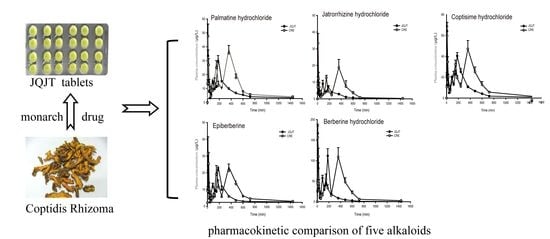
Development and Validation of an UPLC-MS/MS Method for Pharmacokinetic Comparison of Five Alkaloids from JinQi Jiangtang Tablets and Its Monarch Drug Coptidis Rhizoma
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Animals
2.3. UPLC-MS/MS Conditions
2.4. Preparation of Stock Solutions, Calibration Curve, Quality Control Samples, and Biological Plasma Samples
2.5. Preparation of Coptidis Rhizoma Extract
2.6. Method Validation
2.7. Pharmacokinetic Study
3. Results and Discussion
3.1. Method Validation
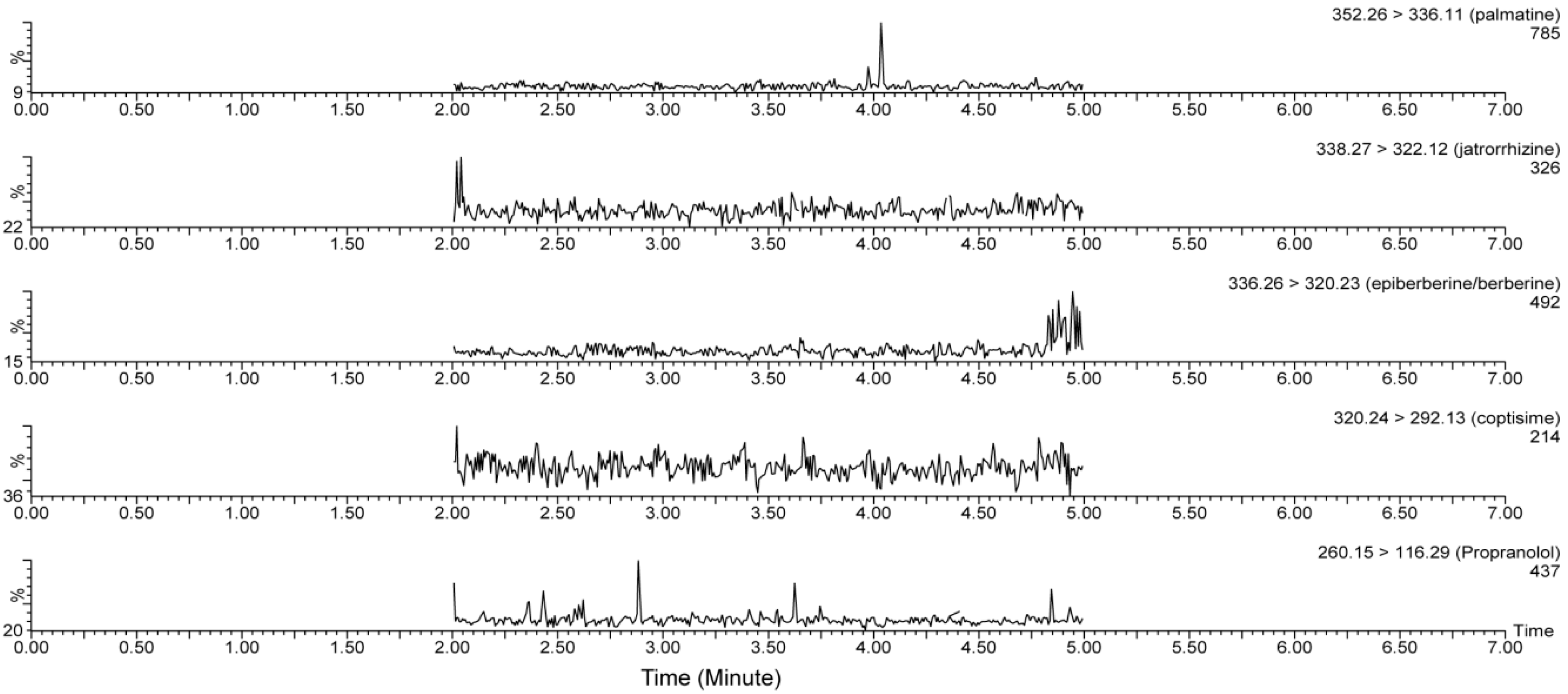
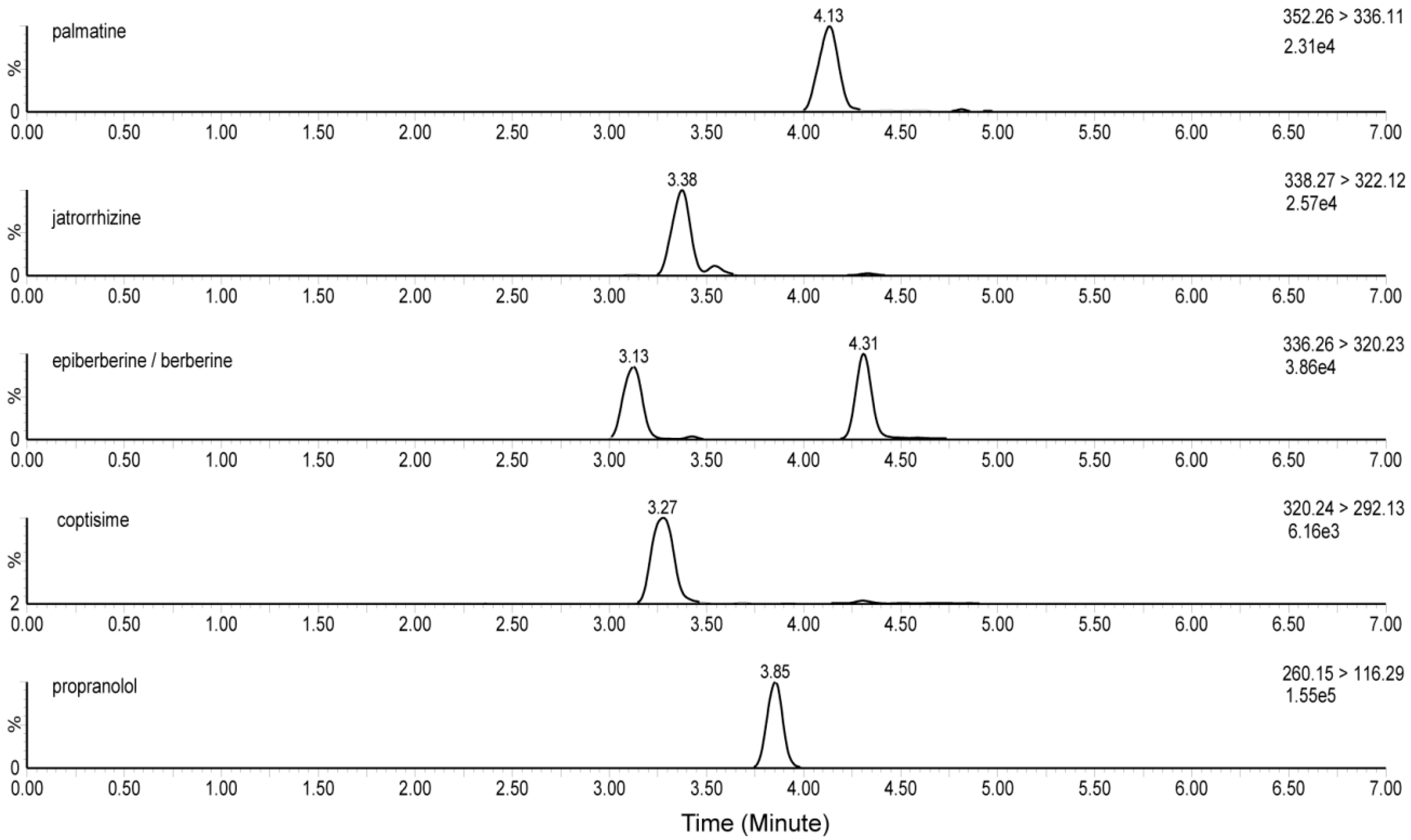
3.1.1. Specificity
3.1.2. Calibration Curves and Sensitivity
3.1.3. Accuracy and Precision
3.1.4. Extraction Recovery and Matrix Effect
3.1.5. Stability
3.2. Pharmacokinetic Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Normile, D. Asian medicine. The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, M.; Du, Q.; Yao, X.; Zhang, P.; Chen, X.; Xie, W.; Li, Z.; Ma, Y.; Zhu, Y. An integrated anti-arrhythmic target network of a Chinese medicine compound, Wenxin Keli, revealed by combined machine learning and molecular pathway analysis. Mol. Biosyst. 2017, 13, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.; Hu, B.; So, P.K.; Chan, C.O.; Mok, D.K.; Xin, G.Z.; Li, P.; Yao, Z.P. Rapid authentication of Gastrodiae rhizoma by direct ionization mass spectrometry. Anal. Chim. Acta 2016, 938, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People’s Republic of China, 2015th ed.; China Medical Science and Technology Press: Beijing, China, 2015; pp. 1076–1077.
- Ding, F.F.; Zhang, X.J.; Sun, Y.; Deng, Y.R. Study on intestinal absorption of Jinqi Jiangtang Tablets by rat everted gut sac. Chin. Hosp. Pharm. J. 2015, 35, 185–190. (In Chinese) [Google Scholar]
- Chang, Y.X.; Ge, A.H.; Donnapee, S.; Li, J.; Bai, Y.; Liu, J.; He, J.; Song, L.J.; Zhang, B.L.; Gao, X.M. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, L.P.; Shang, H.C.; Ren, M.; Wang, Y.; Huo, D.; Lei, X.; Wang, H.; Zhai, J.B. The cost-effectiveness analysis of JinQi Jiangtang tablets for the treatment on prediabetes: A randomized, double-blind, placebo-controlled, multicenter design. Trials 2015, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T.; Wu, J.X.; Wu, Z.; Guo, L.P. Anti-diabetic effect and mechanism of components in Jinqi Jiangtang tablet in Vitro. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 106–109. (In Chinese) [Google Scholar]
- Zhao, Y.H.; Yang, Y.; Xie, Z.S.; Xu, X.J.; Liu, L. Protective effects of Jinqi Jiangtang tablets on diabetic complications of cardiovascular diseases in Drosophila. J. China Pharm. Univ. 2016, 47, 348–352. (In Chinese) [Google Scholar]
- Kim, E.; Ahn, S.; Rhee, H.; Lee, D. Coptis chinensis Franch. extract up-regulate type I helper T-cell cytokine through MAPK activation in MOLT-4 T cell. J. Ethnopharmcol. 2016, 189, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Jin, C.; Xiao, X.; Dong, X. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. J. Biochem. Biophys. Methods 2008, 70, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chan, K.; Pusparajah, P.; Duangjai, A.; Saokaew, S.; Mehmood Khan, T.; Lee, L.; Goh, B. Rhizoma coptidis: A potential cardiovascular protective agent. Front. Pharmacol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, J.; Lee, F.S.; Wang, X.; Yang, H. Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra-performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections. Anal. Chim. Acta 2008, 613, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.P.; Zhang, X.X.; Wang, H.P.; Li, P.L.; Liu, Y.X.; Li, S.J. Rapid Analysis of components in Coptis chinensis Franch by ultra-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. Pharmacogn. Mag. 2017, 13, 175–179. [Google Scholar] [PubMed]
- US Food Drug Administration. Guidance for Industry, Bioanalytical Methods Validation. 2013. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm368107.pdf (accessed on 28 January 2017).
- Pan, Y.; Qian, D.; Liu, P.; Zhang, Y.; Zhu, Z.; Zhang, L.; Duan, J. The influence of essential oils from Xiang-Fu-Si-Wu Decoction on its non-volatile components and its application for pharmacokinetics in normal rats. J. Chromatogr. B 2017, 1060, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Zhang, P.; Yang, B.; Wu, H.; Guo, X.; Li, Y.; Zhang, Y. Sensitive UHPLC–MS/MS quantitation and pharmacokinetic comparisons of multiple alkaloids from Fuzi-Beimu and single herb aqueous extracts following oral delivery in rats. J. Chromatogr. B 2017, 1058, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, D.; Guo, K.; Li, M.; Lv, C.; Wang, J.; Jia, L.; Lu, J. Validated UPLC-MS/MS method for simultaneous quantification of eight saikosaponins in rat plasma: Application to a comparative pharmacokinetic study in depression rats after oral administration of extracts of raw and vinegar-baked Bupleuri Radix. J. Chromatogr. B 2017, 1060, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Chen, L.; Ma, H.; Chen, Y.; Han, F.M. Effects of Jinqi Jiangtang tablet in compatibility on in vivo pharmacokinetics of berberine in rats. Chin. Tradit. Herb. Drugs 2016, 47, 4231–4234. (In Chinese) [Google Scholar]
- He, W.; Liu, G.H.; Cai, H.; Sun, X.M.; Hou, W.; Zhang, P.T.; Xie, Z.Y.; Liao, Q.F. Integrated pharmacokinetics of five protoberberine-type alkaloids in normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan. J. Ethnopharmacol. 2014, 154, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhou, H.; Liu, Z.M.; Ma, Y.M.; Wang, T.M.; Liu, Y.Y.; Wang, C.H. Influence of Coptis Chinensis on pharmacokinetics of flavonoids after oral administration of Radix Scutellariae in rats. Biopharm. Durg Dispos. 2009, 30, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Ye, X.L.; Li, X.M.; Chu, H.F.; Liu, G.Y.; Zhu, Y.; Chen, X.; Zhou, J.Z.; Li, X.G. Effect of additives on absorption of Coptis chinensis total alkaloids and pharmacokinetics in mice. China J. Chin. Mater. Med. 2009, 34, 344–348. (In Chinese) [Google Scholar]


| Compound Name | Parent (m/z) | Daughter (m/z) | Cone (V) | Collision (eV) |
|---|---|---|---|---|
| Propranolol (IS) | 260.15 | 116.29 | 35 | 18 |
| Coptisime chloride | 320.24 | 292.13 | 44 | 29 |
| Epiberberine chloride | 336.26 | 320.23 | 47 | 31 |
| Berberine chloride | 336.26 | 320.23 | 47 | 31 |
| Jatrorrhizine chloride | 338.27 | 322.12 | 38 | 29 |
| Palmatine chloride | 352.26 | 336.11 | 36 | 30 |
| Compound | Calibration Curves | r a | Linear Ranges (ng/mL) |
|---|---|---|---|
| Palmatine chloride | Y = 0.0381X + 0.0034 | 0.9981 | 0.2–100 |
| Jatrorrhizine chloride | Y = 0.0341X + 0.0034 | 0.9987 | 0.2–100 |
| Epiberberine chloride | Y = 0.0534X + 0.0053 | 0.9978 | 0.2–100 |
| Berberine chloride | Y = 0.0581X + 0.00052 | 0.9985 | 0.2–100 |
| Coptisime chloride | Y = 0.0108X − 0.0013 | 0.9976 | 0.2–100 |
| Analyte | Concentration (ng/mL) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Accurary (RE %) | Precision (RSD %) | Accurary (RE %) | Precision (RSD %) | ||
| Palmatine chloride | 1 | −3.33 | 5.97 | 6.70 | 5.41 |
| 5 | 1.33 | 10.13 | 2.84 | 2.00 | |
| 50 | 1.60 | 2.98 | 2.50 | 3.56 | |
| Jatrorrhizine chloride | 1 | −4.00 | 5.51 | −3.30 | 5.97 |
| 5 | 4.67 | 2.21 | −1.30 | 2.34 | |
| 50 | 1.27 | 1.46 | 2.70 | 1.66 | |
| Epiberberine chloride | 1 | −8.50 | 2.84 | −6.70 | 6.19 |
| 5 | 8.00 | 1.85 | 4.00 | 3.85 | |
| 50 | −0.53 | 0.81 | 3.70 | 5.03 | |
| Berberine chloride | 1 | 3.33 | 5.59 | −6.70 | 6.19 |
| 5 | 3.33 | 2.96 | 4.00 | 3.33 | |
| 50 | −2.07 | 1.39 | 3.10 | 5.83 | |
| Coptisime chloride | 1 | −4.80 | 4.74 | −6.67 | 6.19 |
| 5 | 2.00 | 8.55 | −1.33 | 2.34 | |
| 50 | 2.80 | 7.42 | 7.13 | 0.96 | |
| Analyte | Concentration | Room temperature, 2 h | 4 °C, 24 h | Three Freeze-Thaw Cycles | |||
|---|---|---|---|---|---|---|---|
| RSD % | RE % | RSD % | RE % | RSD % | RE % | ||
| Palmatine chloride | 0.5 | 5.49 | 0.33 | 5.62 | −1.00 | 9.08 | −0.67 |
| 5.0 | 5.19 | 2.00 | 0.011 | 6.67 | 4.11 | 1.33 | |
| 80.0 | 3.81 | 1.67 | 4.11 | 0.83 | 2.27 | 0.04 | |
| Jatrorrhizine chloride | 0.5 | 5.88 | −0.33 | 2.98 | 2.67 | 7.63 | −1.00 |
| 5.0 | 2.44 | −5.33 | 3.08 | −0.67 | 4.11 | 1.33 | |
| 80.0 | 2.79 | 5.25 | 4.23 | 3.17 | 1.17 | 2.75 | |
| Epiberberine chloride | 0.5 | 6.65 | 2.33 | 1.96 | 2.00 | 6.98 | 0.67 |
| 5.0 | 3.03 | 0.67 | 6.26 | 2.67 | 6.07 | 0.67 | |
| 80.0 | 5.67 | 1.46 | 4.75 | −1.96 | 2.70 | 0.25 | |
| Berberine chloride | 0.5 | 8.19 | −1.33 | 13.97 | −4.67 | 1.96 | 2.00 |
| 5.0 | 1.14 | 1.33 | 2.96 | 3.33 | 5.00 | 0.67 | |
| 80.0 | 3.74 | 3.96 | 5.51 | −1.75 | 2.43 | 1.13 | |
| Coptisime chloride | 0.5 | 5.88 | −0.33 | 4.44 | 1.67 | 6.19 | −1.33 |
| 5.0 | 3.14 | −2.67 | 3.90 | 6.67 | 8.14 | −0.67 | |
| 80.0 | 1.68 | 7.50 | 3.64 | 1.25 | 3.24 | 6.04 | |
| Analyte | Group | Cmax (ug/L) | AUC0–tn (ug/L h) | MRT0–tn (h) | VRT0–tn (h2) |
|---|---|---|---|---|---|
| Palmatine chloride | CRE | 47.65 ± 8.69 | 204.86 ± 12.17 | 6.60 ± 0.50 | 21.23 ± 5.55 |
| JQJT | 35.1 ± 2.96 * | 107.51 ± 6.32 ** | 5.20 ± 0.26 ** | 21.87 ± 3.20 | |
| Jatrorrhizine chloride | CRE | 33.35 ± 5.82 | 96.58 ± 21.69 | 6.14 ± 0.30 | 15.45 ± 1.26 |
| JQJT | 11.35 ± 2.48 ** | 279.70 ± 83.40 ** | 5.08 ± 0.42 ** | 24.55 ± 5.42 ** | |
| Epiberberine chloride | CRE | 32.60 ± 8.77 | 125.03 ± 12.84 | 6.48 ± 0.29 | 19.61 ± 2.20 |
| JQJT | 22.75 ± 2.15 | 68.58 ± 5.48 ** | 4.31 ± 0.19 ** | 16.09 ± 3.01 | |
| Berberine chloride | CRE | 156.55 ± 27.85 | 571.59 ± 44.41 | 6.46 ± 0.18 | 18.44 ± 2.84 |
| JQJT | 166.40 ± 22.36 | 269.36 ± 13.91 ** | 3.28 ± 0.12 ** | 10.43 ± 2.19 ** | |
| Coptisime chloride | CRE | 60.85 ± 7.34 | 309.59 ± 27.06 | 6.94 ± 0.35 | 21.51 ± 3.40 |
| JQJT | 64.10 ± 8.50 | 177.50 ± 9.83 ** | 5.179 ± 0.18 ** | 21.78 ± 2.54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Ding, F.; You, G.; Liu, H.; Wang, M.; Ren, X.; Deng, Y. Development and Validation of an UPLC-MS/MS Method for Pharmacokinetic Comparison of Five Alkaloids from JinQi Jiangtang Tablets and Its Monarch Drug Coptidis Rhizoma. Pharmaceutics 2018, 10, 4. https://doi.org/10.3390/pharmaceutics10010004
Sun L, Ding F, You G, Liu H, Wang M, Ren X, Deng Y. Development and Validation of an UPLC-MS/MS Method for Pharmacokinetic Comparison of Five Alkaloids from JinQi Jiangtang Tablets and Its Monarch Drug Coptidis Rhizoma. Pharmaceutics. 2018; 10(1):4. https://doi.org/10.3390/pharmaceutics10010004
Chicago/Turabian StyleSun, Lili, Feifei Ding, Guangjiao You, Han Liu, Meng Wang, Xiaoliang Ren, and Yanru Deng. 2018. "Development and Validation of an UPLC-MS/MS Method for Pharmacokinetic Comparison of Five Alkaloids from JinQi Jiangtang Tablets and Its Monarch Drug Coptidis Rhizoma" Pharmaceutics 10, no. 1: 4. https://doi.org/10.3390/pharmaceutics10010004