Formulation, Development, and In Vitro Evaluation of a CD22 Targeted Liposomal System Containing a Non-Cardiotoxic Anthracycline for B Cell Malignancies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Formulation Optimization of LCLA (Untargeted Long Circulating Liposomal AD 198)
2.3. Removal of Un-Encapsulated AD 198
2.4. Analysis of Liposomal Encapsulated Drug Content
2.5. Determination of Phospholipid Concentration
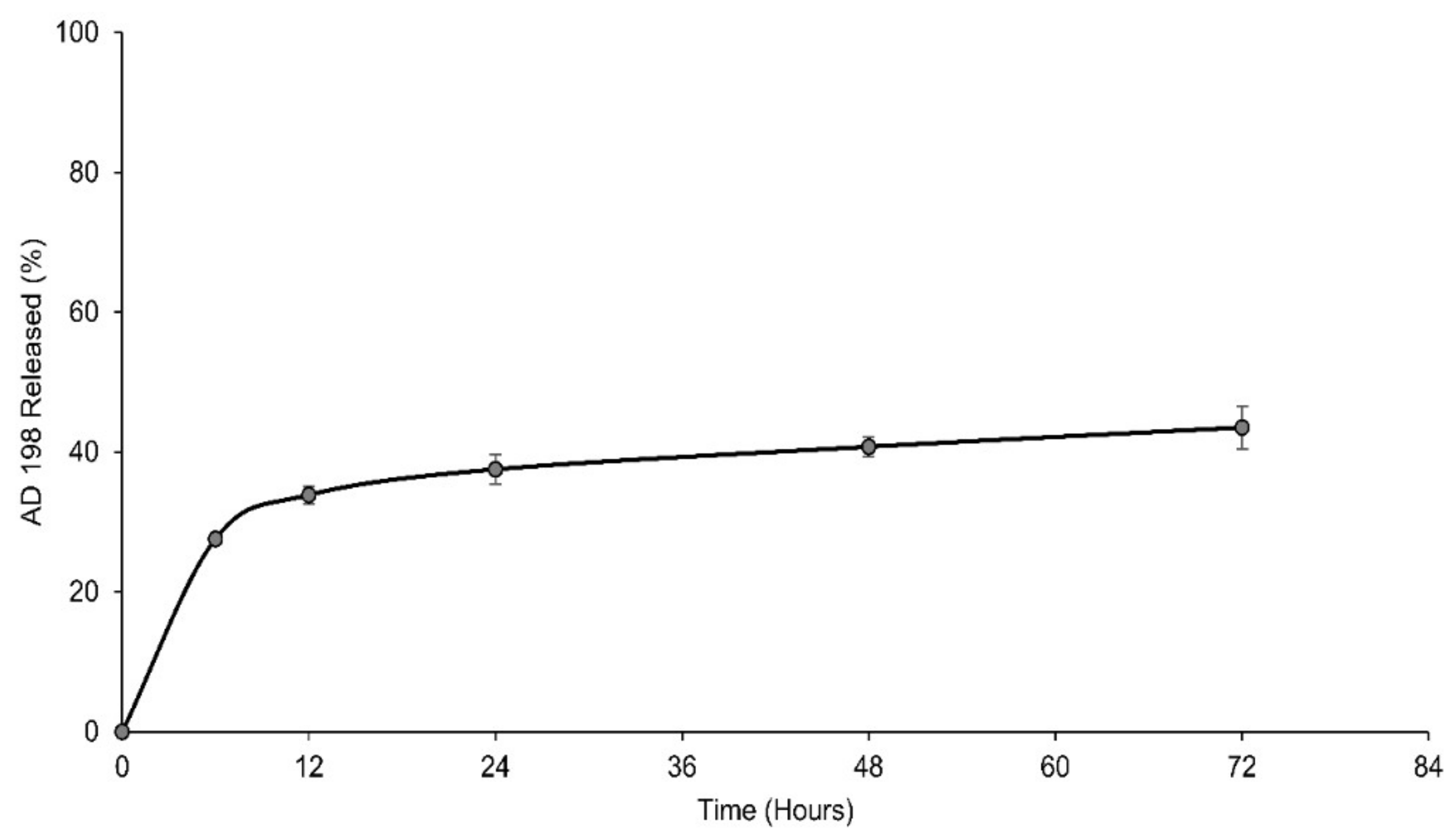
2.6. LCLA AD 198 Release Study
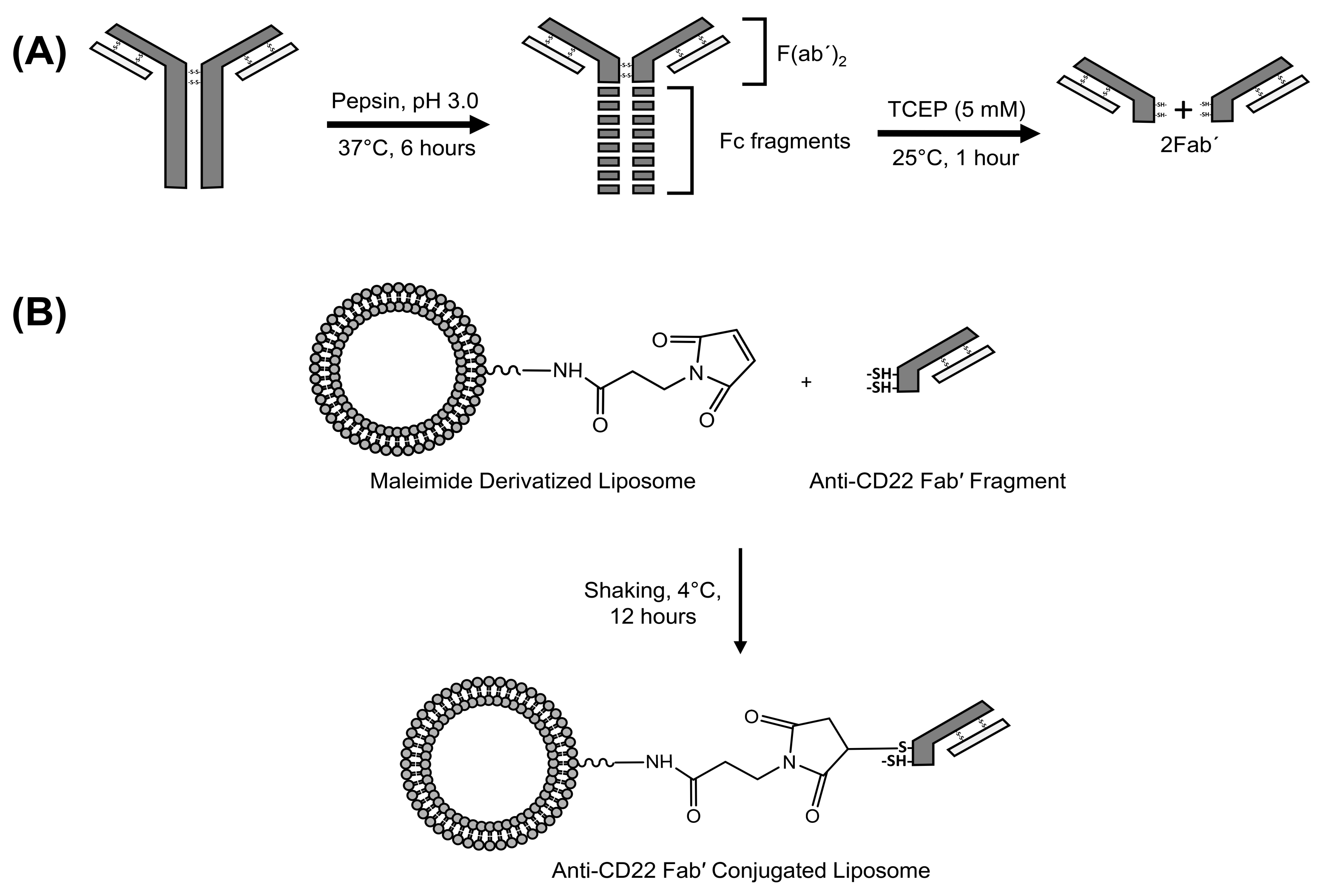
2.7. Fab’ (Antigen Binding Fragment) Generation from Whole Anti-CD22 Antibody
2.8. Conjugation of Fab’ to Liposomes to Give Long Circulating CD22 Targeted Liposomal AD 198 (LCCTLA)
2.9. Verification of Conjugation
2.10. Calculation of Number of Antibody Molecules per Liposome
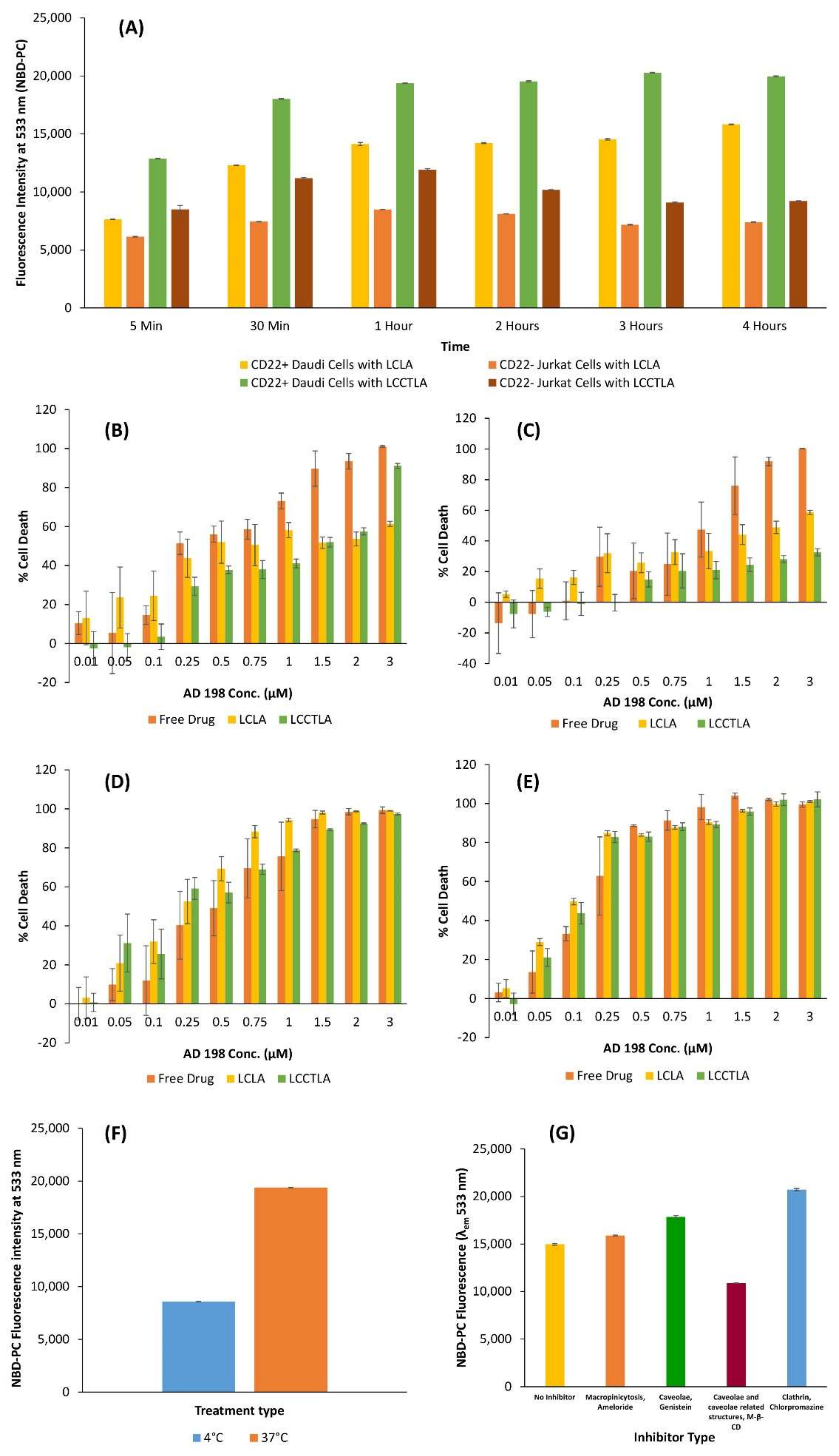
2.11. Cellular Uptake of LCLA and LCCTLA by Flow Cytometry
2.12. Evaluation of LCCTLA Cytotoxicity
2.13. Energy Dependent or Independent Pathway for LCCTLA Internalization
2.14. Route of Uptake of LCCTLA into Daudi Cells
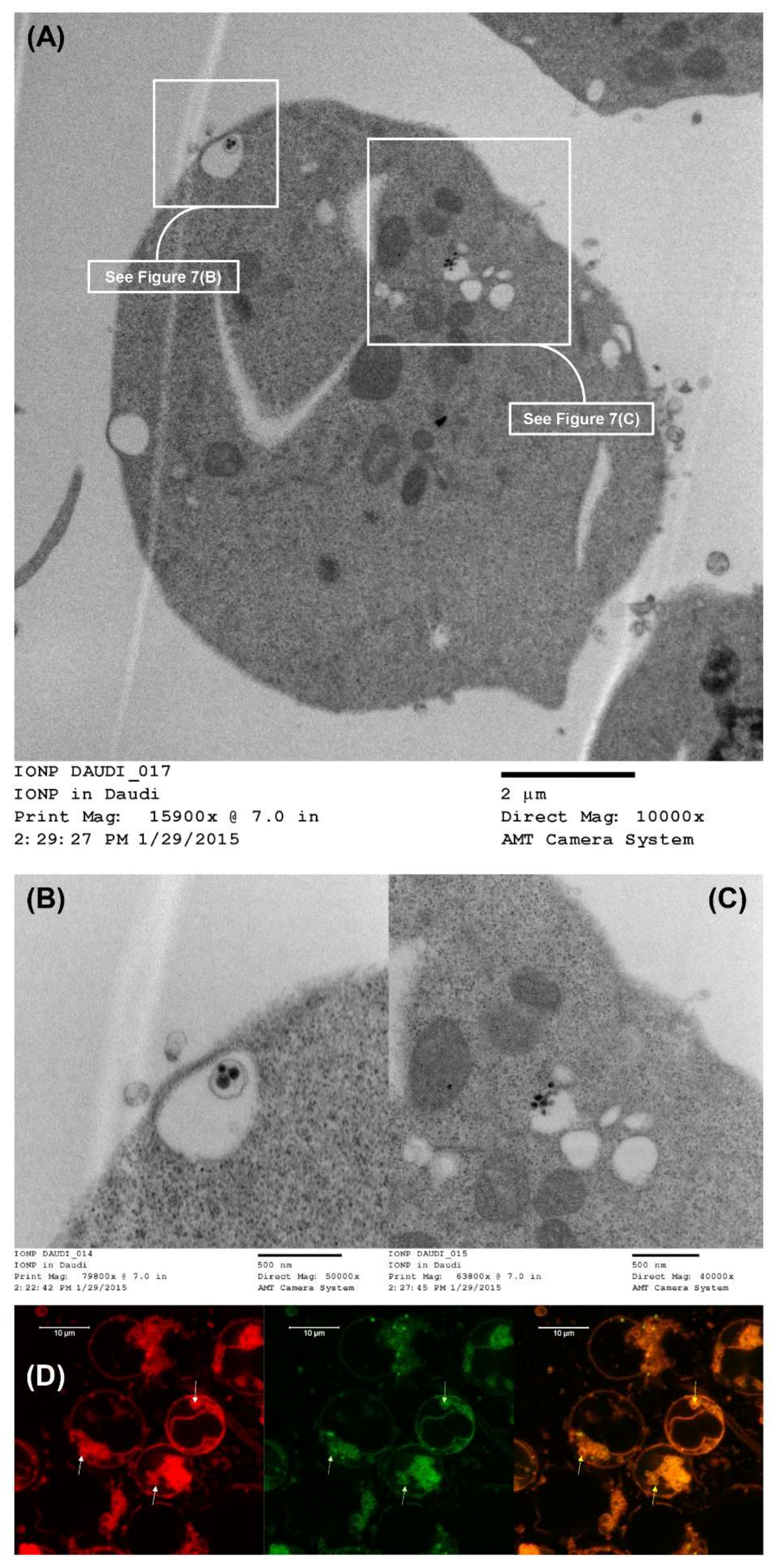
2.15. Intracellular Trafficking of LCCTLA by TEM
2.15.1. MLV Preparation and Treatment of Daudi Cells
2.15.2. TEM Sample Preparation
2.16. Intracellular Trafficking of LCCTLA by CLSM
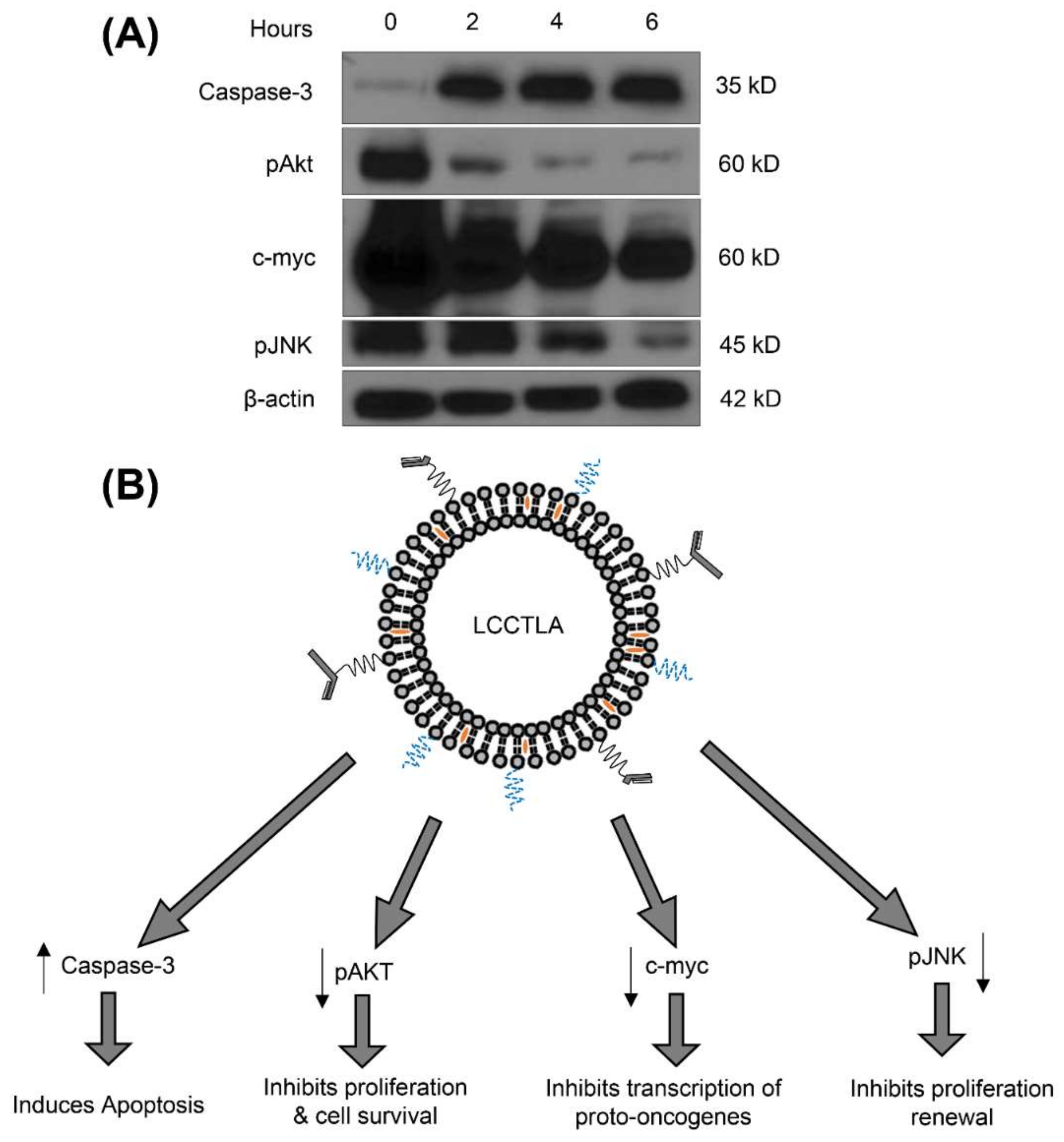
2.17. Effect of LCCTLA on Cell Cycle Regulatory Molecules by Western Blot
2.17.1. Sample preparation
2.17.2. Polyacrylamide Gel Electrophoresis (PAGE)
2.17.3. Blotting
2.17.4. Primary and Secondary Antibody Probing
2.17.5. Analysis
2.17.6. Membrane Stripping
2.18. Statistical Methodology
3. Results
3.1. This Section Effect of Lipid Composition in the Bilayer
3.2. Effect of Total Phospholipids on AD 198 Encapsulation, Liposomal Size and ζ-Potential
3.3. Effect of Cholesterol Concentration on AD 198 Encapsulation and Liposomal Size
3.4. Effect of AD 198 Concentration on AD 198 Encapsulation and ζ-Potential
3.5. Optimization of mPEG2000-DSPE Concentration
3.6. LCLA Drug Release
3.7. Number of AD 198 Molecules/Liposome
3.8. Verification of Anti-CD22 Fab’ Conjugation
3.9. Number of Anti-CD22 Fab’ and Maleimide per LCCTLA
3.10. Cellular Uptake of LCLA and LCCTLA
3.11. Analysis of Cytotoxicity of LCCTLA
3.12. Cellular Association
3.13. LCCTLA Particles Are Endocytosed into Cells by a Clathrin- and Caveolae-Independent Pathway
3.14. LCCTLA Nanoparticles Were Localized Intracellularly in Endosomes
3.15. The Endosomes Fuse with Lysosomes to Give Endolysosomes
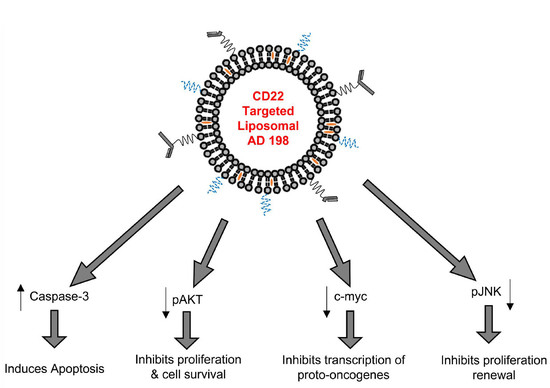
3.16. LCCTLA Activates Classical Apoptotic Pathways
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Szoka, F.C., Jr. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Petre, C.E.; Dittmer, D.P. Liposomal daunorubicin as treatment for kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 277–288. [Google Scholar]
- Deitcher, O.R.; O’Brien, S.; Deitcher, S.R.; Thomas, D.A.; Kantarjian, H.M. Single-agent vincristine sulfate liposomes injection (marqibo®) compared to historical single-agent therapy for adults with advanced, relapsed and/or refractory philadelphia chromosome negative acute lymphoblastic leukemia. Blood 2011, 118, 2592. [Google Scholar]
- Rodriguez, M.; Pytlik, R.; Kozak, T.; Chhanabhai, M.; Gascoyne, R.; Lu, B.; Deitcher, S.R.; Winter, J.N. Vincristine sulfate liposomes injection (marqibo) in heavily pretreated patients with refractory aggressive non-hodgkin lymphoma. Cancer 2009, 115, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Mousa, S.A. Emerging nanomedicines for early cancer detection and improved treatment: Current perspective and future promise. Pharmacol. Ther. 2010, 128, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Immunoliposomes and pegylated immunoliposomes: Possible use for targeted delivery of imaging agents. ImmunoMethods 1994, 4, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Mumbengegwi, D.R.; Charrois, G.J.R. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine b-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin. Cancer Res. 2005, 11, 3567–3573. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Sapra, P.; Moase, E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell. Mol. Biol. Lett. 2002, 7, 217–219. [Google Scholar] [PubMed]
- Cheng, W.W.; Allen, T.M. Targeted delivery of anti-CD19 liposomal doxorubicin in b-cell lymphoma: A comparison of whole monoclonal antibody, fab’ fragments and single chain fv. J. Control. Release 2008, 126, 50–58. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, D.E.L.; Kirchmeier, M.J.; Gagne, J.F.; Pilarski, L.M.; Allen, T.M. Cellular trafficking and cytotoxicity of anti-CD19-targeted liposomal doxorubicin in b lymphoma cells. J. Liposome Res. 1999, 9, 199–228. [Google Scholar] [CrossRef]
- Pillai, G. Nanomedicines for cancer therapy: An update of fda approved and those under various stages of development. SOJ Pharm. Pharm. Sci. 2014, 1, 1–13. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Polanska, H.; Rodrigo, M.A.M.; Guran, R.; Kulich, P.; Kopel, P.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Kizek, R. Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes. Sci. Rep. 2016, 6, 33379. [Google Scholar] [CrossRef] [PubMed]
- Dothager, R.S.; Piwnica-Worms, D. Nano in cancer: Linking chemistry, biology, and clinical applications in vivo. Cancer Res. 2011, 71, 5611–5615. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Salim, K.; Rather, M.A.; Wani, W.A.; Haque, A. Advances in nano drugs for cancer chemotherapy. Curr. Cancer Drug Targ. 2011, 11, 135–146. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; Paulson, J.C. In vivo targeting of b-cell lymphoma with glycan ligands of cd22. Blood 2010, 115, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, U.; Errante, D.; Van Glabbeke, M.; Teodorovic, I.; Kluin-Nelemans, J.; Thomas, J.; Bron, D.; Rosti, G.; Somers, R.; Zagonel, V. Chop is the standard regimen in patients > or= 70 years of age with intermediate-grade and high-grade non-hodgkin’s lymphoma: Results of a randomized study of the european organization for research and treatment of cancer lymphoma cooperative study group. J. Clin. Oncol. 1998, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.K.; Bhattacharjee, H.; Mandal, B.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Targeted liposomal drug delivery systems for the treatment of b cell malignancies. J. Drug Targ. 2014, 22, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Binsky, I.; Haran, M.; Starlets, D.; Gore, Y.; Lantner, F.; Harpaz, N.; Leng, L.; Goldenberg, D.M.; Shvidel, L.; Berrebi, A.; et al. Il-8 secreted in a macrophage migration-inhibitory factor- and cd74-dependent manner regulates b cell chronic lymphocytic leukemia survival. Proc. Natl. Acad. Sci. USA 2007, 104, 13408–13413. [Google Scholar] [CrossRef] [PubMed]
- DiJoseph, J.F.; Dougher, M.M.; Kalyandrug, L.B.; Armellino, D.C.; Boghaert, E.R.; Hamann, P.R.; Moran, J.K.; Damle, N.K. Antitumor efficacy of a combination of cmc-544 (inotuzumab ozogamicin), a cd22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-hodgkin’s b-cell lymphoma. Clin. Cancer Res. 2006, 12, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Beers, R.; Fitzgerald, D.J.; Pastan, I. Differential cellular internalization of anti-cd19 and -cd22 immunotoxins results in different cytotoxic activity. Cancer Res 2008, 68, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Loomis, K.; Smith, B.; Feng, Y.; Garg, H.; Yavlovich, A.; Campbell-Massa, R.; Dimitrov, D.S.; Blumenthal, R.; Xiao, X.; Puri, A. Specific targeting to b cells by lipid-based nanoparticles conjugated with a novel cd22-scfv. Exp. Mol. Pathol. 2010, 88, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Allen, T.M. Improved outcome when b-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the cd19 and cd20 epitopes. Clin. Cancer Res. 2004, 10, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Tuscano, J.M.; Martin, S.M.; Ma, Y.; Zamboni, W.; O’Donnell, R.T. Efficacy, biodistribution, and pharmacokinetics of cd22-targeted pegylated liposomal doxorubicin in a b-cell non–hodgkin’s lymphoma xenograft mouse model. Clin. Cancer Res. 2010, 16, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.T.; Martin, S.M.; Ma, Y.; Zamboni, W.C.; Tuscano, J.M. Development and characterization of cd22-targeted pegylated-liposomal doxorubicin (il-pld). Investig. New Drugs 2010, 28, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Allen, T.M. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002, 62, 7190–7194. [Google Scholar] [PubMed]
- Sapra, P.; Moase, E.H.; Ma, J.; Allen, T.M. Improved therapeutic responses in a xenograft model of human b lymphoma (namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via anti-cd19 igg2a or fab’ fragments. Clin. Cancer Res. 2004, 10, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H.; Sung, H.M.; Yee, H.C.M.; Liu, L.L.; Einzig, A.I.; Dutcher, J.; Keefe, D. Cardiovascular toxicity with cancer chemotherapy. Curr. Probl. Cardiol. 1996, 21, 233–286. [Google Scholar] [CrossRef]
- Jensen, B.; Skovsgaard, T.; Nielsen, S. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002, 13, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Speyer, J.; Wasserheit, C. Strategies for reduction of anthracycline cardiac toxicity. Semin. Oncol. 1998, 25, 525–537. [Google Scholar] [PubMed]
- Binaschi, M.; Bigioni, M.; Cipollone, A.; Rossi, C.; Goso, C.; Maggi, C.A.; Capranico, G.; Animati, F. Anthracyclines: Selected new developments. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. Cancer Therapeutics: Experimental and Clinical Agents; Springer Science & Business Media: Berlin, Germany, 1996. [Google Scholar]
- He, Y.; Liu, J.; Durrant, D.; Yang, H.S.; Sweatman, T.; Lothstein, L.; Lee, R.M. N-benzyladriamycin-14-valerate (AD198) induces apoptosis through protein kinase C-delta-induced phosphorylation of phospholipid scramblase 3. Cancer Res. 2005, 65, 10016–10023. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.A.; Israel, M.; Koseki, Y.; Laskin, J.; Gray, J.; Janik, A.; Sweatman, T.W.; Lothstein, L. N-benzyladriamycin-14-valerate (AD 198): A non-cardiotoxic anthracycline that is cardioprotective through pkc-epsilon activation. J. Pharmacol. Exp. Ther. 2007, 323, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lothstein, L.; Morrison, R.R.; Hofmann, P.A. Protection from doxorubicin-induced cardiomyopathy using the modified anthracycline N-benzyladriamycin-14-valerate (AD 198). J. Pharmacol. Exp. Ther. 2010, 335, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lothstein, L.; Savranskaya, L.; Barrett, C.M.; Israel, M.; Sweatman, T.W. N-benzyladriamycin-14-valerate (AD 198) activates protein kinase c-δ holoenzyme to trigger mitochondrial depolarization and cytochrome c release independently of permeability transition pore opening and Ca2+ influx. Anti-Cancer Drugs 2006, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Rathore, K.; Cekanova, M. A novel derivative of doxorubicin, AD198, inhibits canine transitional cell carcinoma and osteosarcoma cells in vitro. Drug Des. Dev. Ther. 2015, 9, 5323–5335. [Google Scholar]
- Edwards, S.K.; Han, Y.; Liu, Y.; Kreider, B.Z.; Liu, Y.; Grewal, S.; Desai, A.; Baron, J.; Moore, C.R.; Luo, C. Signaling mechanisms of bortezomib in traf3-deficient mouse b lymphoma and human multiple myeloma cells. Leuk. Res. 2016, 41, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.K. Design, Development, Characterization and Testing of CD22 Targeted Long Circulating Liposomal Drug Delivery Systems for β Cell Malignancies. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2015. [Google Scholar]
- Bangham, A.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Lopes de Menezes, D.E.; Pilarski, L.M.; Allen, T.M. In vitro and in vivo targeting of immunoliposomal doxorubicin to human b-cell lymphoma. Cancer Res. 1998, 58, 3320–3330. [Google Scholar] [PubMed]
- Juliano, R.L.; Stamp, D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 1975, 63, 651–658. [Google Scholar] [CrossRef]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta-Biomembr. 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Lothstein, L.; Rodrigues, P.J.; Sweatman, T.W.; Israel, M. Cytotoxicity and intracellular biotransformation of N-benzyladriamycin-14-yalerate (AD 198) are modulated by changes in 14-O-acyl chain length. Anti-Cancer Drugs 1998, 9, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Radovic-Moreno, A.F.; Alexis, F.; Gu, F.X.; Basto, P.A.; Bagalkot, V.; Jon, S.; Langer, R.S.; Farokhzad, O.C. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle–aptamer bioconjugates. ChemMedChem 2007, 2, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Schiffelers, R.M.; van der Veeken, J.; van der Meel, R.; Vongpromek, R.; en Henegouwen, P.M.V.B.; Storm, G.; Roovers, R.C. Downregulation of EGFR by a novel multivalent nanobody-liposome platform. J. Control. Release 2010, 145, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Huth, U.S.; Schubert, R.; Peschka-Süss, R. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J. Control. Release 2006, 110, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Douglas, K.L.; Piccirillo, C.A.; Tabrizian, M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur. J. Pharm. Biopharm. 2008, 68, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Shen, S.; Pang, Z.; Jiang, X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Zambre, A.; Chanda, N.; Hoffman, T.J.; Smith, C.J.; Robertson, J.D.; Kannan, R. Bombesin peptide conjugated gold nanocages internalize via clathrin mediated endocytosis. Bioconjug. Chem. 2014, 25, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Päuser, S.; Reszka, R.; Wagner, S.; Wolf, K.J.; Buhr, H.J.; Berger, G. Liposome-encapsulated superparamagnetic iron oxide particles as markers in an MRI-guided search for tumor-specific drug carriers. Anticancer Drug Des. 1997, 12, 125–135. [Google Scholar] [PubMed]
- Wu, L.; Yu, X.; Feizpour, A.; Reinhard, B.M. Nanoconjugation: A materials approach to enhance epidermal growth factor induced apoptosis. Biomater. Sci. 2014, 2, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gan, L.; Zhu, C.; Dong, Y.; Liu, J.; Gan, Y. Cationic core-shell liponanoparticles for ocular gene delivery. Biomaterials 2012, 33, 7621–7630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A nano-in-nano polymer–dendrimer nanoparticle-based nanosystem for controlled multidrug delivery. Mol. Pharm. 2017, 14, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Rao, P.S.; Smith, D.J.; Wheelock, M.J.; Johnson, K.R.; Hemstreet, G.P.; Balaji, K. E-cadherin phosphorylation by protein kinase D1/protein kinase Cμ is associated with altered cellular aggregation and motility in prostate cancer. Cancer Res. 2005, 65, 483–492. [Google Scholar] [PubMed]
- Lian, T.; Ho, R.J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Senior, J. The phospholipid component of small unilamellar liposomes controls the rate of clearance of entrapped solutes from the circulation. FEBS Lett. 1980, 119, 43–46. [Google Scholar] [CrossRef]
- Senior, J.H. Fate and behavior of liposomes in vivo: A review of controlling factors. Crit. Rev. Ther. Drug Carr. Syst. 1987, 3, 123–193. [Google Scholar]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–743. [Google Scholar] [PubMed]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid–PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C.; Matthay, K.K.; Newman, M.S.; Hidayat, J.E.; Collins, L.R.; Redemann, C.; Martin, F.J.; Papahadjopoulos, D. Versatility in lipid compositions showing prolonged circulation with sterically stabilized liposomes. Biochim. Biophys. Acta 1992, 1105, 193–200. [Google Scholar] [CrossRef]
- Gramse, G.; Dols-Perez, A.; Edwards, M.A.; Fumagalli, L.; Gomila, G. Nanoscale measurement of the dielectric constant of supported lipid bilayers in aqueous solutions with electrostatic force microscopy. Biophys. J. 2013, 104, 1257–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.G.; Carneiro, A.L.; Rosada, R.S.; Silva, C.L.; Santana, M.H.A. A mathematical model describing the kinetic of cationic liposome production from dried lipid films adsorbed in a multitubular system. Br. J. Chem. Eng. 2007, 24, 477–486. [Google Scholar] [CrossRef]
- Budha, N.R.; Lee, R.B.; Hurdle, J.G.; Lee, R.E.; Meibohm, B. A simple in vitro PK/PD model system to determine time–kill curves of drugs against mycobacteria. Tuberculosis 2009, 89, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.L.; Carter, L.L. ATP is required for receptor-mediated endocytosis in intact cells. J. Cell Biol. 1990, 111, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Finver, S.N.; Nishikura, K.; Finger, L.R.; Haluska, F.G.; Finan, J.; Nowell, P.C.; Croce, C.M. Sequence analysis of the MYC oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered. Proc. Natl. Acad. Sci. USA 1988, 85, 3052–3056. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mtor complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.K.; Moore, C.R.; Liu, Y.; Grewal, S.; Covey, L.R.; Xie, P. N-benzyladriamycin-14-valerate (AD 198) exhibits potent anti-tumor activity on TRAF3-deficient mouse b lymphoma and human multiple myeloma. BMC Cancer 2013, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Bubici, C.; Papa, S. Jnk signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef] [PubMed]



| Study Duration | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| Formulation Type | Free Drug | LCLA | LCCTLA | Free Drug | LCLA | LCCTLA |
| Cancer Cell Type | ||||||
| Daudi | 0.243 | 0.481 | 1.443 | 0.199 | 0.101 | 0.114 |
| Jurkat | 1.054 | 2.046 | 4.608* | 0.509 | 0.238 | 0.438 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, N.K.; Mandal, B.; Balabathula, P.; Setua, S.; Janagam, D.R.; Lothstein, L.; Thoma, L.A.; Wood, G.C. Formulation, Development, and In Vitro Evaluation of a CD22 Targeted Liposomal System Containing a Non-Cardiotoxic Anthracycline for B Cell Malignancies. Pharmaceutics 2018, 10, 50. https://doi.org/10.3390/pharmaceutics10020050
Mittal NK, Mandal B, Balabathula P, Setua S, Janagam DR, Lothstein L, Thoma LA, Wood GC. Formulation, Development, and In Vitro Evaluation of a CD22 Targeted Liposomal System Containing a Non-Cardiotoxic Anthracycline for B Cell Malignancies. Pharmaceutics. 2018; 10(2):50. https://doi.org/10.3390/pharmaceutics10020050
Chicago/Turabian StyleMittal, Nivesh K., Bivash Mandal, Pavan Balabathula, Saini Setua, Dileep R. Janagam, Leonard Lothstein, Laura A. Thoma, and George C. Wood. 2018. "Formulation, Development, and In Vitro Evaluation of a CD22 Targeted Liposomal System Containing a Non-Cardiotoxic Anthracycline for B Cell Malignancies" Pharmaceutics 10, no. 2: 50. https://doi.org/10.3390/pharmaceutics10020050