1. Introduction
Following oral administration, a drug must pass through the gastrointestinal lumen, penetrate through the gut wall, and resist metabolic degradation by intestinal and hepatic enzymes, and biliary excretion [
1]. In this process, the oral drug absorption is dependent on various factors including the pH, solubility and dissolution of a drug in the intestinal fluid, permeability across the intestinal membrane, presystemic metabolism, and drug transporters. Moreover, these factors vary depending on the location of the gastrointestinal tract [
2]. Due to the interplays of these regional differences in the gastrointestinal environment and the physicochemical properties of a drug, the orally administered drug may have a favorable region for absorption. A better understanding of the dynamic and variable absorption process is essential for the successful development of oral dosage formulations. It helps to rationally design drug formulations with optimized bioavailability. Moreover, information regarding the regional differences of the gastrointestinal physiology and the factor-controlling absorption is especially critical to design controlled-release formulations with specific drug release pattern in the gastrointestinal tract.
Fimasartan is the 9th angiotensin II receptor antagonist approved for the treatment of mild to moderate hypertension with the brand name of Kanarb
®. As a pyrimidine-4(3
H)-one derivative of losartan, fimasartan provides greater potency and efficacy than losartan in parallel with the rapid onset of antihypertensive effects [
3,
4,
5]. Following oral administration, fimasartan is known to be rapidly absorbed with an oral bioavailability of 32.8–44.7% in rats (solution), 8.0–17.3% in dogs (solution), and 18.6% ± 7.2% in humans (tablet) [
6,
7,
8]. More than 90% of circulating fimasartan moieties in the plasma is the parent form suggesting fimasartan is metabolically stable, and fecal elimination and biliary excretion are the predominant elimination pathways of fimasartan [
6]. Fimasartan has been licensed out to various countries worldwide including 13 Latin American countries as well as Russia and China. Recently, fixed dose combination tablets of fimasartan with another class drug, such as hydrochlorothiazide, amlodipine, and rosuvastatin, have been launched, and various preclinical and clinical studies are also ongoing to develop new formulations of fimasartan.
Several experimental models are currently available to determine the intestinal absorption of a drug and the controlling mechanisms of absorption [
9]. For example, immobilized artificial membrane (IAM) chromatography [
10] and parallel artificial membrane permeability assay (PAMPA) [
11] provide relatively simple and efficient screening tools to predict passive intestinal transport in the drug discovery stage. Various in vitro methods have been used to evaluate the intestinal absorption potential of drug candidates, which include animal tissue-based methods, such as everted gut techniques [
12], Ussing chambers [
13], and isolated membrane vesicles [
14], and cell-based methods such as Caco-2 cells [
15] and Madin-Darby canine kidney cells [
16]. On the other hand, in vivo evaluation of drug absorption in animals is commonly used to predict the extent of absorption of drug candidates in humans. These experimental models have their own advantages and disadvantages, and the judicious use of the various techniques at the right stage of drug discovery and development is important. Furthermore, exciting and novel approaches have been extensively investigated to overcome the hurdles associated with poor gastrointestinal stability and absorption of biological drugs [
17,
18]. Accurate assessment of oral absorption by using proper experimental tools is also critical for the successful development of formulations and oral delivery strategies for biological drugs.
Among the experimental models, in situ single-pass perfusion is a frequently used method to evaluate the regional intestinal permeability as well as the absorption kinetics of drugs [
19,
20,
21]. In this method, the compound of interest is monitored in a perfusate and the difference between inlet and outlet concentrations, i.e., the loss of the compound, is attributed to the permeability. It has been suggested that the extent of absorption in humans can be predicted from single-pass intestinal perfusion studies in rats [
21,
22,
23]. The major advantage of the single-pass perfusion method is the presence of intact blood and nerve supply in the experimental animals, which provides conditions close to the physiological state following oral administration [
9]. The control of the factors, such as concentration, pH, and intestinal perfusion rate [
20], is another strength of the in situ single-pass perfusion method. Moreover, it provides a unique ability to study regional differences in the gastrointestinal tract by using different gastrointestinal segments [
24].
Nevertheless, the in situ single-pass perfusion method has limitations in that perfusion may disturb the normal physiology of the gastrointestinal tract and it does not consider other factors affecting drug concentrations in the intestinal lumen. It is assumed that the disappearance of the drug from the intestinal lumen is attributed to the intestinal permeability. However, the decrease of the drug concentration in the perfusate may not be entirely dependent on the absorption of the drug into the systemic circulation, but also on the drug metabolism in the gastrointestinal tract. Thus, the in situ single-pass perfusion method may overestimate the intestinal permeability and absorption of drugs undergoing intestinal metabolism. Moreover, a significant amount of drug may be necessary to conduct the in situ single-pass perfusion method, because continuous perfusion is needed until the steady state is reached. Thus, it may not be appropriate in the early stage of drug development for candidate screening purpose.
In the present study, an improved in situ absorption method has been developed to assess the regional absorption of fimasartan and compared the results with those obtained by the in situ single-pass perfusion method. The improved in situ absorption model evaluated the absorption by measuring the resulting drug plasma concentrations after an injection of a drug into a specific segment of the gastrointestinal tract, instead of measuring the disappearance of a drug in the perfusate during perfusion. Therefore, it allowed evaluation of net absorption in the different gastrointestinal segments in the more physiological condition, where drug absorption occurs sequentially as the drug solution passes through the gastrointestinal tract.
2. Materials and Methods
2.1. Chemicals and Reagents
Fimasartan and the internal standard (BR-A-563) were provided by Boryung Pharm. Co., Ltd. (Seoul, Korea). High performance liquid chromatography (HPLC) grade acetonitrile, methanol, and distilled water were products of Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA). Formic acid was obatined from Aldrich Chemicals (Milwaukee, WI, USA).
2.2. Animals
The animal studies were approved by the ethics committee for the treatment of laboratory animals at the Catholic University of Daegu (IACUC-2012-005). Male Sprague–Dawley rats, weighing 250–300 g, were housed in a temperature of 22–24 °C and a relative humidity of 50% ± 10% with a standard 12-h light/dark cycle.
2.3. Determination of Hepatic First-Pass Metabolism and Gastrointestinal Bioavailability (FX·FG)
After anesthetized by an intraperitoneal injection of urethane (1 g/kg), the rats were cannulated with a polyethylene (PE) tubing (0.58 mm i.d., 0.96 mm o.d., Natsume, Tokyo, Japan) in the right jugular vein. For drug administration, the animals were also cannulated in the intended routes of administration. The femoral vein and the portal vein was cannulated for intravenous injection and portal vein injection, respectively. For portal venous injection, the portal vein was exposed by an abdominal incision and a PE tube (0.28 mm i.d., 0.61 mm o.d., Natsume, Tokyo, Japan) was inserted into the portal vein, and the wound was closed by applying epoxy glue (Krazy Glue, IL, USA). An abdominal incision was also made in rats receiving an intravenous injection to maintain the same experimental conditions. Fimasartan was dissolved in distilled water and injected at doses of 0.1 and 0.3 mg/kg into the femoral or portal vein. Blood samples were collected from the jugular vein before and at 2 min, 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, and 8 h after the fimasartan administration. Plasma samples were harvested by centrifugation of the blood samples at 1500× g for 10 min.
The plasma concentration of fimasartan vs. time data were analyzed by the noncompartmental method using the Phoenix
® WinNonlin
® software (Certara, L.P., Princeton, NJ, USA). Fractions of the administered dose that escaped the first-pass metabolism by the liver (
FH) was calculated as follows:
where
AUC represents the area under the plasma concentration vs. time curve with the time from zero to infinity while
D is the dose; and the subscript refers to the route of administration.
The gastrointestinal bioavailability (
FX·FG) was derived by:
where
FX is the fraction absorbed and
FG is the fraction of the dose that escapes the gut wall metabolism, and
F is the absolute oral bioavailability of 39.85% [
6].
2.4. In Situ Single-Pass Perfusion
After overnight fasting, the rats were anesthetized by an intraperitoneal injection of urethane (1 g/kg). For determination of the permeability in the duodenum, the abdomen was opened and the gastrointestinal segment of the duodenum (11 cm length from the end of the stomach) [
25] was isolated and cannulated at both ends of the segment with a silicone tube (2 mm i.d. Daihan Scientific Co., Wonjoo, Korea). For determination of the permeability in the small intestine and large intestine, the same procedure was used to prepare the small intestine and large intestine segments. The segment of the small intestine (from the end of the duodenum to the caecum) and large intestine (from the caecum to the rectum) was isolated and cannulated. The cannulated segment was rinsed with 37 °C saline to clear the segment before perfusion. Each end of the cannulated segment was attached to the perfusion assembly, which consisted of a syringe pump (KD Scientific, Holliston, MA, USA) for input and a peristaltic pump (EP-1 Econo Pump, Bio-Rad, Hercules, CA, USA) for output.
Fimasartan was dissolved in distilled water at a concentration of 0.1667 mg/mL and perfused with a perfusion rate of 0.2 mL/min. The animal was placed on a heating pad to maintain the body temperature, and the abdominal incision area was stapled to prevent loss of fluid and hypothermia. The outlet perfusates were collected on the ice at 10-min intervals from 50 to 140 min after the perfusion was initiated. The collected outlet samples were stored at −20 °C until analysis. The regional absorptive clearance (
PeA) was estimated by:
where
Qin is the perfusion rate, and
Cin and
Cout are the inlet and outlet concentrations at the steady state, respectively. The regional fraction absorbed (
Fabs) at the segment
i in the duodenum, small intestine, or large intestine was calculated as:
2.5. Improved In Situ Absorption Model
Similar to the in situ single-pass perfusion method, the rats were anesthetized by an intraperitoneal injection of urethane (1 g/kg) after overnight fasting. The abdomen was opened and the gastrointestinal segment of interest, i.e., stomach, duodenum, small intestine, or large intestine, was identified. The starting point of the segment was slightly tied off, the other end of the segment was cannulated with a silicone tube (2 mm i.d. Daihan Scientific Co., Wonjoo, Korea) to prevent the unabsorbed fraction being absorbed in the next segment, and the contents of the segment were removed.
Fimasartan dissolved in distilled water (1.0 mg/mL, 0.59 mL/kg) was injected to the starting point of the gastrointestinal segment, i.e., stomach, duodenum, small intestine, or large intestine at a dose of 0.5 mg/kg. The blood samples were collected from the jugular vein before and at 2 min, 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, and 8 h after the injection of fimasartan. Plasma samples were obtained by centrifugation of the blood samples at 1500×
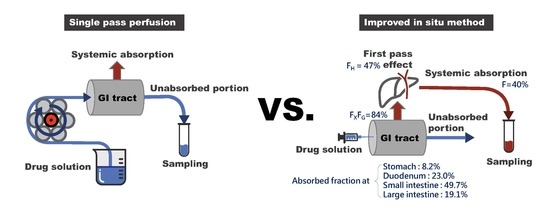
g for 10 min and were stored at −20 °C until analysis. The animal was placed on a heating pad to maintain the body temperature. The experimental set-up for the improved in situ absorption method is illustrated in comparison with that of the single-pass perfusion in
Figure 1.
The relative regional fraction absorbed (
Fabs,relative) at each segment by the improved in situ absorption model was calculated based on the AUC values obtained following the administration of fimasartan into the segment of interest
i:
Since the absorption occurs stepwise as drugs pass through the gastrointestinal tract from stomach to the duodenum, small intestine, and large intestine, the dose available at each segment after oral administration is reduced in the distal gastrointestinal tract. Therefore, the actual fraction absorbed in the specific segment of the gastrointestinal tract (actual
F′abs) was estimated by applying the fraction of the dose arriving at the site of segment (
Farrived). The
Farrived at the segment of interest,
i, was calculated as:
F′abs,i−1 is the actual fraction absorbed prior to the
ith segment, which was estimated as:
where
f is a factor of 1.322, which allows the sum of
F′abs,i to become the average
FX·FG, the gastrointestinal bioavailability estimated by Equation (2).
2.6. LC-MS/MS
The fimasartan concentrations in rat plasma were determined by a previously validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method [
6,
26]. Briefly, an internal standard solution (50 μL, BR-A-563 100 ng/mL in acetonitrile) and blank acetonitrile (200 μL) was added to 50 μL of the plasma samples and mixed on a vortex mixer for 1 min. After centrifugation of the mixture for 10 min at 15,000×
g, 100 μL of the upper layer was mixed with 100 μL of distilled water. A portion (10 μL) was injected into the LC-MS/MS.
The LC-MS/MS instrument comprised an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Toronto, ON, Canada) coupled with an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA). Fimasartan was separated on a Kinetex C18 column (50 × 2.10 mm i.d., 2.6 μm, Phenomenex, Torrence, CA, USA) with a KrundKatcher ultra column inline filter (Phenomenex). The isocratic mobile phase consisted of acetonitrile and 0.05% formic acid (40:60, v/v) at a flow rate of 0.2 mL/min. The column oven temperature was set to 30 °C. The electron spray ionization (ESI) source was operated in a positive mode. The multiple reaction monitoring (MRM) transitions of precursor-to-product ion pairs were m/z 502.7→207.1 for fimasartan and m/z 526.1→207.1 for the internal standard (BR-A-563).
The LC-MS/MS method was fully validated and the lower limit of quantification was 0.2 ng/mL for rat plasma. The assay was linear over a concentration range of 0.2–500 ng/mL with correlation coefficients of >0.999. The intra- and inter-day accuracy and precision ranged from 90.8% to 108.0% and 2.4% to 13.4% for rat plasma.
2.7. Statistical Analysis
Data were presented as mean ± standard deviation (SD) unless otherwise stated. For comparison between the two means of the unpaired data, an unpaired t-test was used. Comparisons among more than two groups were performed using one-way analysis of variance (ANOVA) followed by Scheffe’s post hoc test. Statistical significance was denoted when p < 0.05.
4. Discussion
The regional absorption of fimasartan in the gastrointestinal tract was evaluated by an improved in situ absorption method in rats. The results were also compared with those determined by a conventional in situ single-pass perfusion method. The improved in situ approach measured the drug concentration in the plasma following the injection of fimasartan into a specific part of the gastrointestinal tract (
Figure 1) and provided an accurate assessment of the absorbed fraction of a drug into the systemic circulation across the region of gastrointestinal tract after oral administration.
Before estimating the regional absorption, the gastrointestinal bioavailability (
FX·FG) was determined first based on the ratio of the AUC values following the portal vein and intravenous injections. The
FX·FG consists of the fraction absorbed (
FX) and the fraction that is not metabolized during passage through the gut wall (
FG) [
1]. The estimated gastrointestinal bioavailability (
FX·FG) of fimasartan was 84.1% in rats, whereas over 50% of the dose was eliminated by the first-pass metabolism in the liver (
Table 1). These results are in agreement with the previous studies, which indicated the extensive fecal excretion of fimasartan because of the biliary excretion rather than a low gastrointestinal absorption and an extensive absorption of orally administered fimasartan in the gastrointestinal tract [
6].
To estimate the regional absorption with the improved in situ absorption method, the relative fraction absorbed in each gastrointestinal segment (
Fabs,relative) was determined by using the AUC obtained after injection of fimasartan into the segment. Since absorption occurs sequentially along the gastrointestinal tract, the actual fraction absorbed (
F′abs) was finally estimated by applying the fraction of dose available in each segment (
Farrived). Our results indicated that the orally administered fimasartan was absorbed in the stomach (10.9%), duodenum (27.1%), small intestine (40.7%), and large intestine (5.4%) as the drug passed through the gastrointestinal tract, which added up to the gastrointestinal availability (
FX·FG) of 84.1% (
Table 4). Although the
Fabs,relative indicated that the absorption potential of fimasartan of the large intestine was comparable with that of the duodenum, the actual fraction absorbed in the large intestine (
F′abs) was much less than that in the duodenum. The
F′abs in the large intestine was smaller because only 21.3% of the orally administered drug was available in the large intestine due to the absorption in the stomach, duodenum, and small intestine before the drug entered the large intestine. Taken together, in case of immediate release formulation, the majority of the orally administered fimasartan (67.8%) was predicted to be absorbed in the duodenum and small intestine. However, the comparable absorption potential of the large intestine (
Fabs,relative,LI = 19.1% ± 11.9%) as the upper part of the gastrointestinal tract (
Fabs,relative,duodenum = 23.0% ± 12.1%) suggested that sufficient absorption may be expected in the lower part of the gastrointestinal tract in case of extended release formulation. For the development of extended release formulations, sufficiently high absorption of the drug in both upper and lower parts of the gastrointestinal tract is needed to achieve the desired therapeutic effects [
21].
The regional absorption of fimasartan determined by the improved in situ absorption model was in good agreement with that by the conventional in situ single pass perfusion technique (
Figure 5). In both methods, the highest absorption was predicted through the small intestine while the absorptions in the duodenum and large intestine were similar. There were no significant differences between the regional absorptions of fimasartan determined by the two methods in each gastrointestinal segment. The results of the two methods were comparable, because the model drug in the present study, fimasartan, may be minimally metabolized in the gastrointestinal tract. However, for drugs that undergo extensive first-pass metabolism in the gut wall, the results may be different. As the single-pass perfusion determined the disappearance of the drug in the perfusate, which is a net result of the absorption and metabolic degradation as an indicator of drug absorption, it may overestimate the absorption. On the contrary, the present improved in situ absorption model directly determined the resulting plasma concentrations considering both intestinal permeability and metabolism, leading to more accurate estimations of gastrointestinal bioavailability and regional absorption.
Another advantage of the present improved in situ absorption method is that the drug was injected into the specific part of the gastrointestinal lumen instead of perfusion, which represents a more physiological absorption process. During perfusion, the gastrointestinal lumen is filled with the perfusate, which may disturb the normal physiology of the gastrointestinal tract. Moreover, since the lumen is filled with the drug solution, the drug absorption presumably occurs simultaneously in the whole gastrointestinal tract. However, the real drug absorption in the gastrointestinal tract in vivo is a sequential process along the gastrointestinal tract. In the improved in situ absorption method, therefore, by injecting the drug solution into the starting point of the segment, gradually less amount of drug would be applied to the gastrointestinal tract as the drug solution passes through the gastrointestinal tract after injection, which is close to the real drug absorption condition without disturbing gastrointestinal physiology.
In addition, the improved in situ absorption model has advantages compared to the single-pass perfusion method in terms of the amount of the test drug compound needed. In the improved in situ model, the drug is administered by a single injection into the region of interest, while the single pass perfusion method needs perfusion of a drug until the steady state is reached. Thus, the improved in situ absorption model requires less amount of drug than the single-pass perfusion. The characteristics of the improved in situ absorption method are summarized in comparison with the single-pass perfusion method in
Table 5.
A better understanding of the regional absorption of a drug provides useful insight for the formulation development. The importance of good regional absorption characteristics, which is high and similar absorption throughout the gastrointestinal tract, of a selected compound may be crucial for the development of extended release formulations [
27]. If the absorption is limited in the certain part of the gastrointestinal tract, formulations that make a drug stay for longer time at the absorption site may be designed to increase the absorption time, thereby improving the bioavailability. For example, drugs that are efficiently absorbed in the upper gastrointestinal tract may be formulated as gastroretentive systems to improve oral bioavailability, where extended release formulations may not help.
In summary, a novel improved in situ absorption method was developed for the assessment of regional absorption in the gastrointestinal tract by using fimasartan as a model drug. Instead of measuring drug concentrations in the perfusate while a segment of the intestine is perfused with a drug solution, this method measured the actual plasma drug concentration following the injection of fimasartan into a specific part of the gastrointestinal tract. Thus, the present approach provides more physiological and accurate assessment of the absorbed fraction into the systemic circulation across the region of the gastrointestinal tract after the oral administration. The developed improved in situ absorption model would provide a useful experimental strategy to understand the regional absorption of a drug and a guide to developing new formulations with optimized oral bioavailability.