auto-sampler (4 °C for 24 h) | 106.8 ± 12.1 | 103.2 ± 10.8 |
| Parameter | ATBC | |
|---|---|---|
| IV (10 mg/kg, n = 5) | PO (500 mg/kg, n = 6) | |
| t1/2α (h) | 0.02 ± 0.01 | - |
| t1/2β (h) | 0.58 ± 0.31 | - |
| AUC (ng·h/mL) | 201.3 ± 215.3 | 2757.1 ± 726.3 |
| Tmax (h) | - | 0.4 ± 0.3 |
| Cmax (ng/mL) | - | 478.7 ± 170.9 |
| Vz (L/kg) | 80.4 ± 47.4 | - |
| Cl (L/h/kg) | 75.7 ± 64.4 | - |
| F (%) | - | 27.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Choi, M.S.; Ji, Y.S.; Kim, I.S.; Kim, G.B.; Bae, I.Y.; Gye, M.C.; Yoo, H.H. Pharmacokinetic Properties of Acetyl Tributyl Citrate, a Pharmaceutical Excipient. Pharmaceutics 2018, 10, 177. https://doi.org/10.3390/pharmaceutics10040177
Kim H, Choi MS, Ji YS, Kim IS, Kim GB, Bae IY, Gye MC, Yoo HH. Pharmacokinetic Properties of Acetyl Tributyl Citrate, a Pharmaceutical Excipient. Pharmaceutics. 2018; 10(4):177. https://doi.org/10.3390/pharmaceutics10040177
Chicago/Turabian StyleKim, Hyeon, Min Sun Choi, Young Seok Ji, In Sook Kim, Gi Beom Kim, In Yong Bae, Myung Chan Gye, and Hye Hyun Yoo. 2018. "Pharmacokinetic Properties of Acetyl Tributyl Citrate, a Pharmaceutical Excipient" Pharmaceutics 10, no. 4: 177. https://doi.org/10.3390/pharmaceutics10040177





