Stabilization of the CD81 Large Extracellular Loop with De Novo Disulfide Bonds Improves Its Amenability for Peptide Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Modeling
2.2. Production of Recombinant Proteins
2.3. Biophysical Characterization of the Human CD81 Large Extracellular Loop (hCD81 LEL) Mutants
2.3.1. SDS-PAGE
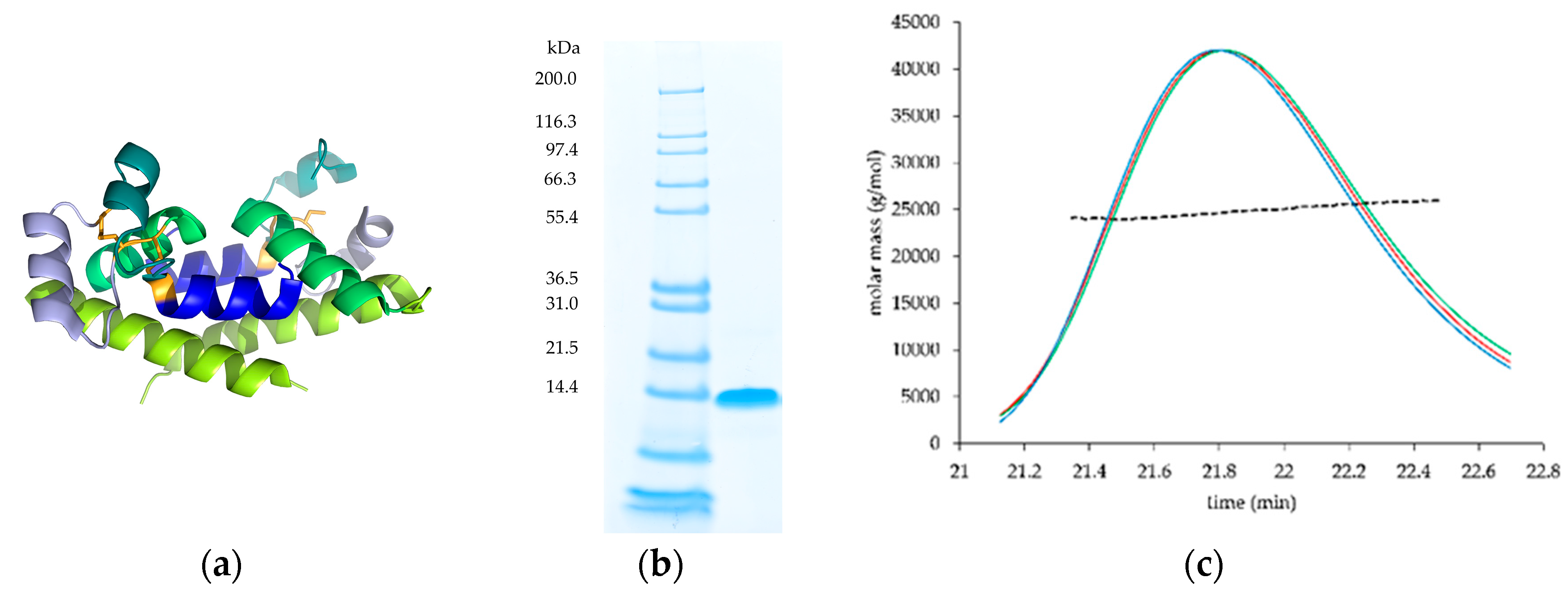
2.3.2. Size Exclusion Chromatography (SEC)-High Press ure Liquid Chromatography (HPLC) and Multi-Angle Light Scattering (MALS)
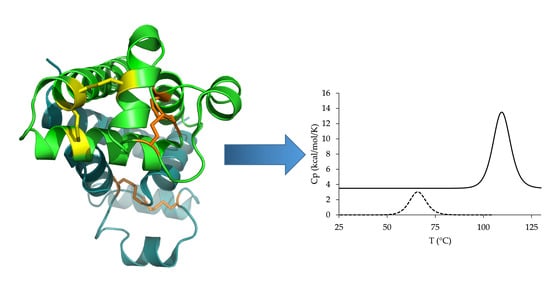
2.3.3. Differential Scanning Chromatography (DSC)
2.3.4. Circular Dichroism (CD) Spectroscopy
2.4. ELISA to Detect the Reactivity with M38 Antibody
2.5. Flow Cytometry
2.6. Receptor-Mediated Internalization into the Target Cell Line
3. Results
3.1. Expression and Purification of the Wild-Type Protein
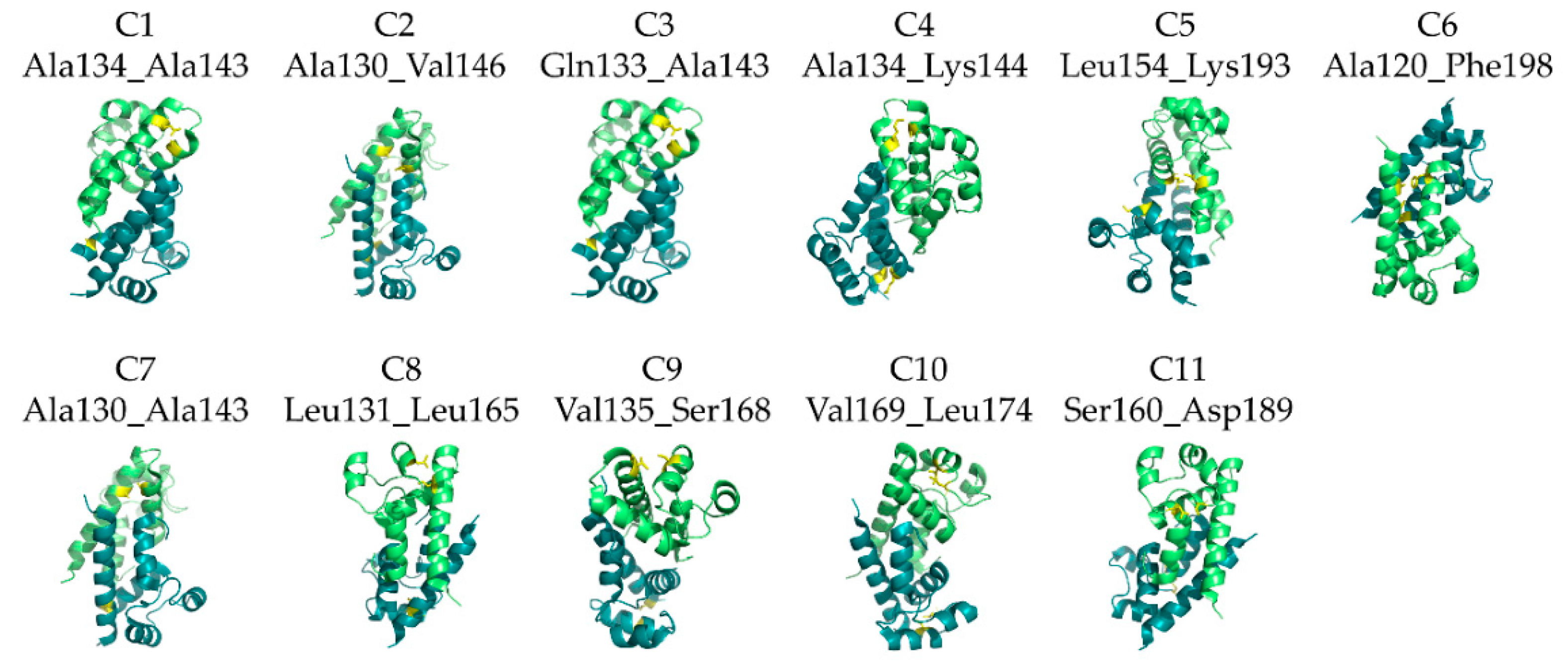
3.2. Design of De Novo Disulfide Bonds and Preliminary Screening of Candidate Mutants for Expression
3.3. Expression and Characterization of the Cysteine-Stabilized Mutants
3.4. Peptide Grafting
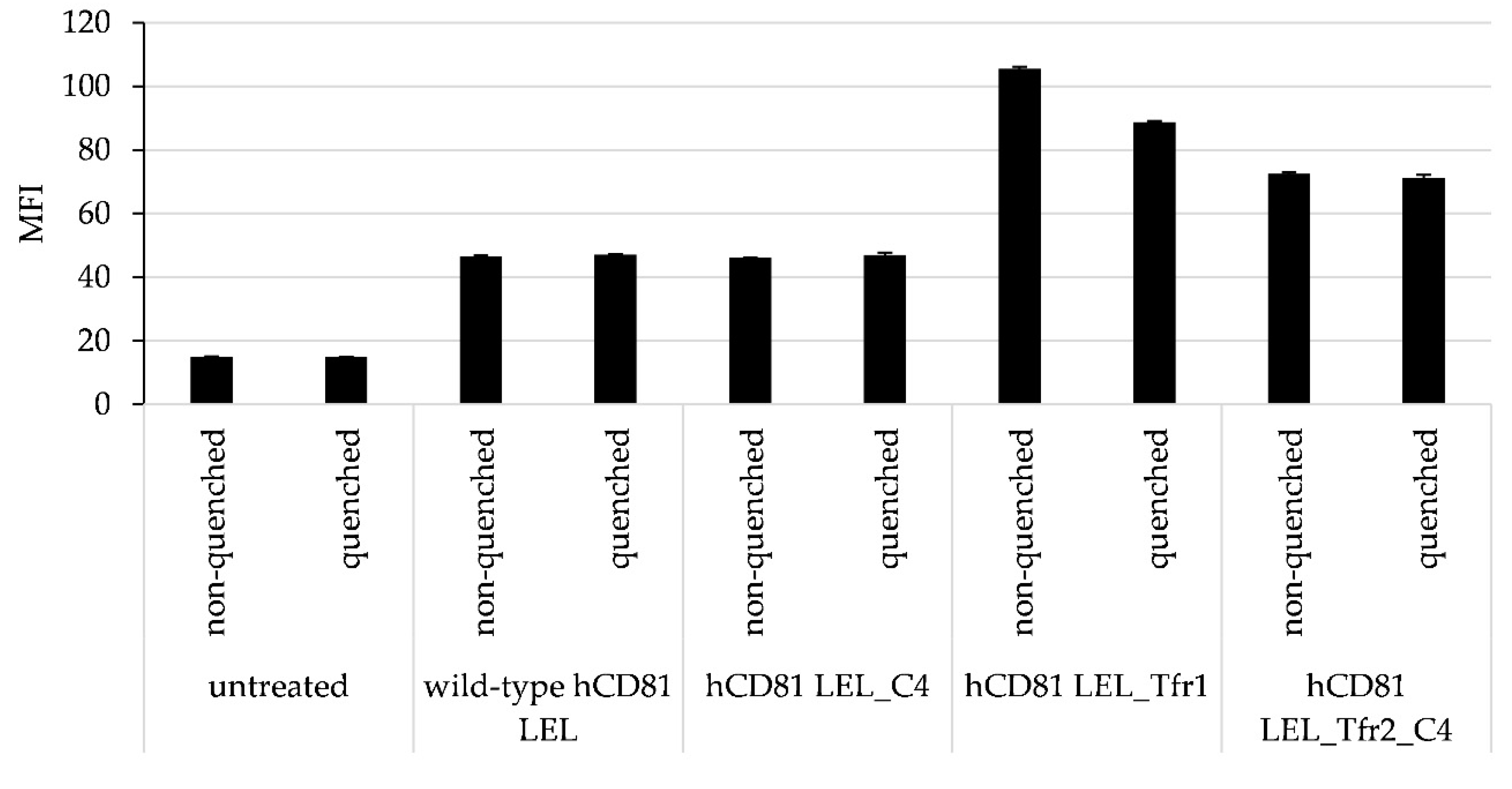
3.5. Internalization of hTfr Reactive Peptide-Engrafted Mutants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Lakhal, S.; Mager, I.; Wood, M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013, 65, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Le Naour, F.; Lagaudriere-Gesbert, C.; Billard, M.; Conjeaud, H.; Boucheix, C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur. J. Immunol. 1996, 26, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Shoham, T. Protein-protein interactions in the tetraspanin web. Physiology 2005, 20, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Morgelin, M.; Belting, M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Odintsova, E. Tetraspanins as regulators of protein trafficking. Traffic 2007, 8, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Kitadokoro, K.; Galli, G.; Petracca, R.; Falugi, F.; Grandi, G.; Bolognesi, M. Crystallization and preliminary crystallographic studies on the large extracellular domain of human CD81, a tetraspanin receptor for hepatitis C virus. Acta Crystallogr. D Biol. Crystallogr. 2001, 57 Pt 1, 156–158. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Ponassi, M.; Galli, G.; Petracca, R.; Falugi, F.; Grandi, G.; Bolognesi, M. Subunit association and conformational flexibility in the head subdomain of human CD81 large extracellular loop. Biol. Chem. 2002, 383, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Kitadokoro, K.; Bordo, D.; Galli, G.; Petracca, R.; Falugi, F.; Abrignani, S.; Grandi, G.; Bolognesi, M. CD81 extracellular domain 3D structure: Insight into the tetraspanin superfamily structural motifs. EMBO J. 2001, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Higginbottom, A.; Quinn, E.R.; Kuo, C.C.; Flint, M.; Wilson, L.H.; Bianchi, E.; Nicosia, A.; Monk, P.N.; McKeating, J.A.; Levy, S. Identification of amino acid residues in CD81 critical for interaction with hepatitis C virus envelope glycoprotein E2. J. Virol. 2000, 74, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Yoshie, O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J. Immunol. 1993, 151, 6470–6481. [Google Scholar] [PubMed]
- Seigneuret, M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 2006, 90, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Sridhar, P.; Tews, B.A.; Feneant, L.; Cocquerel, L.; Ward, D.G.; Berditchevski, F.; Overduin, M. Structural basis of ligand interactions of the large extracellular domain of tetraspanin CD81. J. Virol. 2012, 86, 9606–9616. [Google Scholar] [CrossRef] [PubMed]
- Seigneuret, M.; Delaguillaumie, A.; Lagaudriere-Gesbert, C.; Conjeaud, H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 2001, 276, 40055–40064. [Google Scholar] [CrossRef] [PubMed]
- Homsi, Y.; Schloetel, J.G.; Scheffer, K.D.; Schmidt, T.H.; Destainville, N.; Florin, L.; Lang, T. The extracellular delta-domain is essential for the formation of CD81 tetraspanin webs. Biophys. J. 2014, 107, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.H.; Homsi, Y.; Lang, T. Oligomerization of the Tetraspanin CD81 via the Flexibility of Its delta-Loop. Biophys. J. 2016, 110, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Homsi, Y.; Lang, T. The specificity of homomeric clustering of CD81 is mediated by its delta-loop. FEBS Open Bio 2017, 7, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Engler, J.A.; Collawn, J.F.; Moore, B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001, 268, 2004–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowdhamini, R.; Srinivasan, N.; Shoichet, B.; Santi, D.V.; Ramakrishnan, C.; Balaram, P. Stereochemical modeling of disulfide bridges. Criteria for introduction into proteins by site-directed mutagenesis. Protein Eng. 1989, 3, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Andre, M.; Le Caer, J.P.; Greco, C.; Planchon, S.; El Nemer, W.; Boucheix, C.; Rubinstein, E.; Chamot-Rooke, J.; Le Naour, F. Proteomic analysis of the tetraspanin web using LC-ESI-MS/MS and MALDI-FTICR-MS. Proteomics 2006, 6, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Chelikani, P.; Reeves, P.J.; Zhang, S.; Khorana, H.G. High-level expression, single-step immunoaffinity purification and characterization of human tetraspanin membrane protein CD81. PLoS ONE 2008, 3, e2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellman, J.A. The stability of hydrogen-bonded peptide structures in aqueous solution. C. R. Trav. Lab. Carlsberg Chim. 1955, 29, 230–259. [Google Scholar] [PubMed]
- Oren, R.; Takahashi, S.; Doss, C.; Levy, R.; Levy, S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell. Biol. 1990, 10, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, M.; Horibe, T.; Kohno, M.; Kawakami, K. A novel transferrin receptor-targeted hybrid peptide disintegrates cancer cell membrane to induce rapid killing of cancer cells. BMC Cancer 2011, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Horejsi, V.; Angelisova, P.; Bazil, V.; Kristofova, H.; Stoyanov, S.; Stefanova, I.; Hausner, P.; Vosecky, M.; Hilgert, I. Monoclonal antibodies against human leucocyte antigens. II. Antibodies against CD45 (T200), CD3 (T3), CD43, CD10 (CALLA), transferrin receptor (T9), a novel broadly expressed 18-kDa antigen (MEM-43) and a novel antigen of restricted expression (MEM-74). Folia Biol. 1988, 34, 23–34. [Google Scholar]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlecki-Zaniewicz, L.; Lammermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmullner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wozniak-Knopp, G.; Stadlmann, J.; Ruker, F. Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds. PLoS ONE 2012, 7, e30083. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, X.; Su, F.; Chen, Y.; Xu, L.; Yan, Y. Enhancing the Thermostability of Rhizomucor miehei Lipase with a Limited Screening Library by Rational-Design Point Mutations and Disulfide Bonds. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Schlebach, J.P.; Peng, D.; Kroncke, B.M.; Mittendorf, K.F.; Narayan, M.; Carter, B.D.; Sanders, C.R. Reversible folding of human peripheral myelin protein 22, a tetraspan membrane protein. Biochemistry 2013, 52, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, J.G.; Porat-Shliom, N.; Cohen, L.A. Clathrin-independent endocytosis: A unique platform for cell signaling and PM remodeling. Cell. Signal. 2009, 21, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Kobayashi, N.B.; Takatani-Nakase, T.; Yoshida, T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 2015, 5, 10300. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| hCD81 LEL | Mutated Position 1 | Mutated Position 2 | Tm (°C) | ||
|---|---|---|---|---|---|
| Variant | Located on Segment | Amino Acid | Located on Segment | Amino Acid | |
| wild type | 66.15 ± 0.25 | ||||
| C1 | Helix A | Ala134 | Helix B | Ala143 | n.d. 1 |
| C2 | Helix A | Ala130 | Helix B | Val146 | 67.35 ± 0.05 |
| C3 | Helix A | Gln133 | Helix B | Ala143 | 82.15 ± 0.05 |
| C4 | Helix A | Ala134 | Helix B | Lys144 | 88.95 ± 0.05 |
| C5 | Helix B | Leu154 | Helix E | Lys193 | n.d. |
| C6 | Helix A | Ala120 | Helix E | Phe198 | n.d. |
| C7 | Helix A | Ala130 | Helix B | Ala143 | 76.90 ± 0.00 |
| C8 | Helix A | Leu131 | Helix C | Leu165 | n.d. |
| C9 | Helix A | Val135 | Helix C | Ser168 | 90.45 ± 0.15 |
| C10 | Helix C | Val169 | Unstructured part of segment C | Leu174 | n.d. |
| C11 | Loop preceeding helix C | Ser160 | Start of helix D | Asp189 | 70.25 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, S.; Stadlmayr, G.; Stadlbauer, K.; Sádio, F.; Andorfer, P.; Grillari, J.; Rüker, F.; Wozniak-Knopp, G. Stabilization of the CD81 Large Extracellular Loop with De Novo Disulfide Bonds Improves Its Amenability for Peptide Grafting. Pharmaceutics 2018, 10, 138. https://doi.org/10.3390/pharmaceutics10030138
Vogt S, Stadlmayr G, Stadlbauer K, Sádio F, Andorfer P, Grillari J, Rüker F, Wozniak-Knopp G. Stabilization of the CD81 Large Extracellular Loop with De Novo Disulfide Bonds Improves Its Amenability for Peptide Grafting. Pharmaceutics. 2018; 10(3):138. https://doi.org/10.3390/pharmaceutics10030138
Chicago/Turabian StyleVogt, Stefan, Gerhard Stadlmayr, Katharina Stadlbauer, Flávio Sádio, Peter Andorfer, Johannes Grillari, Florian Rüker, and Gordana Wozniak-Knopp. 2018. "Stabilization of the CD81 Large Extracellular Loop with De Novo Disulfide Bonds Improves Its Amenability for Peptide Grafting" Pharmaceutics 10, no. 3: 138. https://doi.org/10.3390/pharmaceutics10030138
APA StyleVogt, S., Stadlmayr, G., Stadlbauer, K., Sádio, F., Andorfer, P., Grillari, J., Rüker, F., & Wozniak-Knopp, G. (2018). Stabilization of the CD81 Large Extracellular Loop with De Novo Disulfide Bonds Improves Its Amenability for Peptide Grafting. Pharmaceutics, 10(3), 138. https://doi.org/10.3390/pharmaceutics10030138