Photosynthetic Capacity, Stomatal Behavior and Chloroplast Ultrastructure in Leaves of the Endangered Plant Carpinus putoensis W.C.Cheng during Gaseous NO2 Exposure and after Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NO2 Fumigation
2.3. Recovery
2.4. Photosynthetic Parameters
2.5. Measurement of Chl Fluorescence
2.6. Measurement of Chl and Carotenoid Contents
2.7. Scanning Electron Microscopy (SEM)
2.8. Transmission Electron Microscopy (TEM)
2.9. Statistical Analysis
3. Results
3.1. Changes in Leaf Chl Concentration in NO2-Treated and Post-Recovery Plants
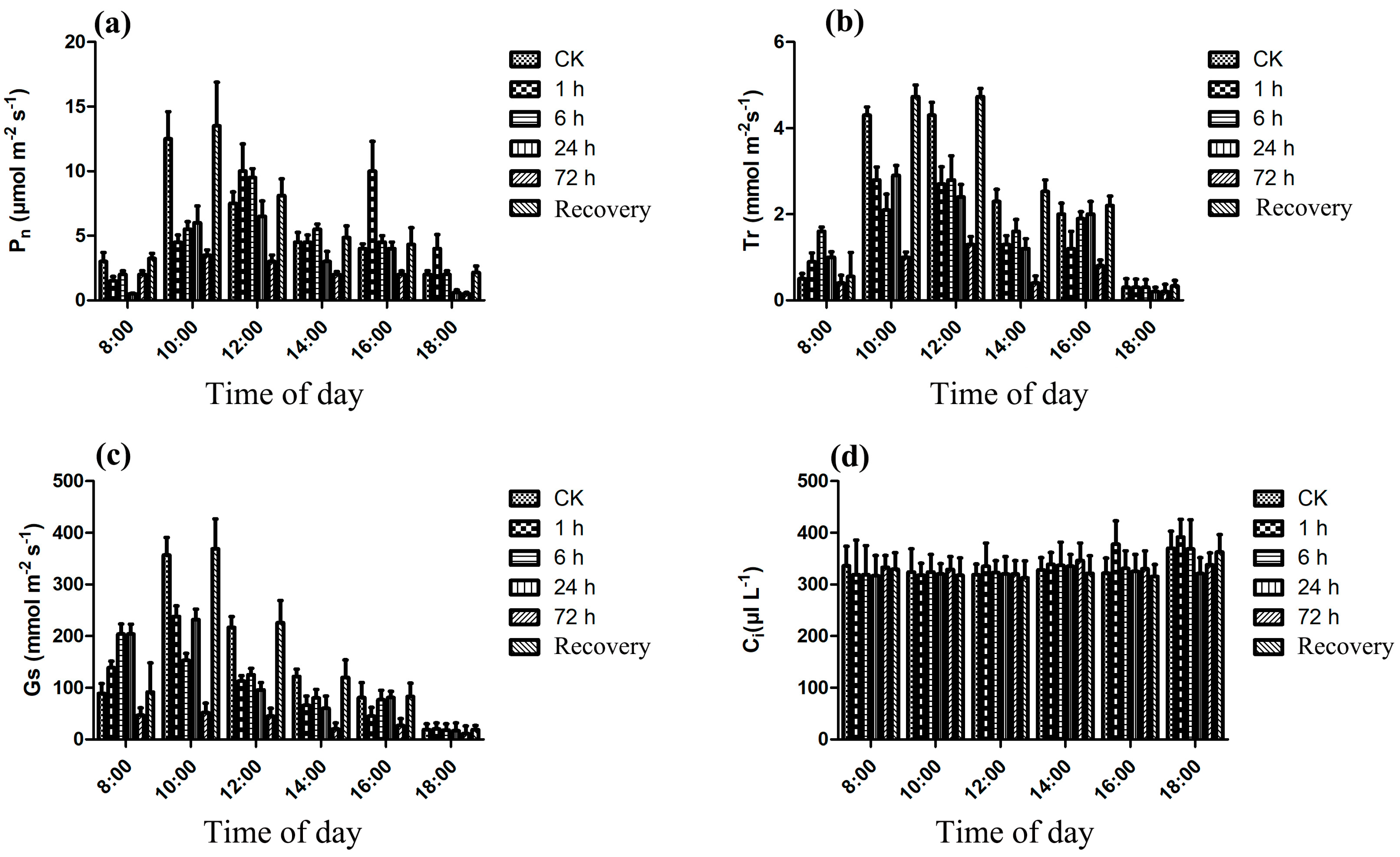
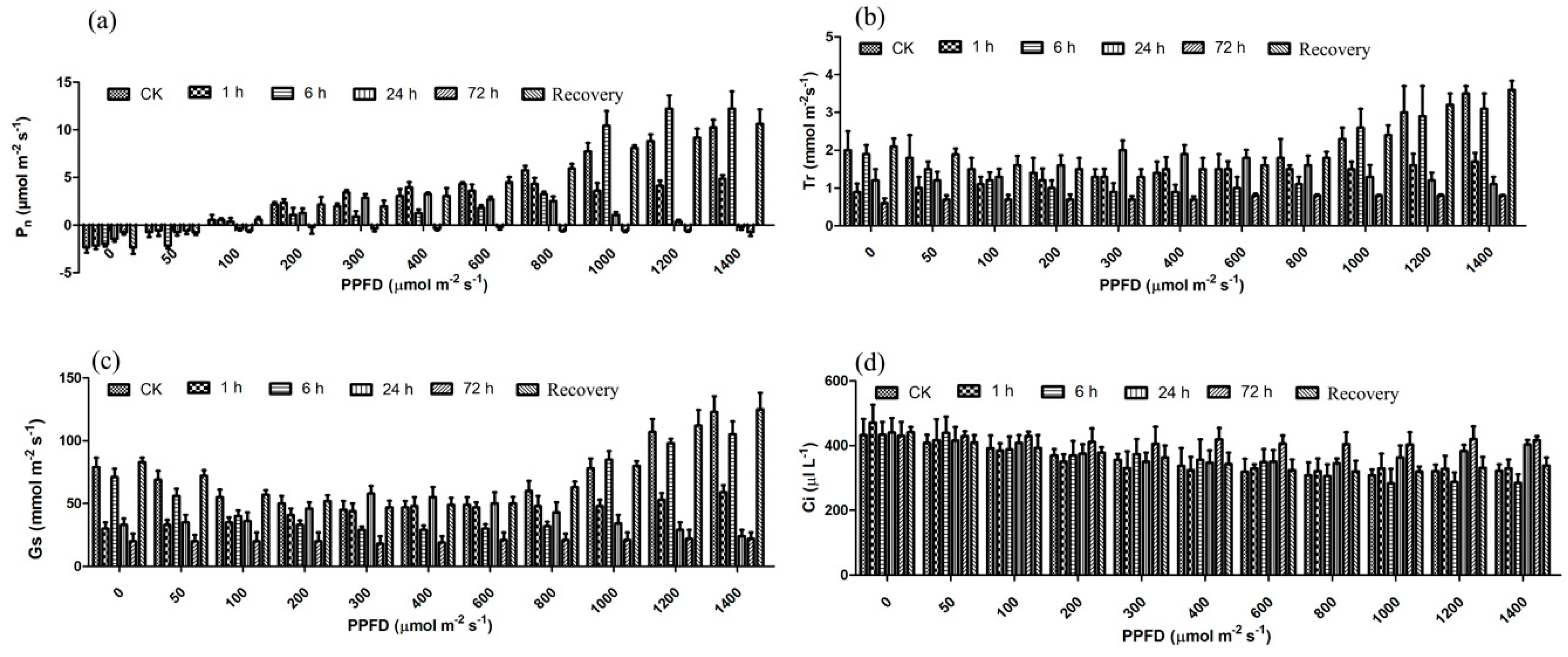
3.2. Photosynthesis
3.3. Chl Fluorescence of NO2-Treated and Post-Recovery Plants
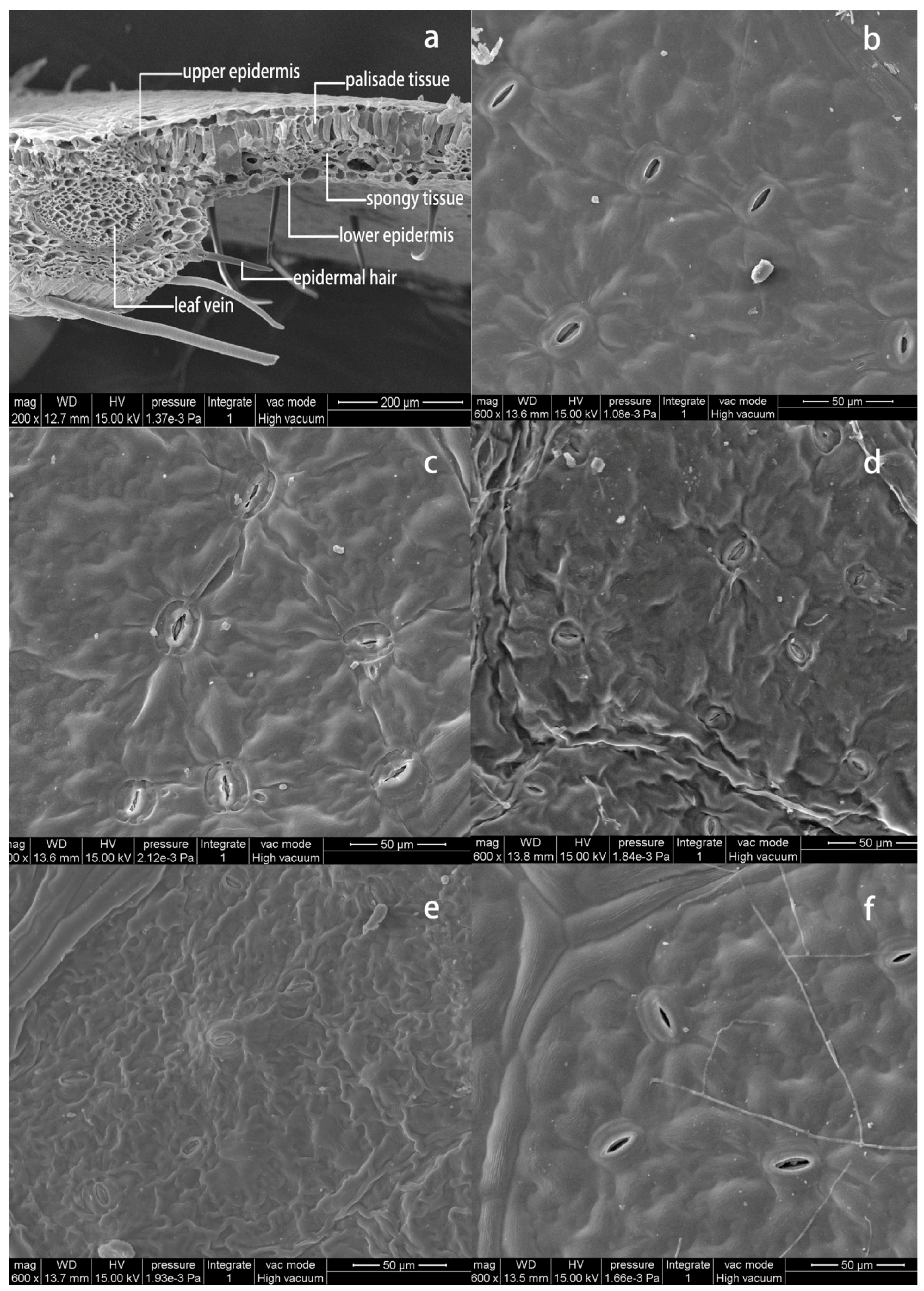
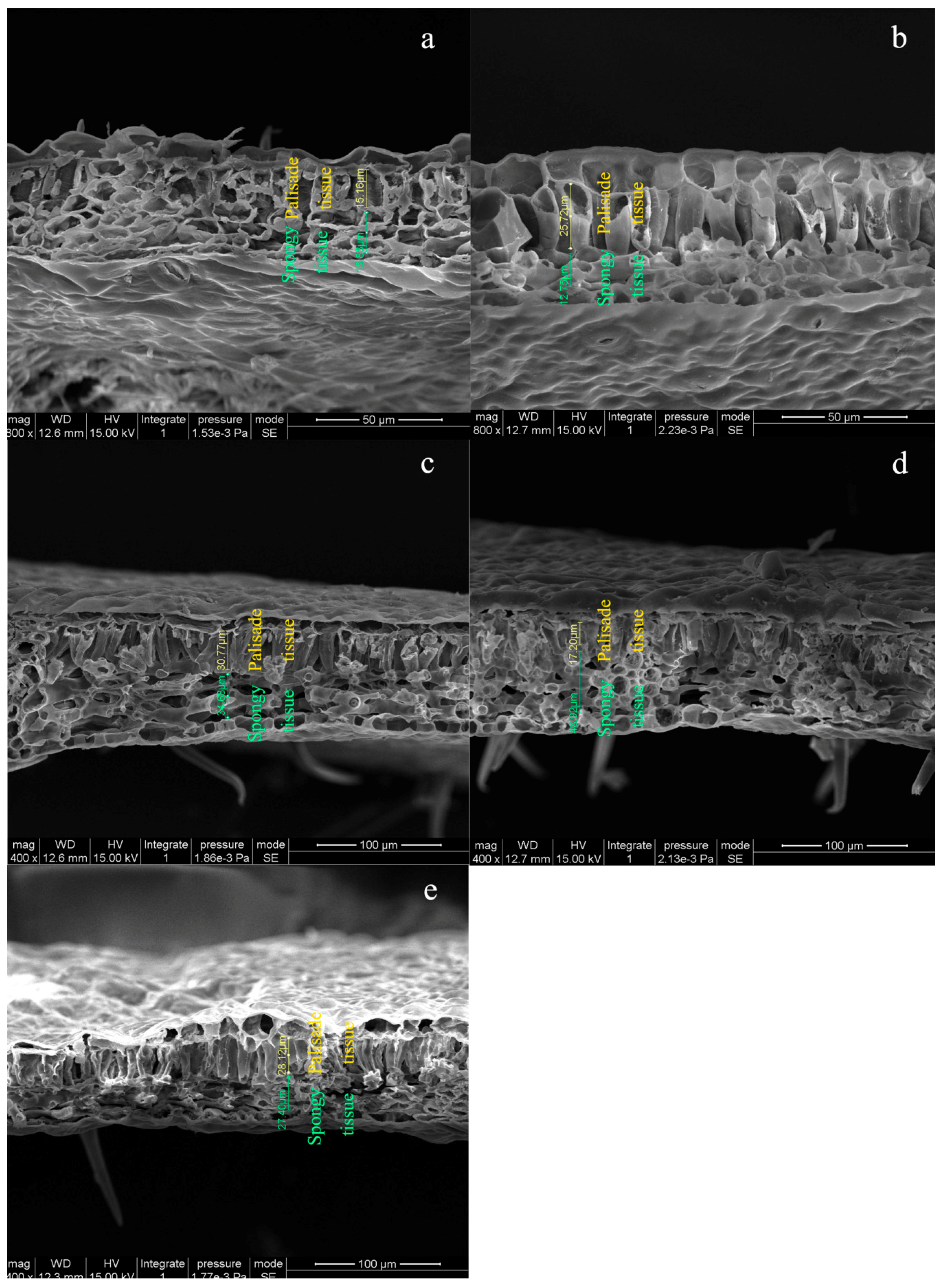
3.4. Leaf Anatomy and Stomatal Morphology in NO2-Treated and Post-Recovery Plants
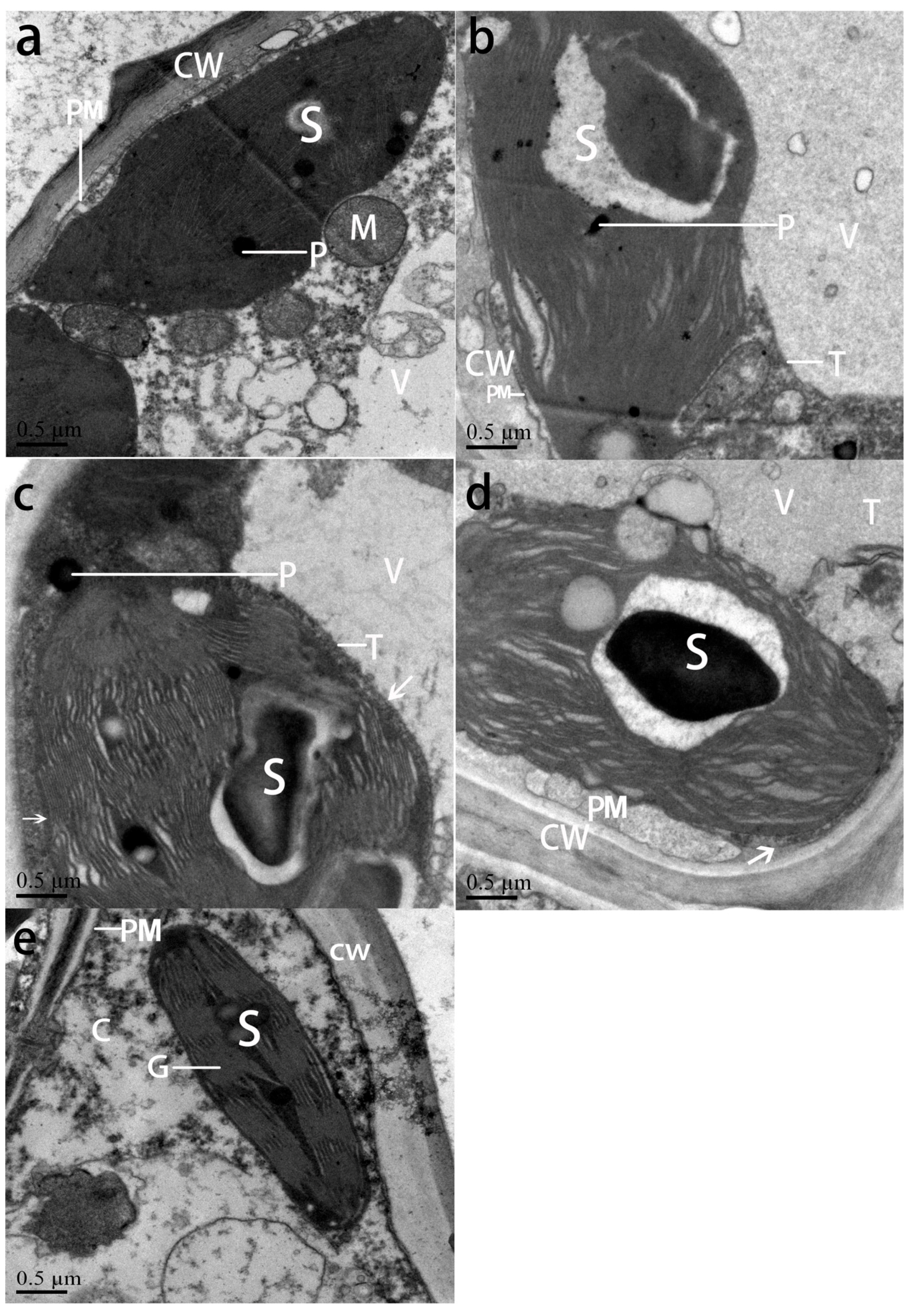
3.5. Ultrastructure of Leaf Chloroplasts in NO2-Treated and Post-Recovery Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahmat, M.; Maulina, W.; Rustami, E.; Azis, M.; Budiarti, D.R.; Seminar, K.B.; Yuwono, A.S.; Alatas, H. Performance in real condition of photonic crystal sensor based NO2 gas monitoring system. Atmos. Environ. 2013, 79, 480–485. [Google Scholar] [CrossRef]
- Bermejo-Orduna, R.; McBride, J.R.; Shiraishi, K.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Biomonitoring of traffic-related nitrogen pollution using Letharia vulpina (L.) Hue in the Sierra Nevada, California. Sci. Total Environ. 2014, 490, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, C.; Meixner, F.X.; Kesselmeier, J. Field investigations of nitrogen dioxide (NO2) exchange between plants and the atmosphere. Atmos. Chem. Phys. 2013, 13, 773–790. [Google Scholar] [CrossRef]
- Lu, M.; Li, Y.J.; Lu, J.P. The study of greening trees on the atmospheric pollutant absorption ability. Urban Environ. Urban Ecol. 2002, 15, 7–9. [Google Scholar]
- Morikawa, H.; Erkin, O.C. Basic processes in phytoremediation and some applications to air pollution control. Chemosphere 2003, 52, 1553–1558. [Google Scholar] [CrossRef]
- Liu, X.F.; Hou, F.; Li, G.K.; Sang, N. Effects of nitrogen dioxide and its acid mist on reactive oxygen species production and antioxidant enzyme activity in Arabidopsis. J. Environ. Sci. 2015, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, W.Q.; Zhang, F.Q.; Gan, X.H.; Wang, M.H.; Li, M.S.; Lin, W.; Peng, Z. Resistance of Rhodoleia championii and other plants to sulfur dioxide and nitrogen dioxide. Ecol. Environ. Sci. 2012, 21, 1851–1858. [Google Scholar]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.Z.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Bellaloui, N.; Tigabu, M.; Wang, J.H.; Diao, J.; Wang, K.; Yang, R.; Sun, G.Y. Gaseous NO2 effects on stomatal behavior, photosynthesis and respiration of hybrid poplar leaves. Acta Physiol. Plant 2015, 37, 39. [Google Scholar] [CrossRef]
- Weber, P.; Rennenberg, H. Dependency of Nitrogen Dioxide (NO2) Fluxes to Wheat (Triticum aestivum L.) Leaves from NO2 Concentration, Light Intensity, temperature and Relative Humidity Determined from Controlled Dynamic Chamber Experiments. Atmos. Environ. 1996, 30, 3001–3009. [Google Scholar] [CrossRef]
- Sparks, J.P. Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 2009, 159, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. Why are atmospheric oxides of nitrogen usually phytotoxic and not alternative fertilizers? New Phytol. 1990, 115, 395–429. [Google Scholar] [CrossRef]
- Huttunen, S.; Soikkeli, S. Effects of various gaseous pollutants on plant cell ultrastructure. In Gaseous Air Pollutants and Plant Metabolism, Proceedings of the 1st International Symposium on Air Pollution and Plant Metabolism, Oxford, UK, 2–5 August 1982; Koziol, M.J., Whatley, F.R., Eds.; Butterworths: London, UK, 1984; pp. 117–127. [Google Scholar]
- Fink, S. Histological and cytological changes caused by air pollutants and other abiotic factors. In Air Pollution and Plant Metabolism, Proceedings of the 2nd International Symposium on Air Pollution and Plant Metabolism, Munich, Germany, 6–9 April 1987; Schulte-Hostede, S., Darrall, N.M., Blank, L.W., Wellburn, A.R., Eds.; Elsevier Applied Science: Barking, UK, 1988; pp. 36–54. [Google Scholar]
- Furukawa, A. Inhibition of Photosynthesis of Populus euramericana and Helianthus annuus by SO2, NO2 and O3. Ecol. Res. 1991, 6, 79–86. [Google Scholar] [CrossRef]
- Schiffgens-Gruber, A.; Lutz, C. Ultrastructure of mesophyll cell chloroplasts of spruce needles exposed to O3, SO2 and NO2 alone and in combination. Environ. Exp. Bot. 1992, 32, 243–254. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Majernik, O.; Wellburn, F.A.M. Effects of SO2 and NO2 Polluted air upon the Ultrastructure of Chloroplasts. Environ. Pollut. 1972, 3, 37–49. [Google Scholar] [CrossRef]
- Chaparro-Suarez, I.G.; Meixner, F.X.; Kesselmeier, J. Nitrogen dioxide (NO2) uptake by vegetation controlled by atmospheric concentrations and plant stomatal aperture. Atmos. Environ. 2011, 45, 5742–5750. [Google Scholar] [CrossRef]
- Li, R.G.; Jiang, Y.G.; Xu, J.; Zhou, B.; Ma, C.; Liu, C.; Yang, C.; Xiao, Y.; Xu, Q.; Hao, L. Synergistic Action of Exogenous Salicylic Acid and Arbuscular Mycorrhizal Fungus Colonization in Avena nuda Seedlings in Response to NO2 Exposure. Bull. Environ. Contam. Toxicol. 2010, 84, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Q.F.; Liu, J.Y.; Zhu, Y.; Hao, L. The preliminary study on exogenous salicylic acid adjusting the response of nitrogen dioxide stress in wheat. J. Shenyang Norm. Univ. (Natl. Sci. Ed.) 2016, 34, 83–87. [Google Scholar]
- Ma, Q.F. The Adjustment of Endogenous Salicylic Acid on NO2 Stress in Arabidopsis; Shenyang Normal University: Shenyang, China, 2016. [Google Scholar]
- Zhang, X.H.; Wang, Z.J.; Li, X.P.; Yu, C.Y.; Chen, Y.P. RAPD analysis of genetic diversity of different progenies from endangered plant Carpinus putoensis. J. Shandong For. Sci. Technol. 2011, 41, 1–5. [Google Scholar]
- Zhong, T.L.; Li, G.Y.; Shi, B.L. Comparison of gas exchange and chlorophyll fluorescence parameters in three endangered species of Zhejiang Province. J. Shanghai Jiaotong Univ. Agric. Sci. 2009, 27, 149–152. [Google Scholar]
- Chen, B.B.; Li, W.P.; Zhou, J.; Wang, A.L. The cloning and analysis of ITS sequences on the four kind of plants of Carpinus genus in Tiantai Mountain. Acta Agric. Zhejiangensis 2011, 23, 1107–1112. [Google Scholar]
- Chen, J.; Wu, F.H.; Liu, T.W.; Chen, L.; Xiao, Q.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Emissions of nitric oxide from 79 plant species in response to simulated nitrogen deposition. Environ. Pollut. 2012, 160, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Xu, X.; Hao, L. The response to NO2 and the regulation of H2O2 in the seedlings of Chinese Cabbage. China’s Agric. Sci. 2007, 40, 2556–2561. [Google Scholar]
- Chen, Z.M.; Chen, Y.X.; Du, G.J.; Wu, X.L.; Li, F. Effects of 60-day NO2 fumigation on growth, oxidative stress and antioxidative response in Cinnamomum camphora seedlings. J. Zhejiang Univ. Sci. B 2010, 11, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Ozone exposure response of beech quanti®ed with a chlorophyll āuores-cence performance index. In Critical Levels for Ozone Ð Level II. Environmental Documentation 115; Fuhrer, J., Achermann, B., Eds.; Swiss Agency for the Environment, Forests and Land-Scape: Bern, Switzerland, 1999; pp. 177–180. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 183–350. [Google Scholar]
- Teng, S.Y.; Chen, Z.M.; Du, G.J. The effect of NO2 exposure on nitrate reductase activity and nitrogen accumulation in Cinnamomum camphora seedlings. Zhejiang For. Sci. Technol. 2010, 20, 70–72. [Google Scholar]
- Hu, Y.; Bellaloui, N.; Sun, G.; Tigabu, M.; Wang, J. Exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide. J. Plant Physiol. 2014, 171, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, J.; Vazquez, M.D.; Poschenrieder, C.H. Structural and ultrastructureal disorders in cadmium-treated bush bean plants (Phaseolous vulgaris L.). New Phytol. 1988, 108, 37–49. [Google Scholar] [CrossRef]
- Beckerson, D.W.; Hofstra, G. Response of leaf diffusive resistance of radish, cucumber and soybean exposed to O3 and SO2 singly or in combination. Atmos. Environ. 1979, 13, 1263–1268. [Google Scholar] [CrossRef]
- Craker, L.E.; Starbuck, J.S. Metabolic injury associated with O3 injury of bean leaves. Can. J. Plant Sci. 1972, 52, 589–597. [Google Scholar] [CrossRef]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 6, 31–43. [Google Scholar] [CrossRef]
- Koziol, M.J.; Whatley, F.R. Gaseous Air Pollutants and Plant Metabolism; Butterworths: London, UK, 1984; pp. 147–168. [Google Scholar]
- Sabaratnam, S.; Gupta, G. Effects of nitrogen dioxide on leaf chlorophyll and nitrogen content of soybean. Environ. Pollut. 1988, 51, 113–120. [Google Scholar] [CrossRef]
- Teklemariam, T.A.; Sparks, J.P. Leaf fluxes of NO and NO2 in four herbaceous plant species: The role of scorbic acid. Atmos. Environ. 2006, 40, 2235–2244. [Google Scholar] [CrossRef]
- Agrawal, M.; Singh, B.; Rajput, M.; Marshall, F.; Bell, J.N.B. Effects of air pollution on peri urban agriculture: A case study. Environ. Pollut. 2003, 126, 323–329. [Google Scholar] [CrossRef]
- Li, D.S.; Sun, X.H.; Li, R.H.; Liu, A.H.; Wang, X.L. Analysis of resistance to sulfur dioxide and nitrate dioxide of economic forest seedling. J. Tianjin Univ. Technol. 2007, 23, 44–46. [Google Scholar]
- Miao, Y.M.; Chen, Z.M.; Chen, Y.F.; Du, G.J. Resistance to and absorbency of gaseous NO2 for 38 young landscaping plants in Zhengjiang Province. J. Zhengjiang Coll. 2008, 25, 765–771. [Google Scholar]
- Dinc, E.; Ceppi, M.G.; Toth, S.Z.; Bottka, S.; Schansker, G. The chl a fluorescence intensity is remarkably insensitive to changes in the chlorophyll content of the leaf as long as the chl a/b ratio remains unaffected. Biochim. Biophys. Acta 1817, 2012, 770–779. [Google Scholar]
- Hsu, B.D.; Leu, K.L. A possible origin of the middle phase of polyphasic chlorophyll fluorescence transient. Funct. Plant Biol. 2003, 30, 571–576. [Google Scholar] [CrossRef]
- Krause, G.H.; Virgo, A.; Winter, K. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta 1995, 197, 583–591. [Google Scholar] [CrossRef]
- Holopainen, T.; Anttonen, S.; Wulff, A.; Palomaki, V.; Kärentampi, L. Comparative evaluation of effects of gaseous pollutants, acidic deposition and mineral deficiencies: Structural changes in the cells of forest plants. Agric. Ecosyst. Environ. 1992, 42, 365–398. [Google Scholar] [CrossRef]
- Grunwald, C. Foliar fatty acids and sterols of soybean field fumigated with SO2. Plant Physiol. 1981, 68, 868–871. [Google Scholar] [CrossRef] [PubMed]

| Chlorophyll a Concentration (mg/g fw) | Chlorophyll b Concentration (mg/g fw) | Chl a/Chl b | Carotenoid Concentration (mg/g fw) | Total Chlorophyll Concentration (mg/g fw) | |
|---|---|---|---|---|---|
| CK | 17.08 ± 1.1 | 7.73 ± 0.6 | 2.21 ± 0.2 | 1.43 ± 0.3 | 24.81 ± 2.1 |
| 1 h | 20.84 ± 5.3 * | 9.95 ± 0.8 * | 2.09 ± 0.1 * | 3.18 ± 0.2 ** | 30.78 ± 3.2 * |
| 6 h | 11.31 ± 1.3 | 4.63 ± 0.2 | 2.44 ± 0.3 | 1.1 ± 0.02 | 15.94 ± 1.2 |
| 24 h | 11.60 ± 1.2 | 5.31 ± 0.5 | 2.18 ± 0.2 | 1.05 ± 0.1 | 16.91 ± 1.2 |
| 72 h | 8.67 ± 0.7 ** | 4.24 ± 0.3 ** | 2.04 ± 0.2 * | 1.91 ± 0.1 ** | 12.92 ± 1.3 ** |
| Recovery | 17.59 ± 1.5 | 7.97 ± 0.5 | 2.27 ± 0.2 | 1.47 ± 0.1 | 25.56 ± 2.3 |
| AQY | Pmax (µmol m−2 s−1) | Rd (µmol m−2 s−1) | LCP (µmol m−2 s−1) | LSP (µmol m−2 s−1) | |
|---|---|---|---|---|---|
| CK | 0.047 ± 0.002 | 13.38 ± 0.781 | −2.34 ± 0.771 | 137.2 ± 36.610 | 1685.6 ± 95.525 |
| 1 h | 0.035 ± 0.001 | 6.44 ± 0.217 | −2.16 ± 0.823 | 64.4 ± 15.609 | 498.4 ± 36.912 |
| 6 h | 0.045 ± 0.002 | 15.26 ± 0.452 | −1.98 ± 0.521 | 151.2 ± 35.431 | 1957.2 ± 97.654 |
| 24 h | 0.031 ± 0.003 | 3.42 ± 0.145 | −1.44 ± 0.433 | 47.6 ± 8.868 | 380.8 ± 14.438 |
| 72 h | 0.033 ± 0.001 | 1.02 ± 0.157 | −0.52 ± 0.217 | 40 ± 10.813 | 403.2 ± 18.652 |
| Recovery | 0.043 ± 0.002 | 14.87 ± 0.521 | −2.34 ± 0.683 | 106.4 ± 24.255 | 1043.5 ± 113.931 |
| Fo | Fm | Fv/Fm | PI | |
|---|---|---|---|---|
| CK | 428.0 ± 34 | 2429.0 ± 164 | 0.824 ± 0.100 | 1.775 ± 0.051 |
| 1 h | 402.5 ± 56 | 2330.6 ± 121 | 0.827 ± 0.125 * | 2.106 ± 0.197 ** |
| 6 h | 542.0 ± 32 | 2442.0 ± 197 | 0.778 ± 0.026 | 0.763 ± 0.098 |
| 24 h | 577.7 ± 38 | 2190.0 ± 176 | 0.736 ± 0.068 | 0.582 ± 0.045 |
| 72 h | 560.0 ± 20 | 2039.0 ± 162 | 0.725 ± 0.057 ** | 0.605 ± 0.031 |
| Recovery | 400 ± 32 | 2180 ± 197 | 0.817 ± 0.069 | 1.667 ± 0.189 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Q.; Zhu, Z. Photosynthetic Capacity, Stomatal Behavior and Chloroplast Ultrastructure in Leaves of the Endangered Plant Carpinus putoensis W.C.Cheng during Gaseous NO2 Exposure and after Recovery. Forests 2018, 9, 561. https://doi.org/10.3390/f9090561
Sheng Q, Zhu Z. Photosynthetic Capacity, Stomatal Behavior and Chloroplast Ultrastructure in Leaves of the Endangered Plant Carpinus putoensis W.C.Cheng during Gaseous NO2 Exposure and after Recovery. Forests. 2018; 9(9):561. https://doi.org/10.3390/f9090561
Chicago/Turabian StyleSheng, Qianqian, and Zunling Zhu. 2018. "Photosynthetic Capacity, Stomatal Behavior and Chloroplast Ultrastructure in Leaves of the Endangered Plant Carpinus putoensis W.C.Cheng during Gaseous NO2 Exposure and after Recovery" Forests 9, no. 9: 561. https://doi.org/10.3390/f9090561




