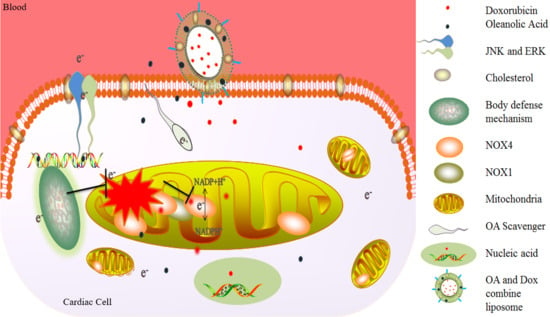
Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animals
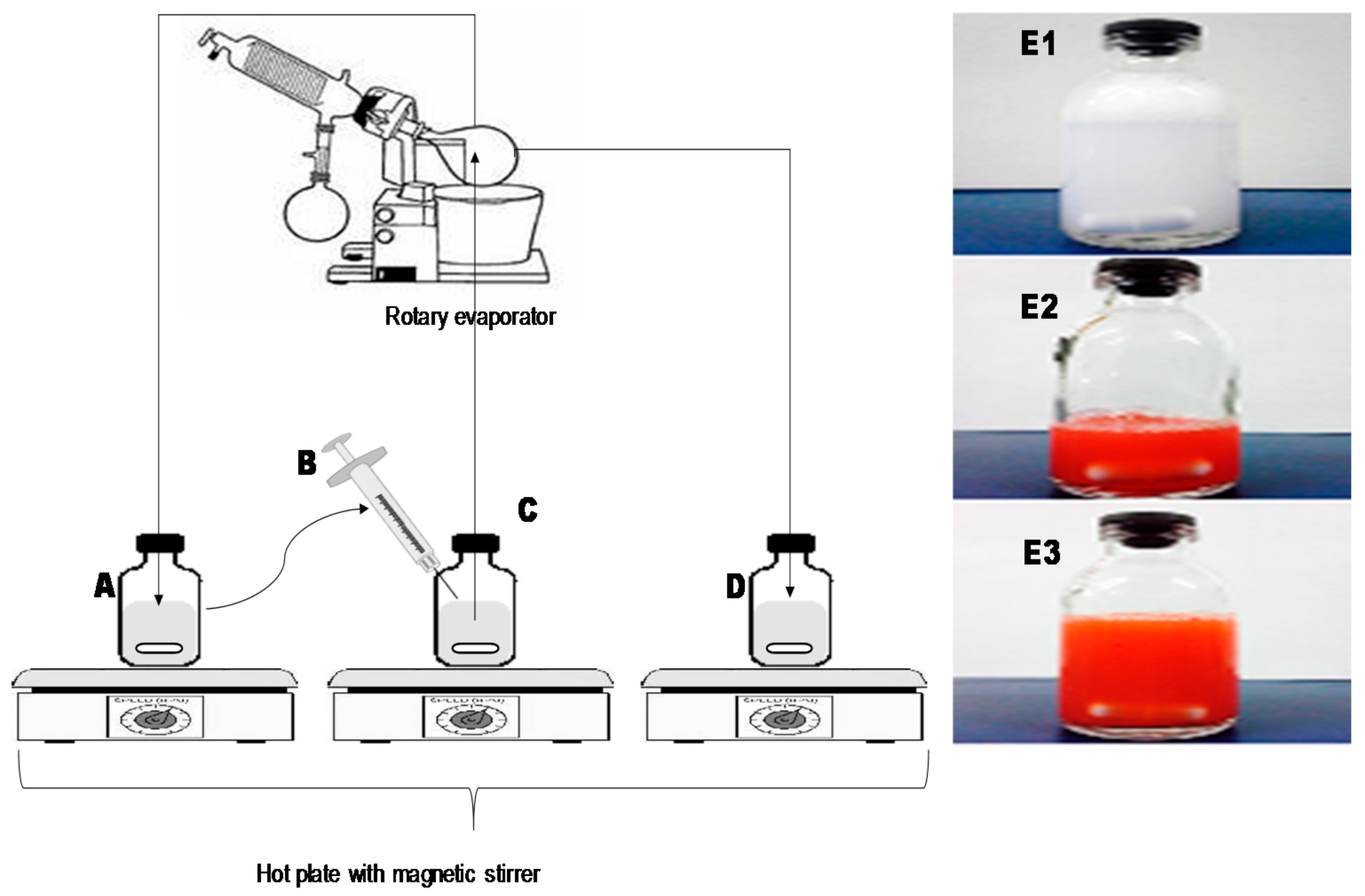
2.4. Preparation of Liposomes
2.4.1. Thin Film Hydration Method (TFH)
2.4.2. Reengineered Ethanolic Injection Method (REIM)
2.5. Method Optimization
2.6. Characterization of Liposomes
2.7. Determination of Drug Loading (DL) and Entrapment Efficiency (EE)
2.8. Stability Studies of Liposomes
2.9. Cell Viability Study
2.10. Cell Uptake Study
2.11. Bio-Distribution Study
2.12. Toxicity Evaluation
2.13. Statistics
3. Results
3.1. Preparation of Liposomes
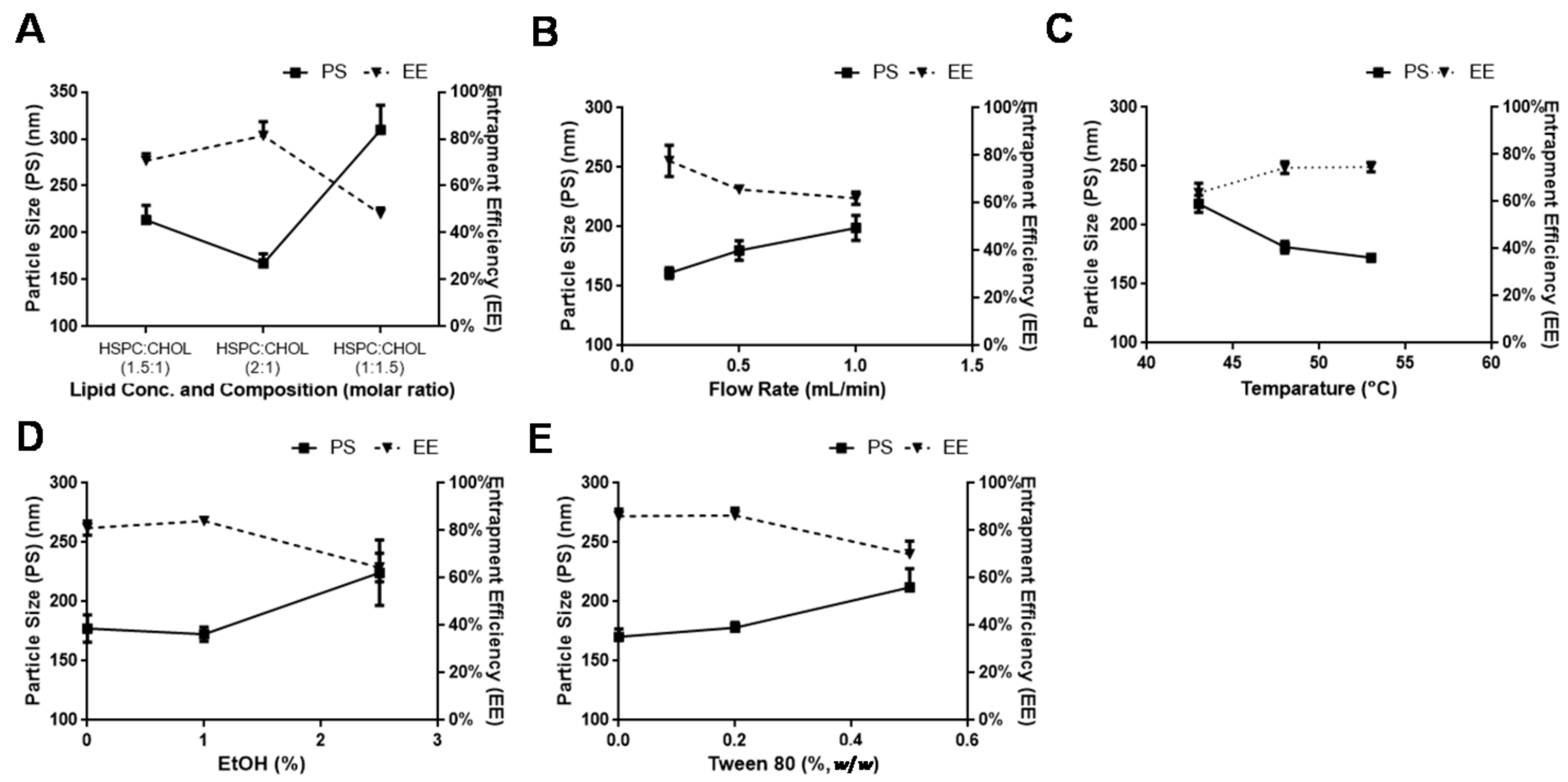
3.2. Method Optimization
3.2.1. Lipid Concentration and Composition
3.2.2. Dispersion Medium
3.2.3. Injection Direction
3.2.4. Rate of Administration
3.2.5. Sonication and Temperature Effect
3.2.6. Effect of Ethanol
3.2.7. Effect of Tween 80
3.3. Characterization of Liposomes
3.4. Stability Study of Liposome
3.5. Cell Viability Study
3.6. Cell Uptake Study
3.7. Bio-Distribution Study
3.8. Toxicity Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.H.; Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 2012, 915375. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Okada, S. Cancer chemoprevention as adjuvant therapy for hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2001, 31, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Kojima-Yuasa, A.; Huang, X.; Matsui-Yuasa, I. Synergistic anticancer activities of natural substances in human hepatocellular carcinoma. Diseases 2015, 3, 260–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xie, C.; Huang, Y.; Lam, C.W.K.; Chow, M.S.S. Overcoming chemotherapy resistance with herbal medicines: Past, present and future perspectives. Phytochem. Rev. 2014, 13, 323–337. [Google Scholar] [CrossRef]
- Tardi, P.G.; Gallagher, R.C.; Johnstone, S.; Harasym, N.; Webb, M.; Bally, M.B.; Mayer, L.D. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Samec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic acid alters multiple cell signaling pathways: Implication in cancer prevention and therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, R.F.; Rajamani, U.; Dlamini, N.; Zungu-Edmondson, M.; Kelly-Laubscher, R.; Shafiullah, M.; Wahab, A.; Hasan, M.Y.; Fahim, M.A.; Rondeau, P.; et al. Oleanolic acid: A novel cardioprotective agent that blunts hyperglycemia-induced contractile dysfunction. PLoS ONE 2012, 7, e47322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Yang, T.; Zhang, W.; Lu, M.; Ma, X.; Xiang, G. In vitro and in vivo antitumor effects of folate-targeted ursolic acid stealth liposome. J. Agric. Food Chem. 2014, 62, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposomes in drug delivery: How it all happened. Pharmaceutics 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Barcellos, J.P.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.S.; Chitneni, M.; Lee, S.K.; Ming, C.L.; Yuen, H.K. Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, W.D.; Yang, T.; Lu, Y.; Lu, M.; Gai, Y.K.; Ma, X.; Xiang, G.Y. Folate receptor-targeted liposomes enhanced the antitumor potency of imatinib through the combination of active targeting and molecular targeting. Int. J. Nanomed. 2014, 9, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Committee for Medicinal Products for Human Use Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK; Available online: http://www.Ema.Europa.Eu/docs/en_gb/document_library/scientific_guideline/2011/08/wc500109686.Pdf (accessed on 4 September 2018).
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Available online: www.Bioagilytix.Com/wp-content/uploads/2016/02/fda-bioanalytical-method-validation-draft-guidance-2013.Pdf (accessed on 4 September 2018).
- Ashraf, M.; Abid, F.; Riffat, S.; Bashir, S.; Iqbal, J.; Sarfraz, M.; Afzal, A.; Zaheer, M. Rationalized and complementary findings of silymarin (milk thistle) in Pakistani healthy volunteers. Acta Pol. Pharm. 2015, 72, 607–614. [Google Scholar] [PubMed]
- Sarfraz, A.; Sarfraz, M.; Ahmad, M. Development and Validation of a Bioanalytical Method for Direct Extraction of Diclofenac Potassium from Spiked Plasma. Trop. J. Pharm. Res. 2011, 10, 663–669. [Google Scholar] [CrossRef]
- Evjen, T.J.; Nilssen, E.A.; Rognvaldsson, S.; Brandl, M.; Fossheim, S.L. Distearoylphosphatidylethanolamine-based liposomes for ultrasound-mediated drug delivery. Eur. J. Pharm. Biopharm. 2010, 75, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote loading of doxorubicin into liposomes by transmembrane pH gradient to reduce toxicity toward H9c2 cells. Saudi Pharm. J. 2016, 24, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Evjen, T.J.; Hagtvet, E.; Nilssen, E.A.; Brandl, M.; Fossheim, S.L. Sonosensitive dioleoylphosphatidylethanolamine-containing liposomes with prolonged blood circulation time of doxorubicin. Eur. J. Pharm. Sci. 2011, 43, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, T.; Zhang, W.; Lu, Y.; Ye, P.; Yang, G.; Li, B.; Qi, S.; Liu, Y.; He, X.; et al. A novel hydrolysis-resistant lipophilic folate derivative enables stable delivery of targeted liposomes in vivo. Int. J. Nanomed. 2014, 9, 4581–4595. [Google Scholar] [CrossRef]
- Nounou, M.M.; El-Khordagui, L.K.; Khalafallah, N. Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles. Acta Pol. Pharm. 2005, 62, 369–379. [Google Scholar] [PubMed]
- Pons, M.; Foradada, M.; Estelrich, J. Liposomes obtained by the ethanol injection method. Int. J. Pharm. 1993, 95, 51–56. [Google Scholar] [CrossRef]
- Trandum, C.; Westh, P.; Jørgensen, K.; Mouritsen, O.G. A thermodynamic study of the effects of cholesterol on the interaction between liposomes andd ethanol. Biophys. J. 2000, 78, 2486–2492. [Google Scholar] [CrossRef]
- Peng, W.; Ding, F.; Jiang, Y.T.; Peng, Y.K. Bioavailability and activity of natural food additive triterpenoids as influenced by protein. J. Agric. Food Chem. 2014, 62, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Hernandez, A.; Martinez, A.; Rivas, F.; Garcia-Mesa, J.A.; Parra, A. Effect of the solvent and the sample preparation on the determination of triterpene compounds in two-phase olive-mill-waste samples. J. Agric. Food Chem. 2015, 63, 4269–4275. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-P.; Kong, T.; Zhang, L.; Tong, S.; Tian, Z.-Y.; Duan, Y.-H.; Zhang, X.-H. Solubilities of ursolic acid and oleanolic acid in four solvents from (283.2 to 329.7) k. J. Chem. Eng. Data 2011, 56, 2723–2725. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Abbas, S.; Feng, B.; Zhang, X.M.; Xia, S.Q. Liposome as a delivery system for carotenoids: Comparative antioxidant activity of carotenoids as measured by ferric reducing antioxidant power, dpph assay and lipid peroxidation. J. Agric. Food Chem. 2014, 62, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Gipmans, M.; Hurst, S.; Layton, R.; Nehra, N.; Pickett, J.; Shah, D.M.; Souza, T.L.; Tripathi, L. Emerging agricultural biotechnologies for sustainable agriculture and food security. J. Agric. Food Chem. 2016, 64, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.Q.; Liu, W.; Liu, W.L.; Liang, R.H.; Li, T.; Liu, C.M.; Cao, Y.L.; Niu, J.; Liu, Z. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J. Agric. Food Chem. 2014, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Wei, X.; Rao, W.; Ai, F.; Zhao, F.; Men, K.; Yang, B.; Liu, X.; Huang, M.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Caddell, R.; Yu, B.; Xu, S.; Theobald, B.; Lee, L.J.; Lee, R.J. Ultrasound-enhanced microfluidic synthesis of liposomes. Anticancer Res. 2010, 30, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Smith, K.E.; Chong, P.L. Effects of alcohol-induced lipid interdigitation on proton permeability in l-alpha-dipalmitoylphosphatidylcholine vesicles. Biophys. J. 1993, 65, 1404–1414. [Google Scholar] [CrossRef]
- Rottenberg, H. Probing the interactions of alcohols with biological membranes with the fluorescent probe prodan. Biochemistry 1992, 31, 9473–9481. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.S.; Kuo, C.C.; Lin, S.H.; Kamaya, H.; Ueda, I. Interfacial dehydration by alcohols: Hydrogen bonding of alcohols to phospholipids. Alcohol 1991, 8, 143–150. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Su, T.T.; Feng, L.L.; Long, Y.Y.; Chen, Z.B. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, F.; Zhang, Z.; Zhu, F.; Xue, J.; Tan, X.; Zhang, L.; Jia, X. Formulation and evaluation of celastrol-loaded liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quesada, C.; Lopez-Biedma, A.; Warleta, F.; Campos, M.; Beltran, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef] [PubMed]
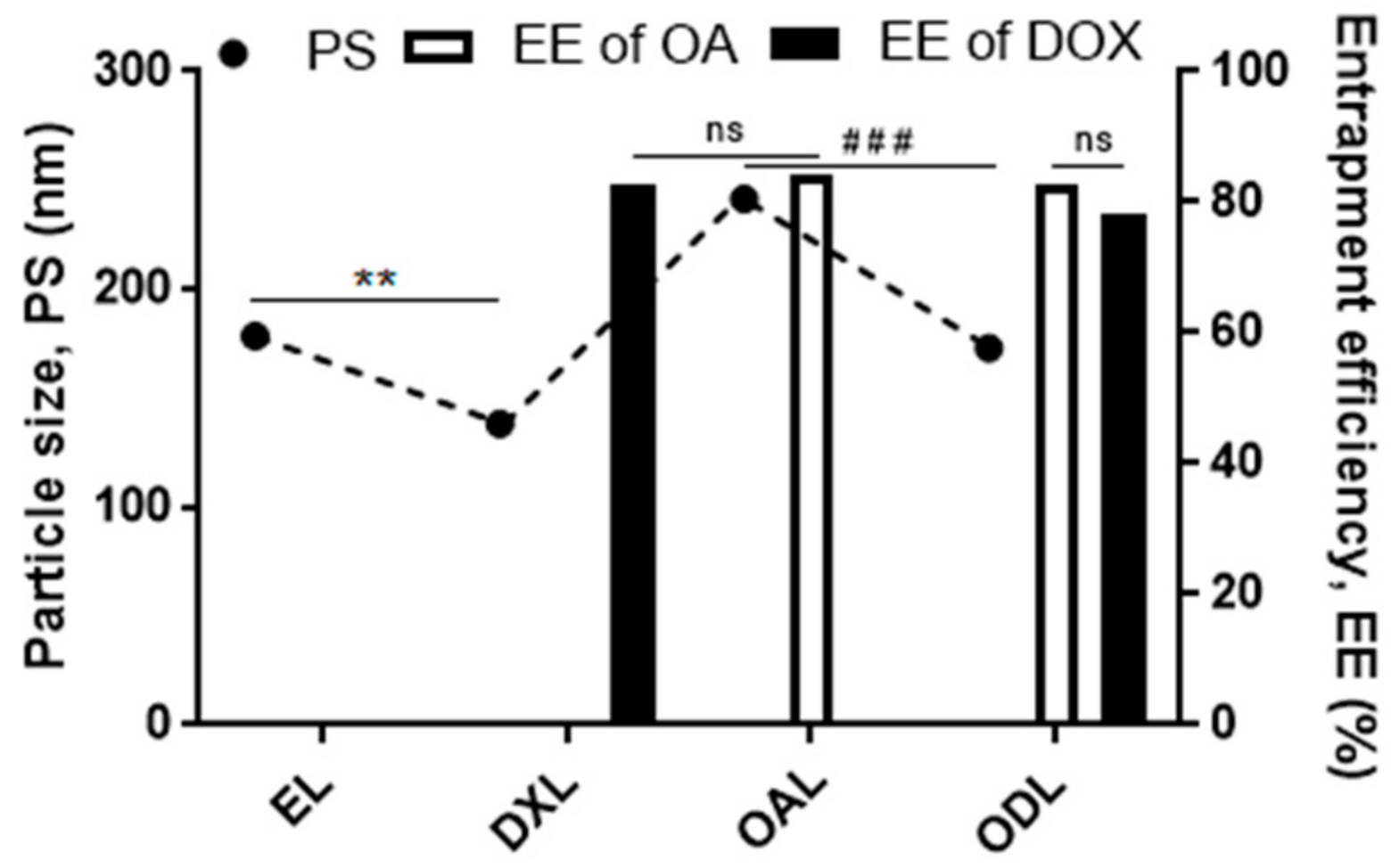


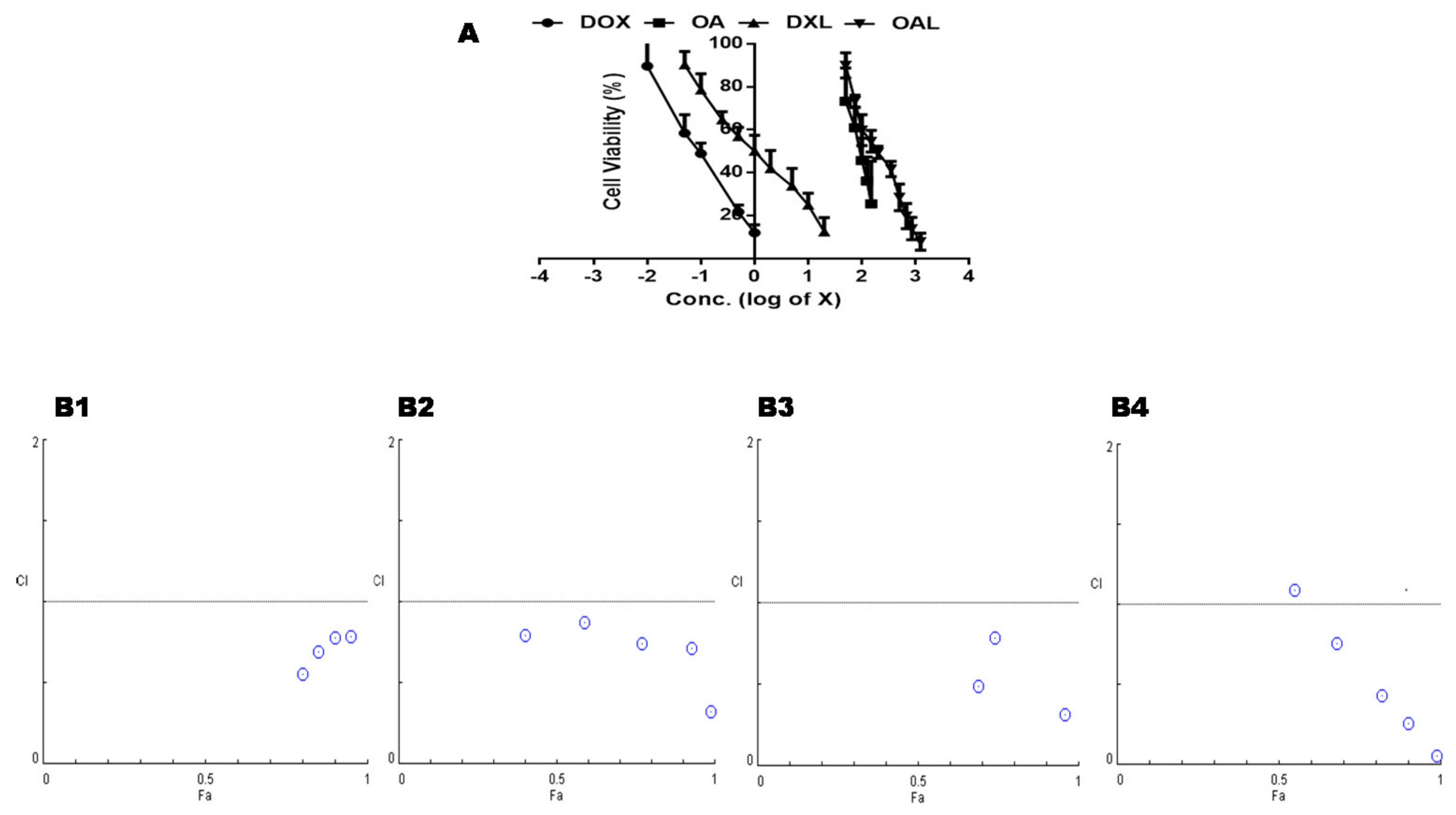
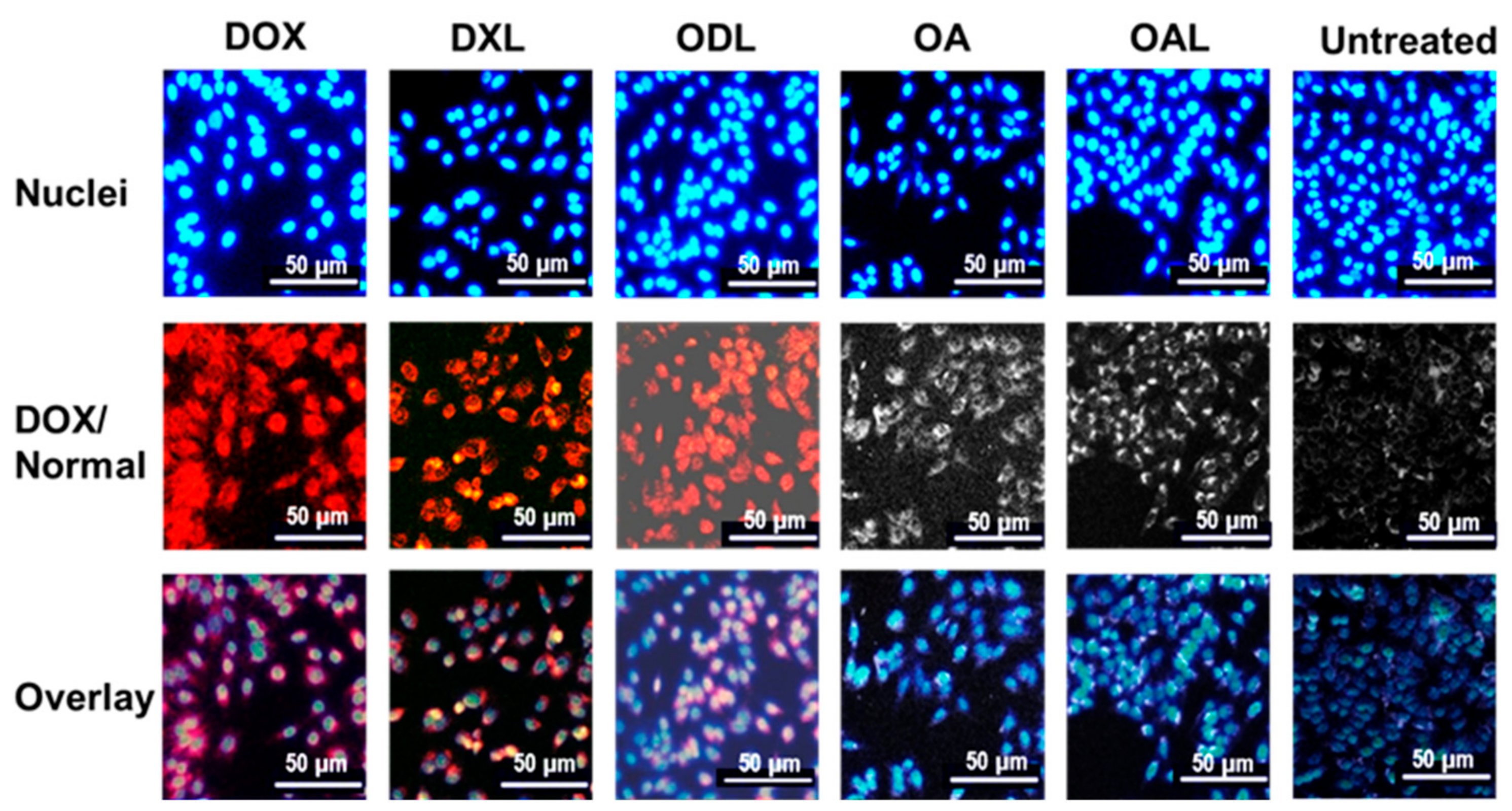
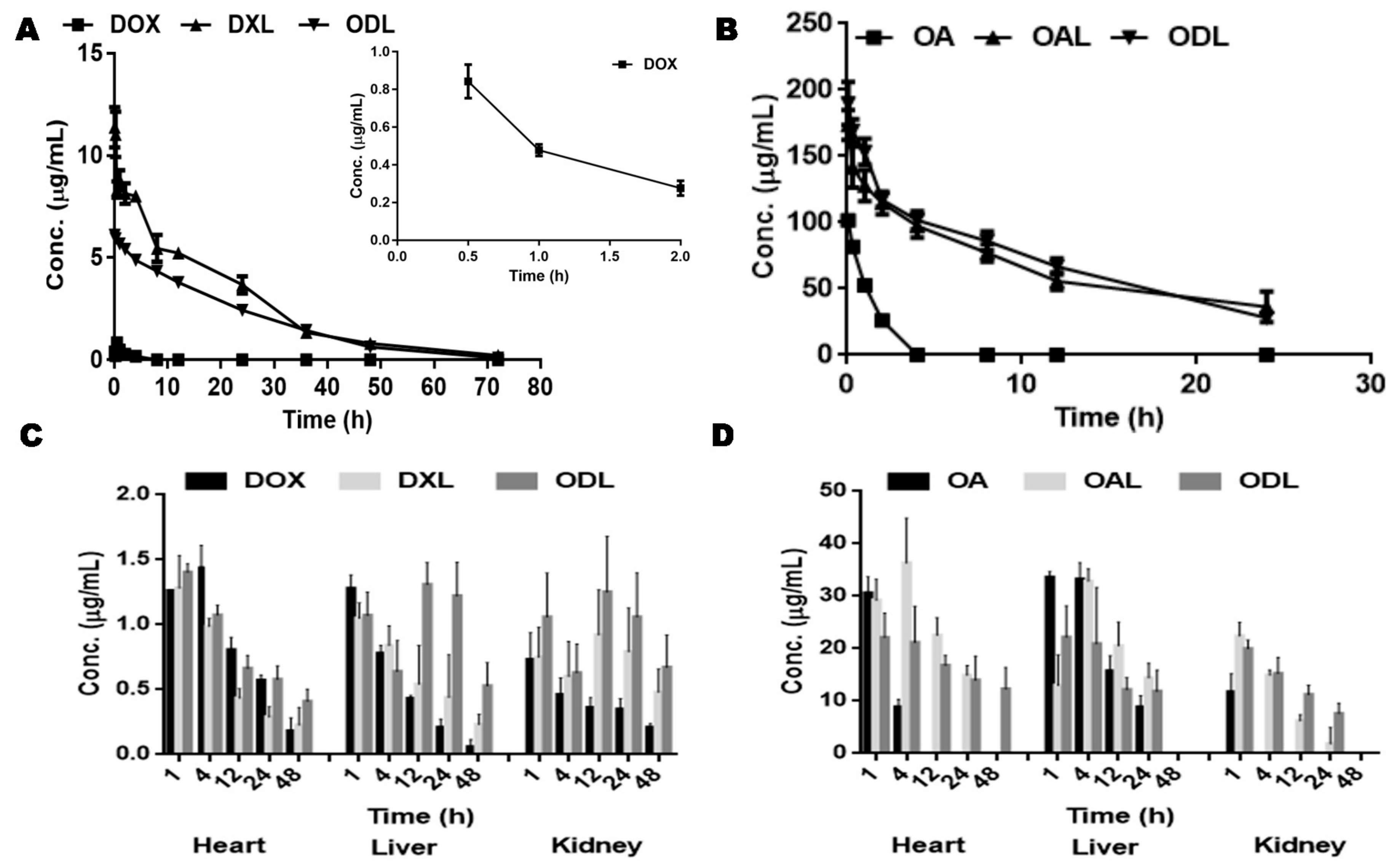
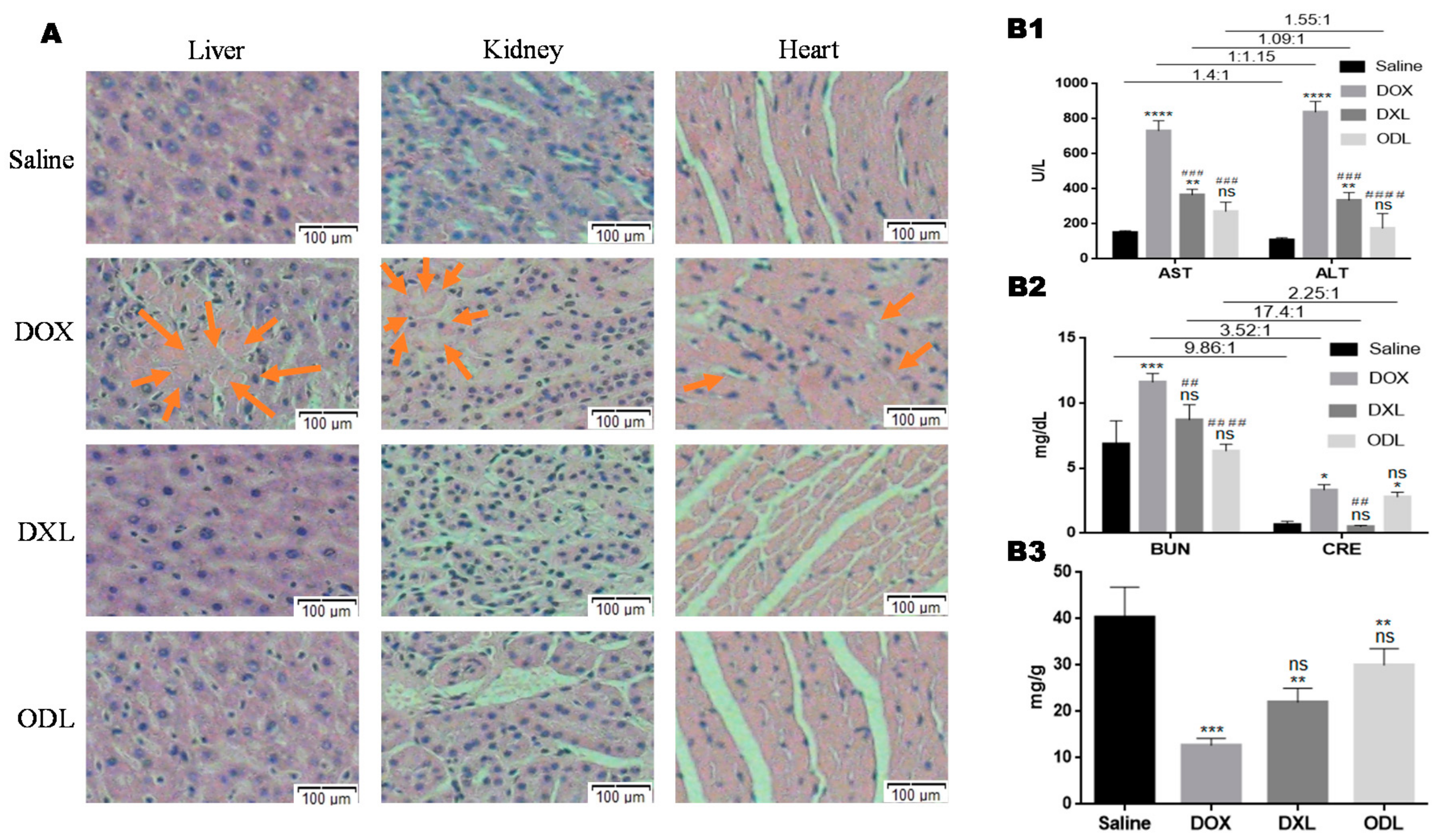
| Batch ID | Before Extrusion | After Extrusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS | PDI | ZP | %EEOA | %EEDOX | PS | PDI | ZP | %EEOA | %EEDOX | |
| OAL | 314.33 ± 30.92 | 0.18 ± 3.06 | −18.33 ± 3.06 | 77.11 ± 3.72 | 241.33 ± 20.13 | 0.19 ± 0.02 | −18.33 ± 4.16 | 66.11 ± 3.22 | ||
| DXL | 155 ± 11.53 | 0.17 ± 0.06 | −27.66 ± 8.96 | 84.82 ± 2.86 | 135.33 ± 8.5 | 0.15 ± 0.03 | −19.33 ± 1.53 | 78.93 ± 1.4 | ||
| ODL | 225.33 ± 28.02 | 0.19 ± 0.03 | −24.33 ± 2.52 | 70.97 ± 3.80 | 63.21 ± 4.12 | 199 ± 10.54 | 0.15 ± 0.03 | −18.33 ± 7.57 | 63.81 ± 2.25 | 61.03 ± 1.76 |
| Batch ID | PS | ZP | PDI | %EEOA | %EEDOX | %EEComb. |
|---|---|---|---|---|---|---|
| OAL | 127 ± 11.14 | −19.33 ± 3.51 | 0.14 ± 0.07 | 95.67 ± 3.06 | ||
| DXL | 136.33 ± 7.02 | −11.33 ± 3.05 | 0.13 ± 0.04 | 91.67 ± 5.7 | ||
| ODL | 169.67 ± 16.01 | −13 ± 5.3 | 0.18 ± 0.03 | 93 ± 2 | 98 ± 1 | 95.1 ± 1.5 |
| PK Parameters | DOX | DXL | OA | OAL | ODL | |
|---|---|---|---|---|---|---|
| DOX | OA | |||||
| Cmax (µg/mL) | 0.91 ± 0.12 | 10.78 ± 1.5 | 90.83 ± 17.11 | 172.81 ± 14.72 | 5.17 ± 0.5 | 130.74 ± 10.07 |
| AUCtot (µg/mL/h) | 1.18 ± 0.15 | 176.54 ± 9.93 | 98.83 ± 24.12 | 2135.24 ± 107.24 | 124.05 ± 2.68 | 1846.38 ± 79.35 |
| T1/2 (h) | 0.6 ± 0.33 | 12 ± 2.25 | 0.6 ± 0.14 | 10.84 ± 1.86 | 8.49 ± 0.65 | 8.86 ± 0.55 |
| Vd (mL) | 84.69 ± 44.08 | 16.68 ± 3.53 | 7.68 ± 0.59 | 6.93 ± 0.82 | 16.87 ± 1.12 | 6.14 ± 0.16 |
| CL (mL/h) | 99.02 ± 15.92 | 0.97 ± 0.17 | 9.34 ± 2.22 | 0.45 ± 0.03 | 1.38 ± 0.03 | 0.48 ± 0.03 |
| MRT (h) | 1.74 ± 0.16 | 19.11 ± 2.61 | 0.88 ± 0.16 | 16.32 ± 2.04 | 18.2 ± 0.92 | 13.04 ± 0.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.M.; Khan, M.W.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. https://doi.org/10.3390/pharmaceutics10030151
Sarfraz M, Afzal A, Yang T, Gai Y, Raza SM, Khan MW, Cheng Y, Ma X, Xiang G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics. 2018; 10(3):151. https://doi.org/10.3390/pharmaceutics10030151
Chicago/Turabian StyleSarfraz, Muhammad, Attia Afzal, Tan Yang, Yongkang Gai, Shahid Masood Raza, Muhammad Waseem Khan, Yao Cheng, Xiang Ma, and Guangya Xiang. 2018. "Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies" Pharmaceutics 10, no. 3: 151. https://doi.org/10.3390/pharmaceutics10030151
APA StyleSarfraz, M., Afzal, A., Yang, T., Gai, Y., Raza, S. M., Khan, M. W., Cheng, Y., Ma, X., & Xiang, G. (2018). Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics, 10(3), 151. https://doi.org/10.3390/pharmaceutics10030151