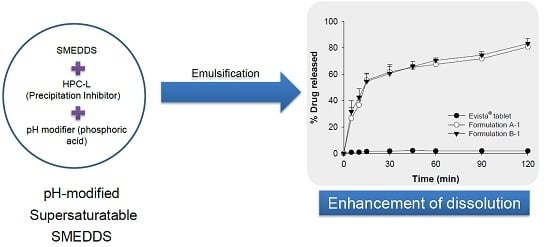
Development and Evaluation of Raloxifene-Hydrochloride-Loaded Supersaturatable SMEDDS Containing an Acidifier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Screening Tests
2.3. Preparation of RLH-Loaded S-SMEDDSs Containing Hydrophilic Polymers
2.4. Selection of an Acidifier/pH Modifier, and In Vitro Dissolution and Stability Testing
3. Results and Discussion
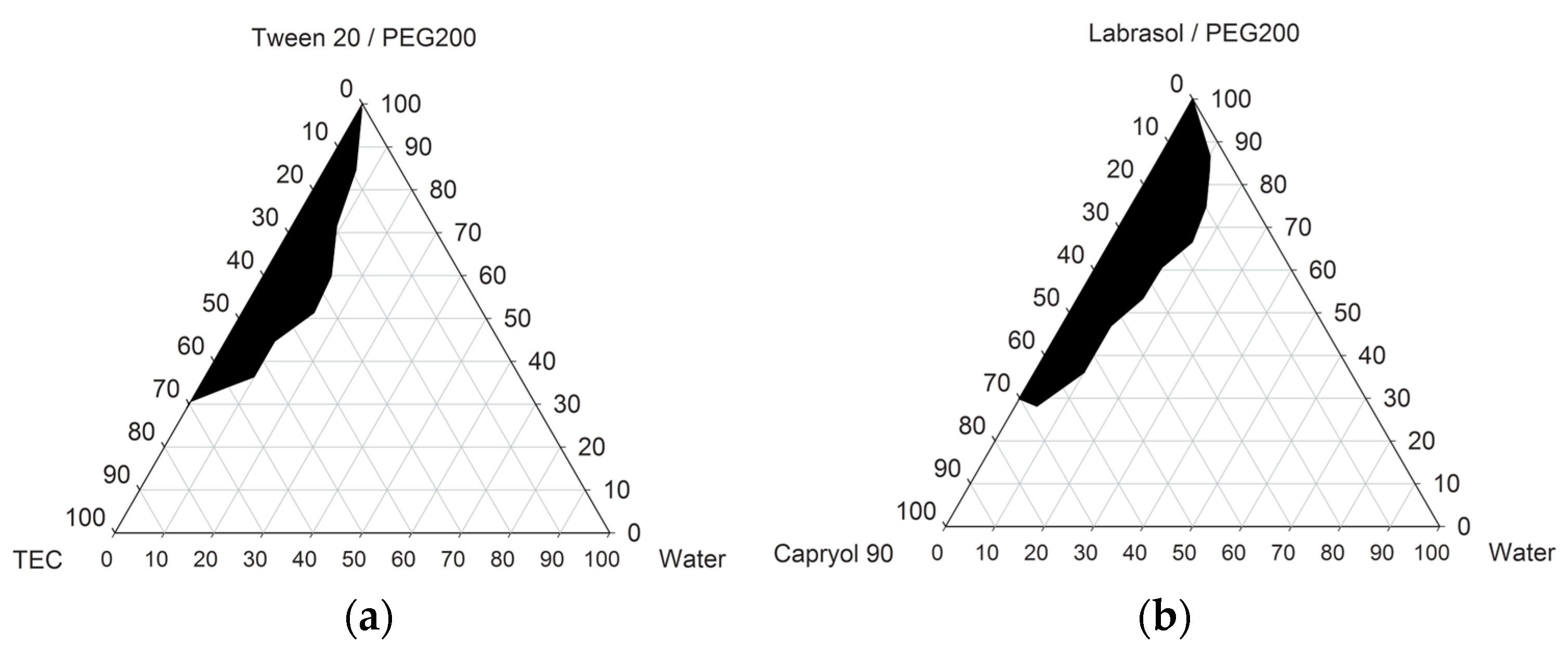
3.1. Solubility Screening Test
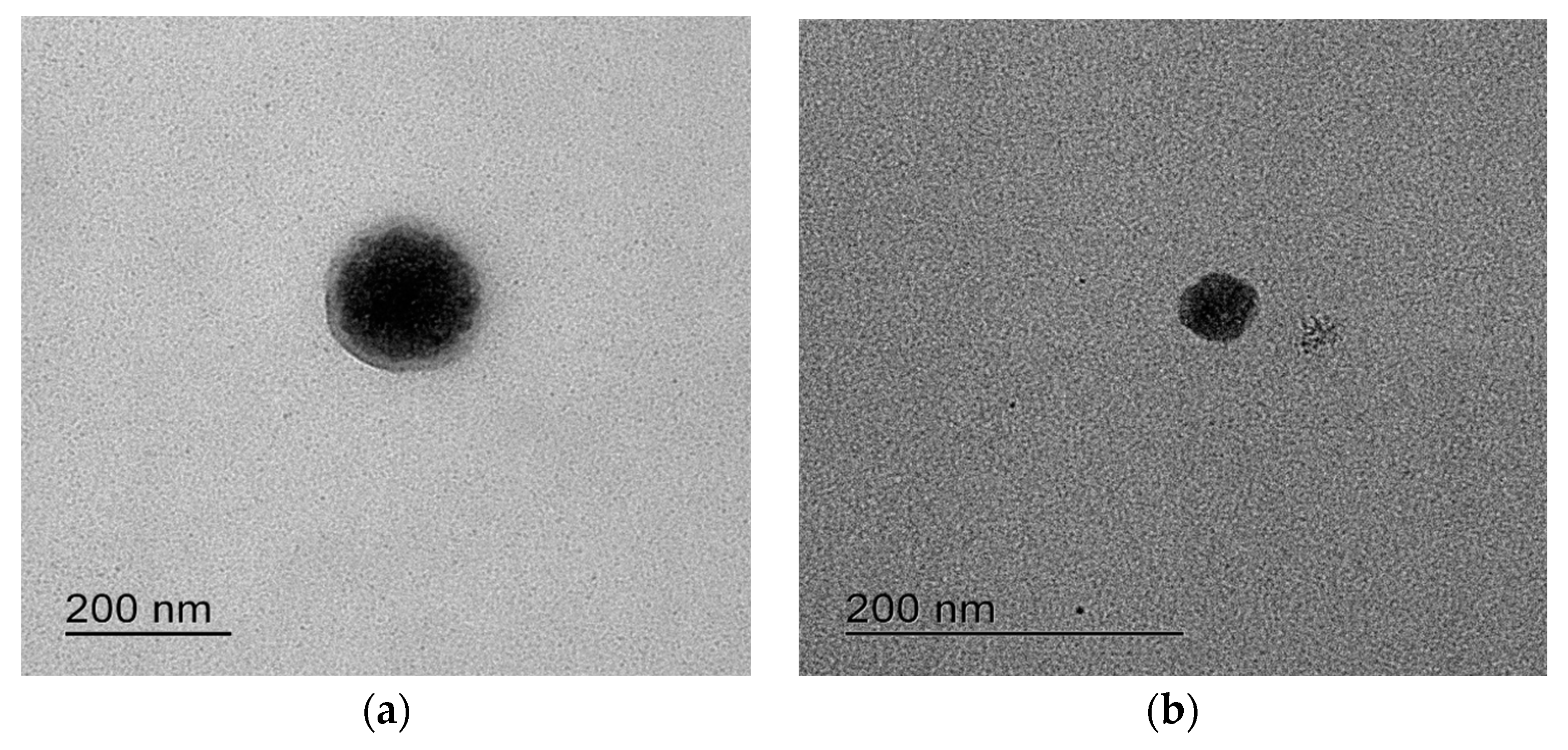
3.2. RLH-Loaded S-SMEDDSs Containing Hydrophilic Polymers
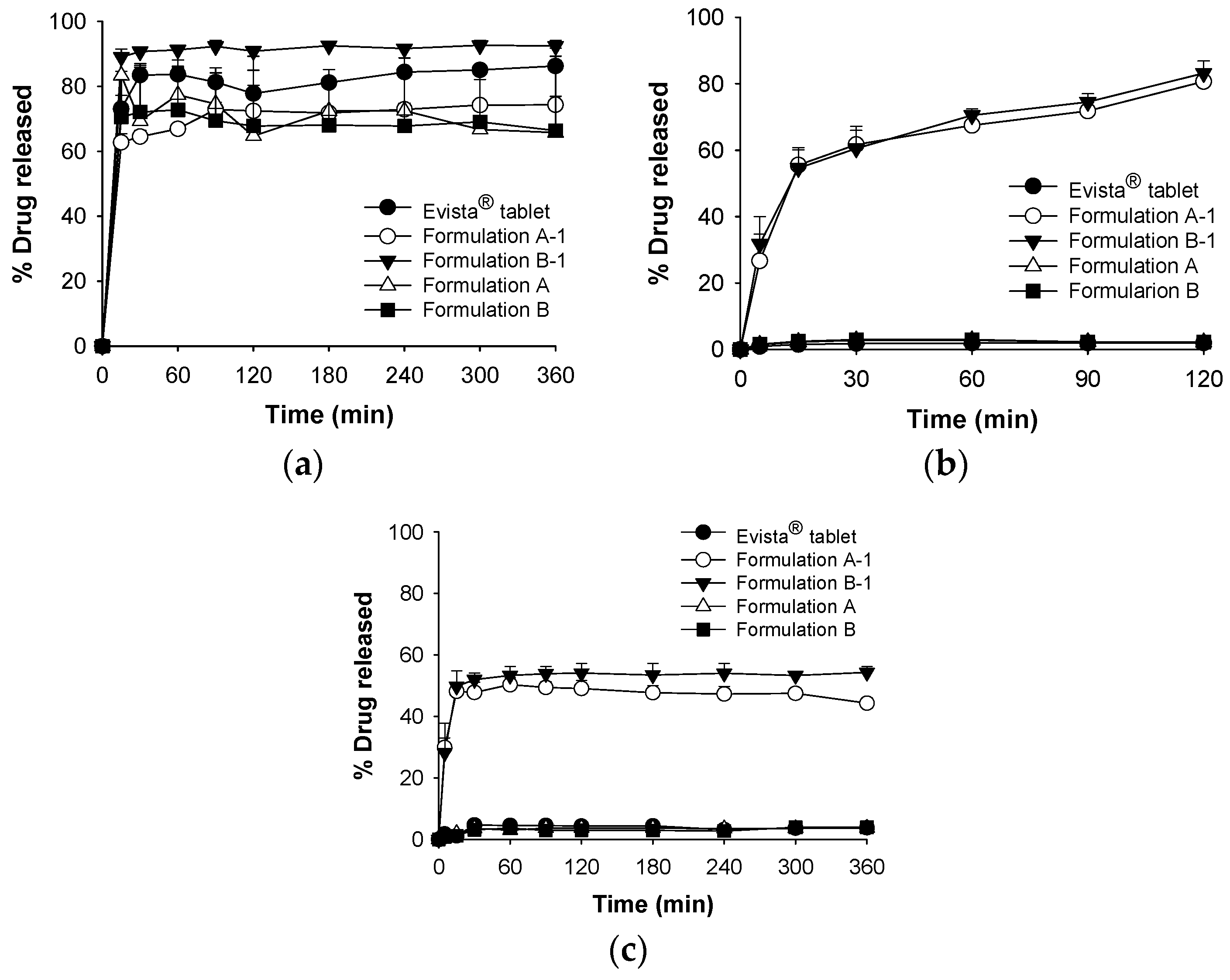
3.3. Improvement of Dissolution Behavior: Acidifier and Stability Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rai, V.; Rajput, B.; Sharma, M.; Agarwal, A.; Gupta, A.; Singh, N. Solubility enhancement of poorly water-soluble drug (raloxifene hydrochloride) by using different hydrophilic binders in solid dosage form. Pharm. Glob. Int. J. Compr. Pharm. 2010, 1, 1–5. [Google Scholar]
- Griffiths, K.A.; Sader, M.A.; Skilton, M.R.; Harmer, J.A.; Celermajer, D.S. Effects of raloxifene on endothelium-dependent dilation, lipoproteins, and markers of vascular function in postmenopausal women with coronary artery disease. J. Am. Coll. Cardiol. 2003, 42, 698–704. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Sugiyama, M.; Mabuchi, S.; Hisamatsu, T.; Nishio, Y.; Ito, K.; Kimura, T.; Kamiura, S.; Morishige, K. Efficacy of raloxifene hydrochloride for the prevention of health care problems in patients who undergo surgery for endometrial cancer: A multicenter randomized clinical trial. Int. J. Gynecol. Cancer 2015, 25, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Messalli, E.M.; Mainini, G.; Scaffa, C.; Cafiero, A.; Salzillo, P.L.; Ragucci, A.; Cobellis, L. Raloxifene therapy interacts with serum osteoprotegerin in postmenopausal women. Maturitas 2007, 56, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Haynes, J.M. Pharmaceutical applications of embryonic stem cells. Adv. Drug Deliv. Rev. 2005, 57, 1918–1934. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (smedds) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoon, K.A.; Hahn, M.; Park, E.S.; Chi, S.C. Preparation and in vitro evaluation of self-microemulsifying drug delivery systems containing idebenone. Drug Dev. Ind. Pharm. 2000, 26, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Sawant, K.K. Self micro-emulsifying drug delivery system: Formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr. Drug Deliv. 2009, 6, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Augustijns, P.; Brewster, M.E. Supersaturating drug delivery systems: Fast is not necessarily good enough. J. Pharm. Sci. 2012, 101, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Iervolino, M.; Raghavan, S.L.; Hadgraft, J. Membrane penetration enhancement of ibuprofen using supersaturation. Int. J. Pharm. 2000, 198, 229–238. [Google Scholar] [CrossRef]
- Pellett, M.A.; Castellano, S.; Hadgraft, J.; Davis, A.F. The penetration of supersaturated solutions of piroxicam across silicone membranes and human skin in vitro. J. Control. Release 1997, 46, 205–214. [Google Scholar] [CrossRef]
- Usui, F.; Maeda, K.; Kusai, A.; Nishimura, K.; Keiji, Y. Inhibitory effects of water-soluble polymers on precipitation of rs-8359. Int. J. Pharm. 1997, 154, 59–66. [Google Scholar] [CrossRef]
- Tran, T.T.; Tran, P.H.; Choi, H.G.; Han, H.K.; Lee, B.J. The roles of acidifiers in solid dispersions and physical mixtures. Int. J. Pharm. 2010, 384, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.I.; Hussain, M.A. Microenvironmental pH modulation in solid dosage forms. J. Pharm. Sci. 2007, 96, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Morozowich, W. Development of supersaturatable self-emulsifying drug delivery system formulations for improving the oral absorption of poorly soluble drugs. Expert Opin. Drug Deliv. 2006, 3, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, G.; Wu, X.; Chen, Z.; Hang, J.; Qin, B.; Chen, S.; Wang, R. Self-microemulsifying drug delivery system (smedds) of vinpocetine: Formulation development and in vivo assessment. Biol. Pharm. Bull. 2008, 31, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Vavia, P. Preparation and in vivo evaluation of smedds (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007, 9, E344–E352. [Google Scholar] [CrossRef] [PubMed]
- De Campo, L.; Yaghmur, A.; Garti, N.; Leser, M.E.; Folmer, B.; Glatter, O. Five-component food-grade microemulsions: Structural characterization by sans. J. Colloid Interface Sci. 2004, 274, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, E.; Acosta, E.; Sabatini, D.A.; Harwell, J.H. A two-state model for selective solubilization of benzene−limonene mixtures in sodium dihexyl sulfosuccinate microemulsions. Langmuir 2004, 20, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.L.; Kiepfer, B.; Davis, A.F.; Kazarian, S.G.; Hadgraft, J. Membrane transport of hydrocortisone acetate from supersaturated solutions; the role of polymers. Int. J. Pharm. 2001, 221, 95–105. [Google Scholar] [CrossRef]
- Kim, D.W.; Kwon, M.S.; Yousaf, A.M.; Balakrishnan, P.; Park, J.H.; Kim, D.S.; Lee, B.J.; Park, Y.J.; Yong, C.S.; Kim, J.O.; et al. Comparison of a solid smedds and solid dispersion for enhanced stability and bioavailability of clopidogrel napadisilate. Carbohydr. Polym. 2014, 114, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.S.; Cho, W.; Cha, K.H.; Park, H.J.; Park, J.; Hwang, S.J. Supersaturatable formulations for the enhanced oral absorption of sirolimus. Int. J. Pharm. 2013, 445, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Liu, Y.; Zhao, J.H.; Wang, L.; Feng, N.P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int. J. Nanomed. 2012, 7, 1115–1125. [Google Scholar]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (snedds) for oral delivery of zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Teeter, J.S.; Meyerhoff, R.D. Environmental fate and chemistry of raloxifene hydrochloride. Environ. Toxicol. Chem. 2002, 21, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.S.; Somvanshi, S.; Jumde, A.B.; Khandelwal, H.M.; Umekar, M.J.; Kotagale, N.R. Ethyl cellulose and hydroxypropyl methyl cellulose buoyant microspheres of metoprolol succinate: Influence of pH modifiers. Int. J. Pharm. Investig. 2013, 3, 163–170. [Google Scholar] [PubMed]
- Rao, V.M.; Engh, K.; Qiu, Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int. J. Pharm. 2003, 252, 81–86. [Google Scholar] [CrossRef]
- Nie, S.; Pan, W.; Li, X.; Wu, X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev. Ind. Pharm. 2004, 30, 627–635. [Google Scholar] [CrossRef] [PubMed]


| Vehicle | Solubility (mg/mL) |
|---|---|
| Oil | |
| Triethyl citrate (TEC) | 0.58 ± 0.02 |
| Capryol™ 90 | 0.52 ± 0.02 |
| Glyceryl triacetate | 0.38 ± 0.04 |
| Labrafil M® 1944 CS | 0.16 ± 0.01 |
| Labrafil M® 2125 CS | 0.15 ± 0.00 |
| Lauroglycol™ 90 | 0.21 ± 0.01 |
| Lauroglycol™ FCC | 0.09 ± 0.00 |
| Capryol™ PGMC | 0.51 ± 0.02 |
| Pluorol® Oleique CC 497 | 0.04 ± 0.00 |
| Surfactant | |
| Cremophor® EL | 0.82 ± 0.11 |
| Cremophor® RH 40 | 1.35 ± 0.48 |
| Labrasol® | 11.59 ± 0.76 |
| Transcutol® P | 8.41 ± 0.62 |
| Span® 20 | 0.08 ± 0.05 |
| Span® 80 | 0.01 ± 0.01 |
| Tween® 20 | 28.38 ± 3.07 |
| Tween® 80 | 18.78 ± 5.44 |
| Brij® 97 | 1.44 ± 0.41 |
| Glyceride | |
| Glycerine | 6.50 ± 1.37 |
| Polyethylene glycol (PEG) | 9.39 ± 2.21 |
| PEG 200 | 31.14 ± 1.54 |
| PEG 400 | 17.86 ± 5.11 |
| Propylene glycol (PG) | 11.74 ± 0.55 |
| Ingredients (w/w, %) | Solubility (mg/g) | ||
|---|---|---|---|
| Tween® 20 | Labrasol® | PEG 200 | |
| 100 | 31.14 ± 1.54 | ||
| 100 | 11.59 ± 0.76 | ||
| 100 | 28.38 ± 3.07 | ||
| 20 | 80 | 39.07 ± 0.71 | |
| 25 | 75 | 30.47 ± 2.19 | |
| 33.3 | 66.7 | 30.88 ± 3.39 | |
| 50 | 50 | 27.42 ± 1.19 | |
| 66.7 | 33.3 | 19.68 ± 2.28 | |
| 75 | 25 | 28.28 ± 1.26 | |
| 80 | 20 | 20.68 ± 1.72 | |
| 20 | 80 | 28.70 ± 0.73 | |
| 25 | 75 | 24.76 ± 0.76 | |
| 33.3 | 66.7 | 19.68 ± 2.63 | |
| 50 | 50 | 11.90 ± 2.07 | |
| 66.7 | 33.3 | 9.16 ± 0.66 | |
| 75 | 25 | 10.10 ± 0.75 | |
| 80 | 20 | 16.12 ± 2.78 | |
| 25 | 25 | 50 | 26.16 ± 1.94 |
| 25 | 50 | 25 | 20.33 ± 2.97 |
| No. | Triethyl Citrate | Capryol 90™ | Tween® 20 | Labrasol® | PEG 200 | Solubility (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 5 | 95 | 23.10 ± 3.07 | |||
| 2 | 10 | 90 | 22.52 ± 0.71 | |||
| 3 | 5 | 19 | 76 | 32.75 ± 1.76 | ||
| 4 | 5 | 20 | 75 | 37.96 ± 1.98 | ||
| 5 | 5 | 40 | 55 | 30.88 ± 0.37 | ||
| 6 | 5 | 75 | 20 | 22.95 ± 0.04 | ||
| 7 | 5 | 30 | 10 | 55 | 31.70 ± 1.87 | |
| 8 | 5 | 27.14 | 13.57 | 54.29 | 15.20 ± 1.44 | |
| 9 | 5 | 23.75 | 23.75 | 47.5 | 23.50 ± 3.19 | |
| 10 | 5 | 20 | 75 | 24.48 ± 2.30 | ||
| 11 | 10 | 10 | 80 | 23.36 ± 0.49 | ||
| 12 | 10 | 18 | 72 | 29.84 ± 0.84 | ||
| 13 | 5 | 20 | 75 | 36.83 ± 1.33 | ||
| 14 | 5 | 19 | 76 | 32.75 ± 1.76 | ||
| 15 | 10 | 18 | 72 | 24.80 ± 1.82 |
| Formulation | Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| A (S-SMEDDS) | 1316.67 ± 72.57 | 0.18 ± 0.04 | 33.80 ± 0.75 |
| B (S-SMEDDS) | 190.73 ± 2.15 | 0.25 ± 0.04 | 54.97 ± 2.14 |
| A-1 (pH-modified S-SMEDDS) | 266.57 ± 3.56 | 0.73 ± 0.01 | 33.80 ± 1.97 |
| B-1 (pH-modified S-SMEDDS) | 195.50 ± 2.90 | 0.22 ± 0.01 | 57.23 ± 1.38 |
| Formulation | Solubility (mg/mL) | ||
|---|---|---|---|
| Water | pH 1.2 | pH 6.8 | |
| A (S-SMEDDS) | 8.89 ± 0.12 | 0.79 ± 0.19 | 0.22 ± 0.03 |
| B (S-SMEDDS) | 13.13 ± 0.35 | 2.86 ± 0.42 | 1.10 ± 0.03 |
| A-1 (pH-modified S-SMEDDS) | 11.57 ± 0.45 | 3.28 ± 0.24 | 1.95 ± 0.89 |
| B-1 (pH-modified S-SMEDDS) | 11.65 ± 0.55 | 6.05 ± 0.41 | 3.46 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, H.H.; Cho, Y.H.; Koo, T.-S.; Lee, G.W. Development and Evaluation of Raloxifene-Hydrochloride-Loaded Supersaturatable SMEDDS Containing an Acidifier. Pharmaceutics 2018, 10, 78. https://doi.org/10.3390/pharmaceutics10030078
Lee J-H, Kim HH, Cho YH, Koo T-S, Lee GW. Development and Evaluation of Raloxifene-Hydrochloride-Loaded Supersaturatable SMEDDS Containing an Acidifier. Pharmaceutics. 2018; 10(3):78. https://doi.org/10.3390/pharmaceutics10030078
Chicago/Turabian StyleLee, Jong-Hwa, Hak Hyung Kim, Young Ho Cho, Tae-Sung Koo, and Gye Won Lee. 2018. "Development and Evaluation of Raloxifene-Hydrochloride-Loaded Supersaturatable SMEDDS Containing an Acidifier" Pharmaceutics 10, no. 3: 78. https://doi.org/10.3390/pharmaceutics10030078