Contribution of Harvest Residues to Nutrient Cycling in a Tropical Acacia mangium Willd. Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Site Description
2.2. Estimation of Biomass and Nutrients in Harvest Residues, Litter and Understory Vegetation
2.3. Rate of Decomposition and Nutrient Release of Harvest Residues (Branches, Leaves, and Bark)
2.4. Plant Nutrient Analysis
2.5. Statistical Analysis
3. Results
3.1. Biomass and Nutrient Content of Harvest Residues, Litter and Understory Vegetation
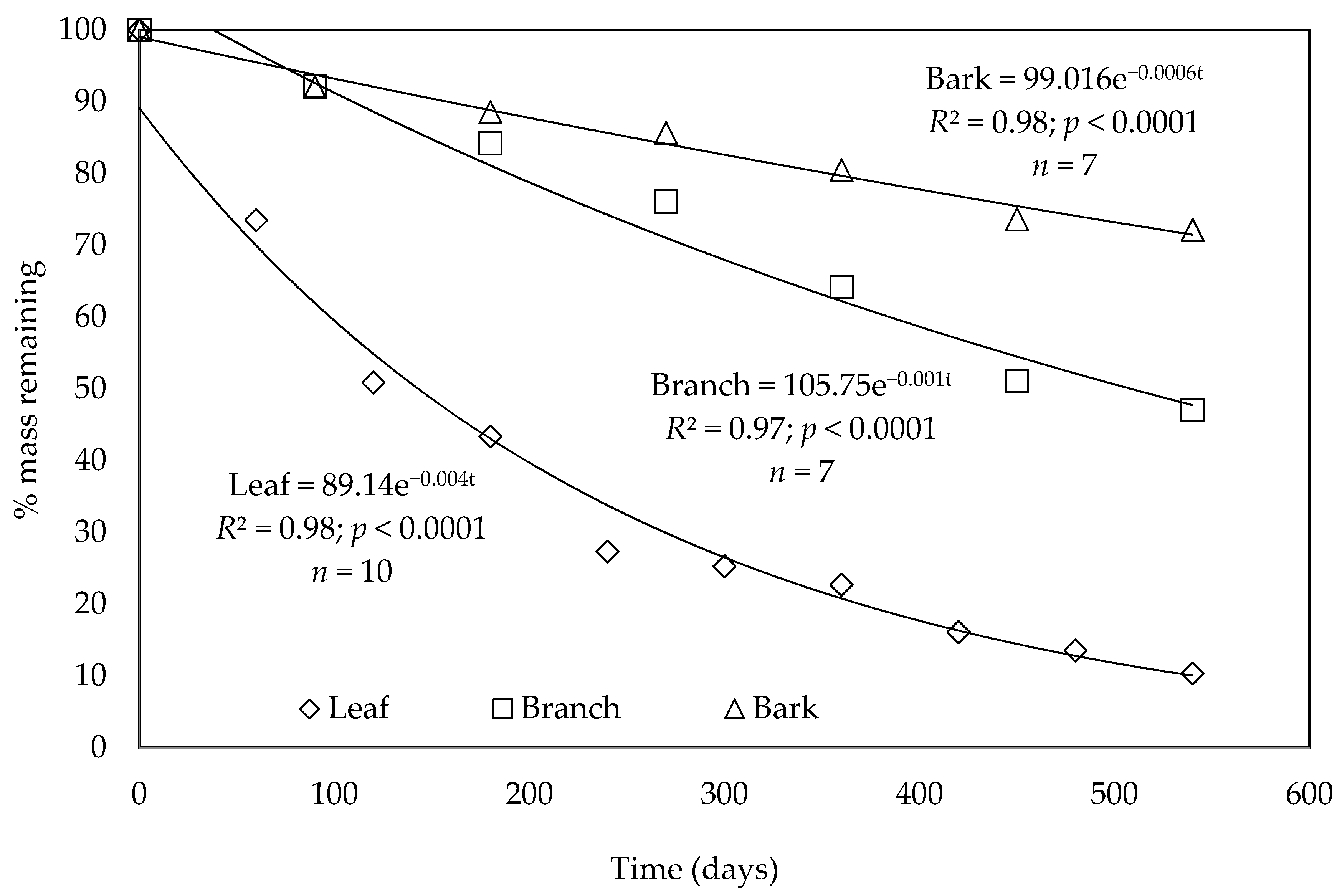
3.2. Decomposition of Harvest Residue Components
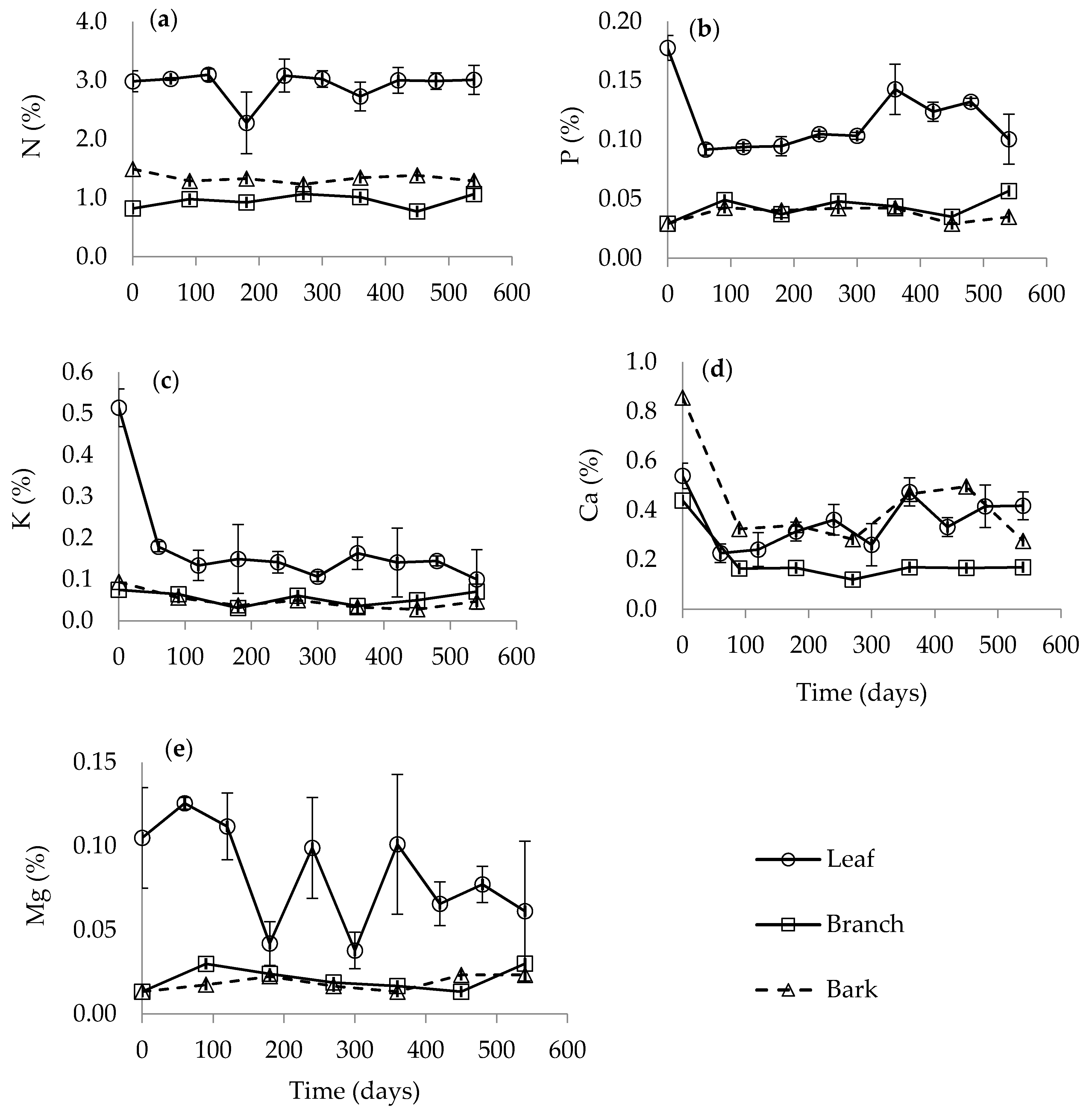
3.3. Nutrient Release During Decomposition of Harvest Residues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huong, V.D.; Nambiar, E.S.; Quang, L.T.; Mendham, D.S.; Dung, P.T. Improving productivity and sustainability of successive rotations of Acacia auriculiformis plantations in South Vietnam. South. For. 2015, 77, 51–58. [Google Scholar] [CrossRef]
- Hai, V.D.; Trieu, D.T.; Tiep, N.H.; Bich, N.V.; Duong, D.T. Biomass Accumulation and Carbon Sequestration in Some Major Types of Plantation Forests in Vietnam; Agriculture Publishing House: Hanoi, Vietnam, 2009. [Google Scholar]
- Bao Bac Giang. Burning Tree Bark Residue to Produce Charcoal: Advantages and Disadvantages. Available online: http://baobacgiang.com.vn/bg/nhip-cau-ban-doc/150436/dot-vo-go-lay-than--loi-it--hai-nhieu.html (accessed on 3 July 2018).
- Bac, V. Producing Compost Fertiliser from Bark of Some Tree Species. Available online: https://www.vietnamplus.vn/san-xuat-phan-bon-huu-co-tu-vo-cay-nguyen-lieu-giay/213711.vnp (accessed on 3 July 2018).
- Anh, N. Producing Wood Glue from Bark of Acacia Tree. Available online: http://www.khoahocphothong.com.vn/tao-keo-dan-go-tu-vo-keo-la-tram-26294.html (accessed on 3 July 2018).
- Nambiar, E.K.S.; Harwood, C.E.; Kien, N.D. Acacia plantations in Vietnam: Research and knowledge application to secure a sustainable future. South. For. 2015, 77, 1–10. [Google Scholar] [CrossRef]
- Goncalves, J.L.D.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.D.; Lima, W.D.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Laclau, J.P.; Levillain, J.; Deleporte, P.; Nzila, J.D.; Bouillet, J.P.; Saint Andre, L.; Versini, A.; Mareschal, L.; Nouvellon, Y.; M’Bou, A.T.; et al. Organic residue mass at planting is an excellent predictor of tree growth in Eucalyptus plantations established on a sandy tropical soil. For. Ecol. Manag. 2010, 260, 2148–2159. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Goncalves, J.L.D.; Gava, J.L.; Godinho, T.D.; Melo, E.A.S.C.; Bazani, J.H.; Hubner, A.; Arthur, J.C.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Raison, R.J.; O’Connell, A.M.; Khanna, P.K.; Keith, H. Effect of repeated fiers on nitrogen and phosphorus budgets and cycling processes in forest ecosystems. In Fire in Mediterranean Ecosystems; Trabaud, L., Prodon, R., Eds.; Commission of the European Communities: Brussels, Belgium, 1993; pp. 347–363. [Google Scholar]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. For. Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Deleporte, P.; Laclau, J.P.; Nzila, J.D.; Kazotti, J.G.; Marien, J.N.; Bouillet, J.P.; Szwarc, M.; D’Annunzio, R.; Ranger, J. Effects of slash and litter management practices on soil chemical properties and growth of second rotation eucalypts in the Congo. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of Workshops, Piracicaba, Brazil, 22–26 November 2004 and Bogor, Indonesia, 6–9 November 2006; Nambiar, E.K.S., Ed.; Center for International Forestry Research: Bogor, Indonesia, 2008; pp. 5–22. [Google Scholar]
- Goncalves, J.L.M.; Wichert, M.C.P.; Gava, J.L.; Masetto, A.V.; Junior, J.C.A.; Serrano, M.I.P.; Mello, S.L.M. Soil fertility and growth of Eucalyptus grandis in Brazil under different residue management practices. South. For. 2007, 69, 95–102. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Giardina, C.P.; Sanford, R.L.; Døckersmith, I.C.; Jaramillo, V.J. The effects of slash burning on ecosystem nutrients during the land preparation phase of shifting cultivation. Plant Soil 2000, 220, 247–260. [Google Scholar] [CrossRef]
- Shammas, K.; O’Connell, A.M.; Grove, T.S.; McMurtrie, R.; Damon, P.; Rance, S.J. Contribution of decomposing harvest residues to nutrient cycling in a second rotation Eucalyptus globulus plantation in south-western Australia. Biol. Fertil. Soils 2003, 38, 228–235. [Google Scholar]
- Hernández, J.; del Pino, A.; Salvo, L.; Arrarte, G. Nutrient export and harvest residue decomposition patterns of a Eucalyptus dunnii Maiden plantation in temperate climate of Uruguay. For. Ecol. Manag. 2009, 258, 92–99. [Google Scholar] [CrossRef]
- De Souza, I.F.; De Barros, N.F.; Da Silva, I.R.; Renier, R.F.; Silva, L.D.; De Novais, R.F. Decomposition of eucalypt harvest residues as affected by management practices, climate and soil properties across southeastern Brazil. For. Ecol. Manag. 2016, 374, 186–194. [Google Scholar] [CrossRef]
- Ferreira, G.W.D.; Soares, E.M.B.; Oliveira, F.C.C.; Silva, I.R.; Dungait, J.A.J.; Souza, I.F.; Vergutz, L. Nutrient release from decomposing Eucalyptus harvest residues following simulated management practices in multiple sites in Brazil. For. Ecol. Manag. 2016, 370, 1–11. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Marques, E.R.G.; Gonçalves, J.L.D.M.; Hübner, A.; Brandani, C.B.; Ferraz, A.D.V.; Moreira, R.M. Decomposition rates of forest residues and soil fertility after clear-cutting of Eucalyptus grandis stands in response to site management and fertilizer application. Soil Used Manag. 2016, 32, 289–302. [Google Scholar] [CrossRef]
- Hardiyanto, E.; Nambiar, S.E. Productivity of successive rotations of Acacia mangium plantations in Sumatra, Indonesia: Impacts of harvest and inter-rotation site management. New For. 2014, 45, 557–575. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Versini, A.; Zeller, B.; Derrien, D.; Mazoumbou, J.-C.; Mareschal, L.; Saint-André, L.; Ranger, J.; Laclau, J.-P. The role of harvest residues to sustain tree growth and soil nitrogen stocks in a tropical Eucalyptus plantation. Plant Soil 2014, 376, 245–260. [Google Scholar] [CrossRef]
- Folster, H.; Khanna, P.K. Dynamics of Nutrient Supply in Plantation Soils. In Management of Soil, Nutrients and Water in Tropical Plantation Forests; Nambiar, E.K.S., Brown, A.G., Eds.; ACIAR: Canberra, Australia, 1997; pp. 339–373. [Google Scholar]
- Sam, D.D.; Binh, N.N. Assessment of Productivity of Forest Lands in Vietnam; Statistical Publishing House: Hanoi, Vietnam, 2001. [Google Scholar]
- Dong, T.L.; Doyle, R.; Beadle, C.L.; Corkrey, R.; Quat, N.X. Impact of short-rotation acacia hybrid plantations on soil properties of degraded lands in central Vietnam. Soil Res. 2014, 52, 271–281. [Google Scholar] [CrossRef]
- Hung, T.T.; Almeida, A.C.; Eyles, A.; Mohammed, C. Predicting productivity of Acacia hybrid plantations for a range of climates and soils in Vietnam. For. Ecol. Manag. 2016, 367, 97–111. [Google Scholar] [CrossRef]
- Meentemeyer, V. Macroclimate the lignin control of litter decomposition rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta. Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: a review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.L.; Klopatek, J.M.; Klopatek, C.C. The effects of litter quality and climate on decomposition along an elevational gradient. Ecol. Appl. 1998, 8, 1061–1071. [Google Scholar] [CrossRef]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Bachega, L.R.; Bouillet, J.-P.; de Cássia Piccolo, M.; Saint-André, L.; Bouvet, J.-M.; Nouvellon, Y.; de Moraes Gonçalves, J.L.; Robin, A.; Laclau, J.-P. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the home field advantage hypothesis. For. Ecol. Manag. 2016, 359, 33–43. [Google Scholar] [CrossRef]
- Tchichelle, S.V.; Epron, D.; Mialoundama, F.; Koutika, L.S.; Harmand, J.M.; Bouillet, J.P.; Mareschal, L. Differences in nitrogen cycling and soil mineralisation between a eucalypt plantation and a mixed eucalypt and Acacia mangium plantation on a sandy tropical soil. South. For. 2017, 79, 1–8. [Google Scholar] [CrossRef]
- Hardiyanto, E.; Anshori, S.; Sulistyono, D. Early results of site management in Acacia mangium plantations at PT. Musi Hutan Persada, South Sumatra, Indonesia. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of Workshop, Congo, July 2001 and China, February 2003; Nambiar, E., Ranger, J., Tiarks, A., Toma, T., Eds.; Center for International Forestry Research: Bogor, Indonesia, 2004; pp. 93–108. [Google Scholar]
- Ngoran, A.; Zakra, N.; Ballo, K.; Kouame, C.; Zapata, F.; Hofman, G.; Van Cleemput, O. Litter decomposition of Acacia auriculiformis Cunn. Ex Benth. and Acacia mangium Willd. under coconut trees on quaternary sandy soils in Ivory Coast. Biol. Fertil. Soils 2006, 43, 102–106. [Google Scholar] [CrossRef]
- Harwood, C.E.; Nambiar, E.K.S. Productivity of acacia and eucalypt plantations in Southeast Asia. 2. trends and variations. Int. For. Rev. 2014, 16, 249–260. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. World Reference Base for Soil Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Bich, N.V.; Mendham, D.; Evans, K.J.; Dong, T.L.; Hai, V.D.; Thanh, H.V.; Mohammed, C. Effect of residue management and fertiliser application on the productivity of young eucalyptus hybrid and Acacia mangium planted on steep slopes in Northern Vietnam. South. For. 2018, in press. [Google Scholar]
- Bärlocher, F. Leaf Mass Loss Estimated by Litter Bag Technique. In Methods to Study Litter Decomposition: A Practical Guide; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 37–42. [Google Scholar]
- O’Connell, A.M. Decomposition of slash residues in thinned regrowth eucalypt forest in Western Australia. J. Appl. Ecol. 1997, 34, 111. [Google Scholar] [CrossRef]
- Berry, W.L.; Johnson, C.M. Determination of calcium and magnesium in plant material and culture solutions, using atomic-absorption spectroscopy. Appl. Spectrosc. 1966, 20, 209–211. [Google Scholar] [CrossRef]
- Harmon, M.E.; Silver, W.L.; Fasth, B.; Chen, H.; Burke, I.C.; Parton, W.J.; Hart, S.C.; Currie, W.S.; Lidet. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: An intersite comparison. Glob. Chang. Biol. 2009, 15, 1320–1338. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–330. [Google Scholar] [CrossRef]
- Siregar, S.T.H.; Nurwahyudi; Mulawarman. Effects of inter-rotation management on site productivity of Acacia mangium in Riau Province, Sumatra, Indonesia. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of Workshops, Piracicaba, Brazil, 22–26, November 2004 and Bogor, Indonesia, 6–9 November 2006; Nambiar, E.K.S., Ed.; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2008; pp. 93–106. [Google Scholar]
- Hai, V.D.; Trieu, D.T.; Thang, H.V.; Bich, N.V.; Cam, N.V.; Dinh, P.X.; Phuong, V.; Dien, P.V.; Tam, D.D.; Tiep, N.H. The Research on Development of Schima wallichi and Schima superba Tree Species in Vietnam. Final Project Report No. VAFS 43/2010; Vietnamese Academy of Forest Sciences: Hanoi, Vietnam, 2010; p. 120.
- Santana, R.C.; Barros, N.F.; Comerford, N.B. Above-ground biomass, nutrient content, and nutrient use efficiency of eucalypt plantations growing in different sites in Brazil. N. Z. J. For. Sci. 2000, 30, 225–236. [Google Scholar]
- Du Toit, B.; Dovey, S.B.; Fuller, G.M.; Job, R.A. Effects of harvesting and site management on nutrient pools and stand growth in a South African euclypt plantation. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of Workshops, Congo, July 2001 and China, February 2003; Nambiar, E.K.S., Ranger, J., Tiarks, A., Toma, T., Eds.; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2004; pp. 31–43. [Google Scholar]
- Li, X.; Yi, M.J.; Son, Y.; Park, P.S.; Lee, K.H.; Son, Y.M.; Kim, R.H.; Jeong, M.J. Biomass and carbon storage in an age-sequence of Korean Pine (Pinus koraiensis) plantation forests in Central Korea. J. Plant Biol. 2011, 54, 33–42. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Kurka, A.-M.; Mannerkoski, H.; Piirainen, S.; Starr, M. Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant Soil 2004, 263, 53–67. [Google Scholar] [CrossRef]
- Garrett, L.G.; Kimberley, M.O.; Oliver, G.R.; Pearce, S.H.; Paul, T.S.H. Decomposition of woody debris in managed Pinus radiata plantations in New Zealand. For. Ecol. Manag. 2010, 260, 1389–1398. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, W. Control of climate and litter quality on leaf litter decomposition in different climatic zones. J. Plant Res. 2015, 128, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Z.; Jiang, H.; Peng, C.H.; Zhou, G.M.; Chang, S.X.; Yu, S.Q.; Ma, Y.D.; Peng, S.L.; Wei, X.H. Leaf litter decomposition along the temperate-tropical transect (East China): The influence of stand succession, litter quality and climate. Pol. J. Ecol. 2012, 60, 265–276. [Google Scholar]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Titus, B.D. Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forests. Plant Soil 2011, 339, 163–175. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soils: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 2nd ed.; John Wiley and Sons Ltd.: New York, NY, USA, 1999; p. 427. ISBN 0471320714. [Google Scholar]
- Heal, O.; Anderson, J.; Swift, M. Plant litter quality and decomposition: An historical overview. In Driven By Nature. Plant Litter Quality and Decomposition; Cadisch, G., Giller, K., Eds.; CAB International: Wallingford, CT, USA, 1997; pp. 3–30. [Google Scholar]
- Voigtlaender, M.; Laclau, J.P.; Goncalves, J.L.D.; Piccolo, M.D.; Moreira, M.Z.; Nouvellon, Y.; Ranger, J.; Bouillet, J.P. Introducing Acacia mangium trees in Eucalyptus grandis plantations: Consequences for soil organic matter stocks and nitrogen mineralization. Plant Soil 2012, 352, 99–111. [Google Scholar] [CrossRef]
- Sánchez, G.; Pino, A.d.; Hernández, J. Decomposition of Eucalyptus sp. and Pinus taeda harvest residues under controlled temperature and moisture conditions. Open J. For. 2018, 8, 18. [Google Scholar]
- Harwood, C.E.; Nambiar, E.K.S.; Dinh, P.X.; Toan, L.X.; Quang, L.T. Managing wood production from small grower Acacia hybrid plantations on eroded soils in central Vietnam. Aust. For. 2017, 80, 286–293. [Google Scholar] [CrossRef]
- Mendham, D.S.; Hardiyanto, E.B.; Wicaksono, A.; Nurudin, M. Nutrient management of contrasting Acacia mangium genotypes and weed management strategies in South Sumatra, Indonesia. Aust. For. 2017, 80, 127–134. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus and nitrogen addition on heterotrophic respiration in an Acacia mangium plantation soil in South Sumatra, Indonesia. Tropics 2013, 22, 83–87. [Google Scholar] [CrossRef]
- Mori, T.; Wachrinrat, C.; Staporn, D.; Meunpong, P.; Suebsai, W.; Matsubara, K.; Boonsri, K.; Lumban, W.; Kuawong, M.; Phukdee, T.; et al. Contrastive effects of inorganic phosphorus addition on soil microbial respiration and microbial biomass in tropical monoculture tree plantation soils in Thailand. ANRES 2016, 50, 327–330. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Brown, A.G. Management of Soil, Nutrients and Water in Tropical Plantation Forests; ACIAR Monograph No.43; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1997; p. 571.
- Tiarks, A.; Ranger, J. Soil properties in tropical plantation forests: Evaluation and effects of site management: A summary. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of workshops, Piracicaba, Brazil, 22–26 November 2004 and Bogor, Indonesia, 6–9 November 2006; Nambiar, E.K.S., Ed.; Center for International Forestry Research: Bogor, Indonesia, 2008; pp. 191–204. [Google Scholar]
- Bich, N.V.; Eyles, A.; Mendham, D.; Evans, K.J.; Dong, T.L.; Hai, V.D.; Thang, H.V.; Thinh, N.V.; Mohammed, C. Effect of harvest residue management on soil properties of eucalyptus hybrid and Acacia mangium plantations planted on steep slopes of northern Vietnam. 2018. Manuscript in preparation. [Google Scholar]
- Ranjbar, F.; Jalali, M. Calcium, magnesium, sodium, and potassium release during decomposition of some organic residues. Commun. Soil Sci. Plant. Anal. 2012, 43, 645–659. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J. For. Res. 2004, 9, 23–31. [Google Scholar] [CrossRef]
- Dauer, J.M.; Perakis, S.S. Calcium oxalate contribution to calcium cycling in forests of contrasting nutrient status. For. Ecol. Manag. 2014, 334, 64–73. [Google Scholar] [CrossRef]
- O’Connell, A.M.; Malajczuk, N.; Gailitis, V. Occurrence of calcium oxalate in karri (Eucalyptus diversicolor F. Muell.) forest ecosystems of south western Australia. Oecologia 1983, 56, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hardiyanto, E.B.; Wicaksono, A. Inter-rotation site management, stand growth and soil properties in Acacia mangium plantations in South Sumatra, Indonesia. In Site Management and Productivity in Tropical Plantation Forests, Proceedings of Seventh Workshops, Piracicaba, Brazil, 22–26 November 2004 and Bogor, Indonesia, 6–9 November 2006; Nambiar, E.K.S., Ed.; CIFOR: Bogor, Indonesia, 2008; pp. 107–122. [Google Scholar]
- O’Connell, A.M.; Grove, T.S. Biomass production nutrient uptake and nutrient cycling in the Jarrah (Eucalyptus marginata) and Karri (Eucalyptus diversicolor) forests of South-Western Australia. In Nutrition of Eucalypts; Attiwill, P.M., Mark, A.A., Eds.; CSIRO Publishing: Collingwood, Australia, 1996; pp. 155–189. [Google Scholar]
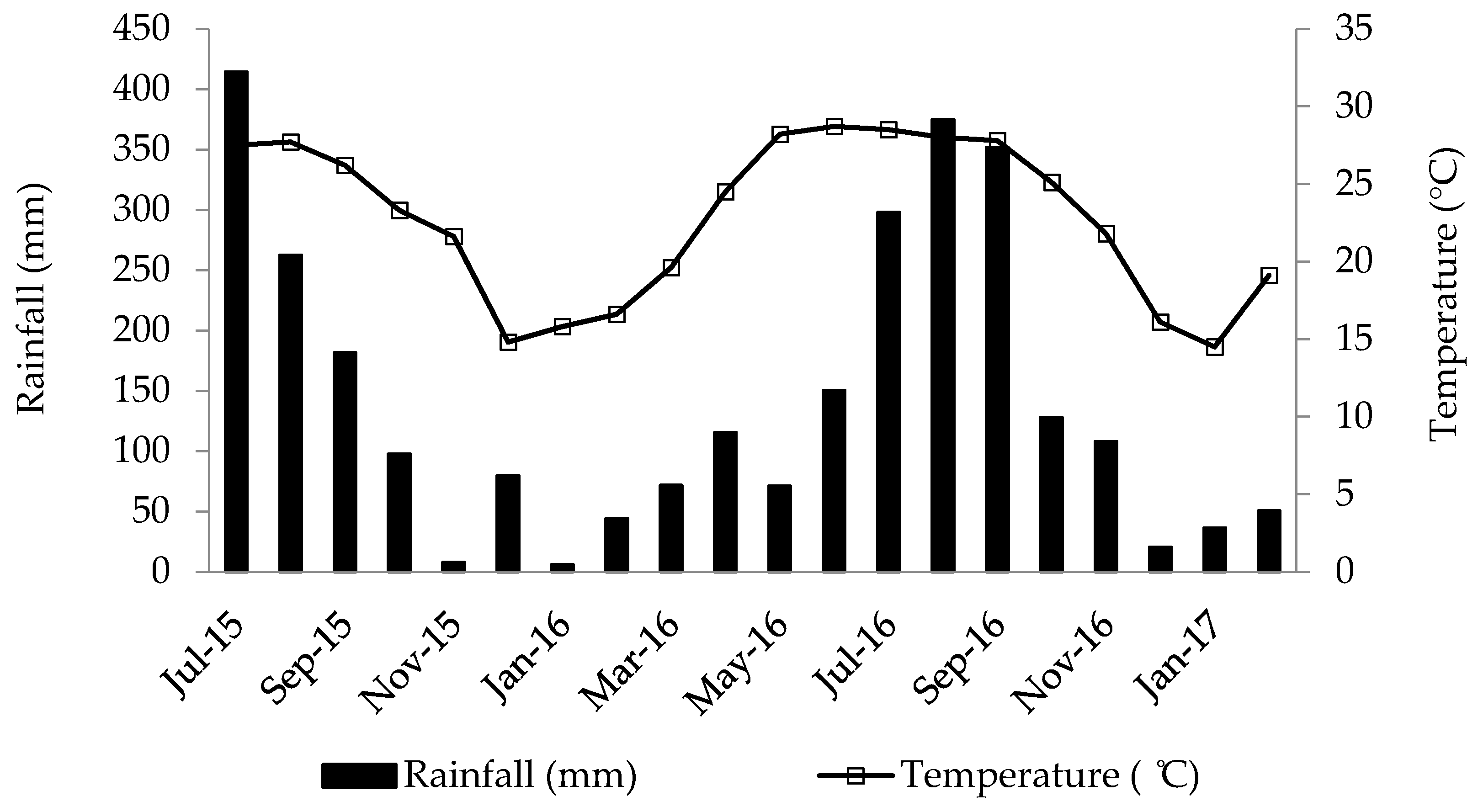

| Soil Depth | pH | Total C | Total N | Total P | Bray Extractable P | Exchangeable Cations (cmolc (+) kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| (cm) | (1:5 Water) | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | K | Ca | Mg |
| 0–10 | 3.79 | 44.36 | 2.10 | 0.09 | 4.04 | 0.12 | 0.70 | 0.45 |
| (0.03) | (2.57) | (0.09) | (0.00) | (0.51) | (0.01) | (0.11) | (0.09) | |
| 10–30 | 3.94 | 27.80 | 1.54 | 0.08 | 2.21 | 0.08 | 0.57 | 0.40 |
| (0.08) | (2.14) | (0.07) | (0.00) | (0.37) | (0.02) | (0.13) | (0.07) | |
| Components | Dry Weight | Nutrient Content (kg ha−1) | ||||
|---|---|---|---|---|---|---|
| (t DM ha−1) | N | P | K | Ca | Mg | |
| Harvest residue components | ||||||
| Bark | 8.9 (0.3) | 133.4 (5.2) | 2.6 (0.1) | 8.4 (0.3) | 76.5 (3.0) | 1.2 (0.1) |
| Branches | 6.6 (0.3) | 53.9 (1.8) | 1.9 (0.1) | 4.9 (0.1) | 28.9 (1.3) | 0.9 (0.1) |
| Leaves | 2.5 (0.1) | 76.0 (3.3) | 4.5 (0.2) | 13.1 (0.6) | 13.7 (0.6) | 2.8 (0.1) |
| Subtotal | 18.1 (0.7) | 263.4 (10) | 9.0 (0.3) | 26.4 (1.0) | 119.2 (4.9) | 4.9 (0.2) |
| Forest floor residue components | ||||||
| Litter | 5.8 (0.6) | 128.9 (13.3) | 5.1 (0.5) | 31.6 (3.3) | 45.4 (4.7) | 13.7 (1.4) |
| Understory | 3.3 (0.4) | 47.3 (5.6) | 0.7 (0.1) | 2.7 (0.3) | 20.4 (2.4) | 1.5 (0.2) |
| Subtotal | 9.1 (0.6) | 176.2 (12) | 5.9 (0.5) | 34.4 (3.1) | 65.8 (4.3) | 15.2 (1.3) |
| Total residue with bark | 27.2 (0.9) | 439.6 (15) | 14.8 (0.6) | 60.7 (3.2) | 185.0 (6.3) | 20.1 (1.2) |
| Total residue without bark | 18.2 (0.7) | 306.1 (12) | 12.2 (0.5) | 52.3 (3.1) | 108.5 (4.5) | 18.9 (1.3) |
| Component | Dry Weight Loss (%) | k (Year−1) | R2 | RMSE | p-Value | t0.5 (Year) |
|---|---|---|---|---|---|---|
| Leaves | 90 | 1.47 | 0.98 | 0.10 | <0.0001 | 0.47 |
| Branches | 53 | 0.54 | 0.97 | 0.05 | <0.0001 | 1.29 |
| Bark | 28 | 0.22 | 0.98 | 0.02 | <0.0001 | 3.09 |
| Components | Dry Weight Loss | Nutrient Release from Decomposing Harvest Residues (kg ha−1) | ||||
|---|---|---|---|---|---|---|
| (t DM ha−1) | N | P | K | Ca | Mg | |
| Leaves | 2.3 (0.1) | 68.1 (2.0) | 4.3 (0.1) | 12.8 (0.1) | 12.6 (0.4) | 2.6 (0.1) |
| Branches | 3.5 (0.3) | 20.9 (1.5) | 0.2 (0.8) | 2.7 (0.1) | 23.7 (2.0) | −0.1 (0.0) |
| Bark | 2.3 (0.1) | 48.0 (2.8) | 0.3 (0.0) | 5.3 (0.2) | 58.2 (1.5) | −0.4 (0.0) |
| Total residue with bark | 8.1 (0.0) | 137.1 (3.1) | 4.7 (0.4) | 20.8 (0.5) | 94.5 (0.6) | 2.2 (0.2) |
| Total residue without bark | 5.8 (0.1) | 89.0 (2.3) | 4.4 (0.4) | 15.6 (0.5) | 36.3 (0.8) | 2.6 (0.2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bich, N.; Eyles, A.; Mendham, D.; Dong, T.L.; Ratkowsky, D.; Evans, K.J.; Hai, V.D.; Thanh, H.V.; Thinh, N.V.; Mohammed, C. Contribution of Harvest Residues to Nutrient Cycling in a Tropical Acacia mangium Willd. Plantation. Forests 2018, 9, 577. https://doi.org/10.3390/f9090577
Van Bich N, Eyles A, Mendham D, Dong TL, Ratkowsky D, Evans KJ, Hai VD, Thanh HV, Thinh NV, Mohammed C. Contribution of Harvest Residues to Nutrient Cycling in a Tropical Acacia mangium Willd. Plantation. Forests. 2018; 9(9):577. https://doi.org/10.3390/f9090577
Chicago/Turabian StyleVan Bich, Nguyen, Alieta Eyles, Daniel Mendham, Tran Lam Dong, David Ratkowsky, Katherine J. Evans, Vo Dai Hai, Hoang Van Thanh, Nguyen Van Thinh, and Caroline Mohammed. 2018. "Contribution of Harvest Residues to Nutrient Cycling in a Tropical Acacia mangium Willd. Plantation" Forests 9, no. 9: 577. https://doi.org/10.3390/f9090577





