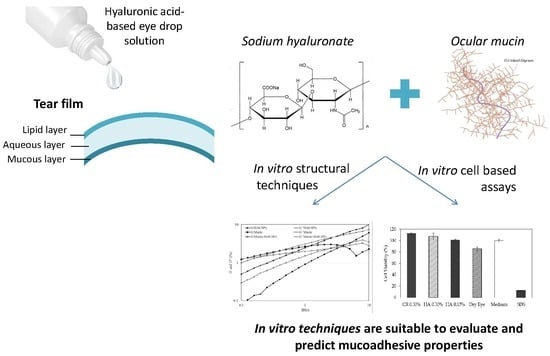
Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of HA 0.15% and HA 0.30%
2.2.2. Mucoadhesion Studies
Viscosity Measurements—Ostwald Viscometer
Rheology Measurements—Rotational Rheometer
Zeta Potential (ZP)
2.2.3. In Vitro Cell-Based Assays
Cell Culture Condition
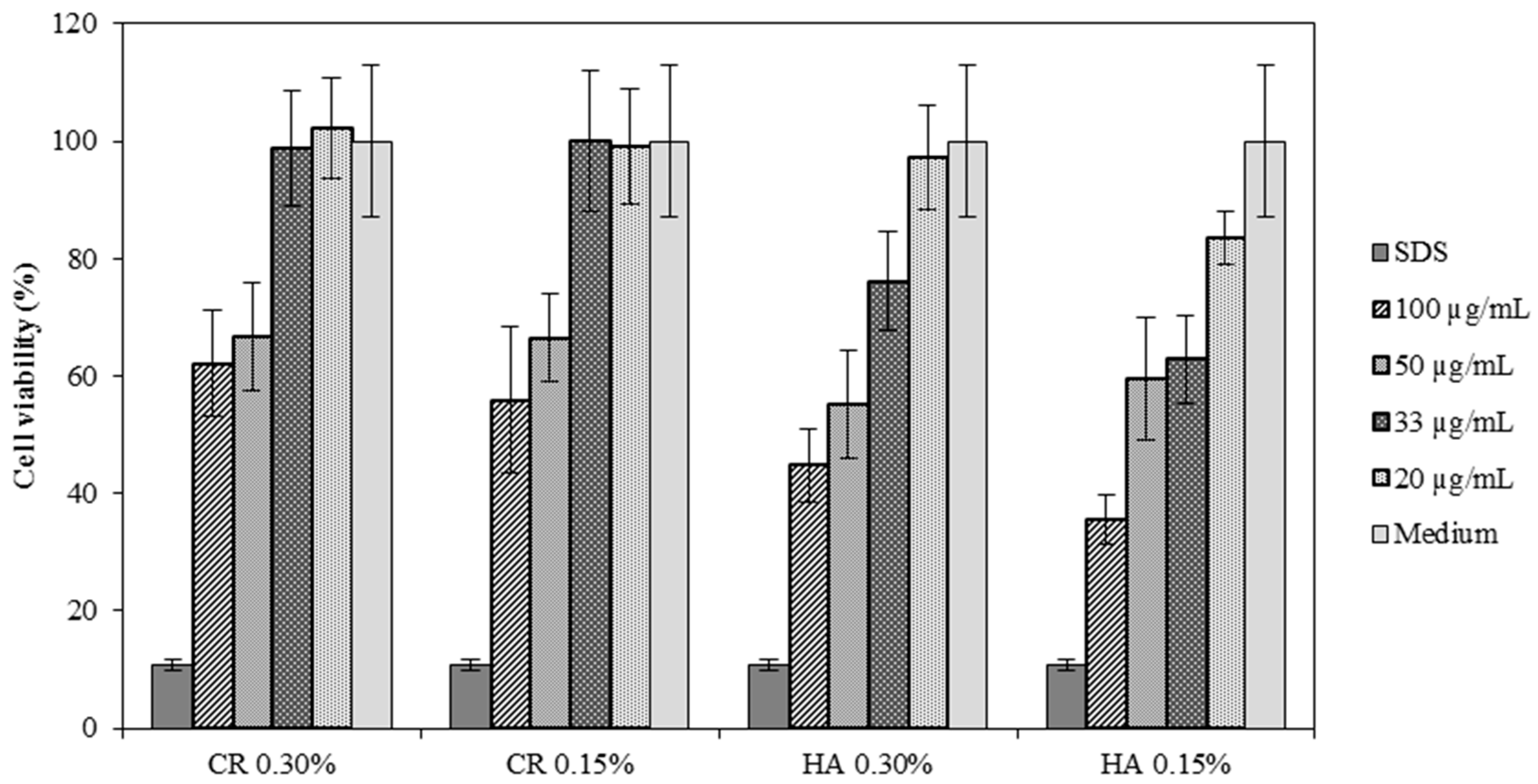
Cell Viability of HA 0.15% and HA 0.30%
2D Model—Evaluation of Cell Morphology and Cell Viability after Dehydration
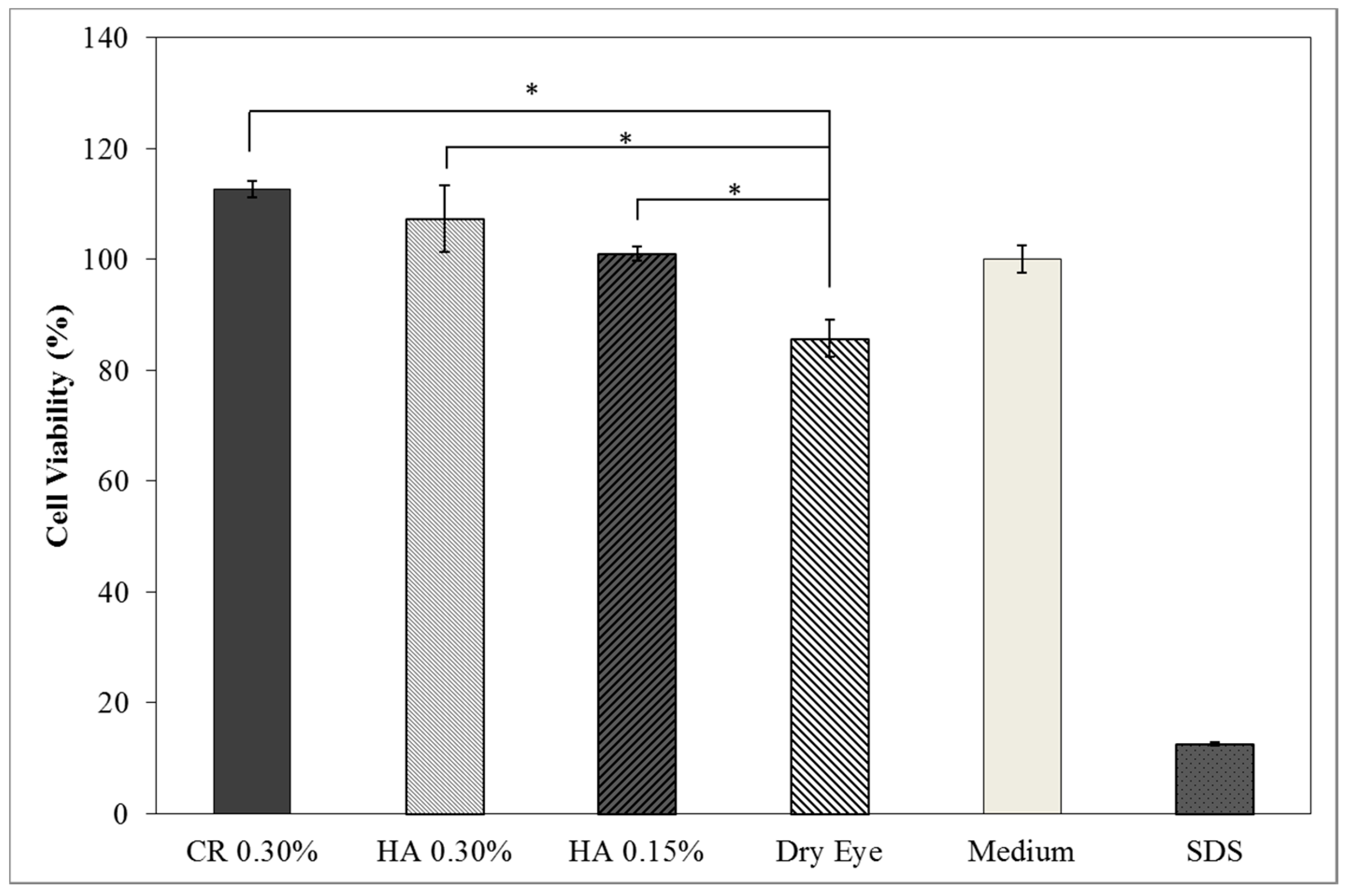
3D Model—Dry Eye Model and Cell Viability
Statistical Data Analysis
3. Results
3.1. HA 0.15% and HA 0.30% (w/v) Formulation Development
3.2. Mucoadhesive Studies
3.2.1. Viscosity Measurements
3.2.2. Rheology Measurements
Oscillation Frequency Sweep
Tackiness Testing
Zeta Potential
3.2.3. In Vitro Cell-Based Assays
Cell Viability of HA 0.15% and HA 0.30%
2D Model—Evaluation of Cell Morphology and Cell Viability after Dehydration
3D Model—Dry Eye Model Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cwiklik, L. Tear film lipid layer: A molecular level view. BBA Biomembr. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.; Fernandes, R.; Bernardes, F.; Bonfioli, A.; Jorge, E.; Soares, C. Dry Eye Disease. Inf. Healthc. 2010, 25, 84–93. [Google Scholar] [CrossRef]
- Javadi, M.; Feizi, S. Dry Eye Syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198. [Google Scholar] [PubMed]
- Foulks, G.N. The Correlation Between the Tear Film Lipid Layer and Dry Eye Disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.; Willcox, M. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul. Surf. 2007, 5, 75–92. Available online: http://linkinghub.elsevier.com/retrieve/pii/S1542012412700812 (accessed on 6 December 2017). [CrossRef]
- Understanding the Tear Film and Dry Eeye. Available online: http://www.collinsoptometrists.com.au/dry-eye-clinic/understanding-the-tear-film-and-dry-eye/ (accessed on 11 October 2017).
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Holly, J. Formation and Rupture of the Tear Film. Exp. Eye Res. 1973, 1, 515–525. [Google Scholar] [CrossRef]
- Colligris, B.; Alkozi, H.A.; Pintor, J. Recent developments on dry eye disease treatment compounds. Saudi J. Ophthalmol. 2014, 28, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Rand, A.L.; York, N. Current Opinion in Ophthalmology Nutritional Supplements for Dry Eye Syndrome. Natl. Inst. Health 2012, 22, 279–282. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Hägerström, H.; Edsman, K. Interpretation of mucoadhesive properties of polymer. J. Pharm. Pharmacol. 2001, 53, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Kashikar, V.S. Ophthalmic Mucoadhesive Polymers—A Literature Review. Ophthalmic Res. 2011, 7, 68–73. [Google Scholar]
- Boddeda, B.; Ratna, J.V.; Battu, H. A review on mucoadhesive polymers in ophthalmics. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 237–245. [Google Scholar]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Donnelly, L.; Jones, D.S.; Curran, R.M.; Morrow, R.J.; Woolfson, A.D. Characterization of the Rheological, Mucoadhesive, and Drug Release Properties of Highly Structured Gel Platforms for Intravaginal Drug Delivery. Biomacromolecules 2009, 10, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; Agostino, A.D.; Filosa, R.; De Rosa, M. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Menchicchi, B.; Fuenzalida, J.P.; Hensel, A.; Swamy, M.J.; David, L.; Rochas, C. Biophysical Analysis of the Molecular Interactions between Polysaccharides and Mucin. Biomacromolecules 2015, 16, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, D.; Wahlgren, M. Comparison of in vitro methods of measuring mucoadhesion: Ellipsometry, tensile strength and rheological measurements. Colloids Surf. B Biointerfaces 2012, 92, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Asadi, M.; Mortazavi-Tabatabaei, S.A.; Omidi, Y. Ocular Drug Delivery; Impact of in vitro Cell Culture Models. J. Ophthalmic Vis. Res. 2009, 4, 238–252. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23198080%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3498862 (accessed on 12 January 2018). [PubMed]
- Grillet, A.M.; Wyatt, N.B.; Gloe, L.M. Polymer Gel Rheology and Adhesion. J. Rheol. 2012, 59–80, 59–80. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Montés-Micó, R.; Cerviño, A.; Ferrer-Blasco, T.; García-Lázaro, S.; Madrid-Costa, D. The Tear Film and the Optical Quality of the Eye. Ocul. Surf. 2010, 8, 185–192. [Google Scholar] [CrossRef]
- Norn, M.S. Tear fluid pH in normals, contact lens wearers, and pathological cases. Acta Ophthalmol. 1988, 66, 485–489. [Google Scholar] [CrossRef]
- Potvin, R.; Makari, S.; Rapuano, C.J. Tear film osmolarity and dry eye disease: A review of the literature. Clin. Ophthalmol. 2015, 9, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Franck, A. Understanding Rheology of Structured Fluids. B TA Instruments. 2004, pp. 1–11. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Understanding+Rheology+of+Structured+Fluids#2%5Cnhttp://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Understanding+rheology+of+structured+fluids#2 (accessed on 20 February 2018).
- Assessing Tackiness and Adhesion Using a Pull Away Test on a ROTATIONAL Rheometer. Malvern Instruments Worldwide 2015. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/application-notes/AN150527AssessingTackinessPullAway.html (accessed on 20 February 2018).
- Fathalla, Z.M.A.; Khaled, K.A.; Hussein, A.K.; Alany, R.G. Formulation and corneal permeation of ketorolac tromethamine-loaded chitosan nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M. Structure of Chitosan Determines Its Interactions with Mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef]
- Tong, L.; Petznick, A.; Lee, S.; Tan, J. Choice of Artificial Tear Formulation for Patients with Dry Eye. Cornea 2012, 31, S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.E.; Gallo, J.M. A Simple Rheological Method for the in Vitro Assessment of Mucin-Polymer Bioadhesive Bond Strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 25, 622. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Comparative mucoadhesive study of hyaluronic acid-based conjugates on different mucosae. J. Appl. Polym. Sci. 2018, 46071, 2–7. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.P.; Jeldres, R.I.; Quezada, G.R.; Concha, F.; Toledo, P.G. Zeta potential and viscosity of colloidal silica suspensions: Effect of seawater salts, pH, flocculant, and shear rate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 210–218. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration Mar. Drugs 2017, 15, 370. [Google Scholar]


| Tests | Specifications | HA 0.15% Eye Drop Solutions | HA 0.3% Eye Drop Solutions |
|---|---|---|---|
| Appearance | Limpid, clear and odorless solution | Limpid, clear and odorless solution | Limpid, clear and odorless solution |
| pH | 7.0–7.6 at 20–25 °C | 7.16 (23.2 °C) | 7.32 (20.8 °C) |
| Osmolality | 280–320 mOsm/Kg | 302 mOsm/Kg | 304 mOsm/Kg |
| Sterility (Ph. Eur. 2.6.1. Sterility) | Absence of growth | Absence of growth | Absence of growth |
| Formulations | Viscosity (mPa·s) | Mucoadhesive Index (%) |
|---|---|---|
| HA 0.15% | 6.8 ± 0.1 | - |
| HA 0.30% | 71.2 ± 4.1 | - |
| Mucin 5% | 25.0 ± 0.6 | - |
| Mucin 5% + HA 0.15% | 53.2 ± 1.1 * | 298.07 ± 19.90 * |
| Mucin 5% + HA 0.30% | 382.2 ± 0.4 * | 67.44 ± 6.24 * |
| Formulations | Peak Normal Force-Normal Force (N) | Area Under Force-Time Curve (N·s) |
|---|---|---|
| HA 0.15% *1 | −0.178 ± 0.003 * | 0.438 ± 0.058 * |
| HA 0.30% *1 | −0.229 ± 0.013 * | 0.775 ± 0.091 * |
| CR 0.15% *1 | −0.168 ± 0.017 | 0.904 ± 0.069 |
| CR 0.30% *1 | −0.220 ± 0.007 | 1.051 ± 0.043 |
| Mucin 5.0% *1 | −0.228 ± 0.004 * | 1.012 ± 0.065 * |
| Mucin 5% + HA 0.15% *1 | −0.216 ± 0.019 * | 0.573 ± 0.152 * |
| Mucin 5% + HA 0.30% *1 | −0.287 ± 0.030 * | 0.747 ± 0.066 * |
| Pig Eye + HA 0.15% *2 | −0.078 ± 0.029 * | 0.891 ± 0.060 * |
| Pig Eye + HA 0.30% *2 | −0.134 ± 0.034 * | 1.010 ± 0.059 * |
| Without Die Magnification 100× | Crystal Violet Magnification 200× | Crystal Violet Magnification 400× | Cell Viability (%) | |
|---|---|---|---|---|
| Dry Eye (negative control) |  |  |  | 50.7 ± 6.8 |
| CR 0.30% |  |  |  | 73.4 ± 12.6 |
| HA 0.15% |  |  |  | 62.7 ± 9.5 |
| HA 0.30% |  |  |  | 72.5 ± 6.2 |
| Medium (positive control) |  |  |  | 100.0 ± 11.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics 2018, 10, 110. https://doi.org/10.3390/pharmaceutics10030110
Graça A, Gonçalves LM, Raposo S, Ribeiro HM, Marto J. Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics. 2018; 10(3):110. https://doi.org/10.3390/pharmaceutics10030110
Chicago/Turabian StyleGraça, Angélica, Lídia Maria Gonçalves, Sara Raposo, Helena Margarida Ribeiro, and Joana Marto. 2018. "Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems" Pharmaceutics 10, no. 3: 110. https://doi.org/10.3390/pharmaceutics10030110
APA StyleGraça, A., Gonçalves, L. M., Raposo, S., Ribeiro, H. M., & Marto, J. (2018). Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics, 10(3), 110. https://doi.org/10.3390/pharmaceutics10030110