Landscape Diversity for Reduced Risk of Insect Damage: A Case Study of Spruce Bud Scale in Latvia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Source
2.3. Data Processing
2.4. Statistical Analyses
3. Results
3.1. SBS Distribution in Forest Areas
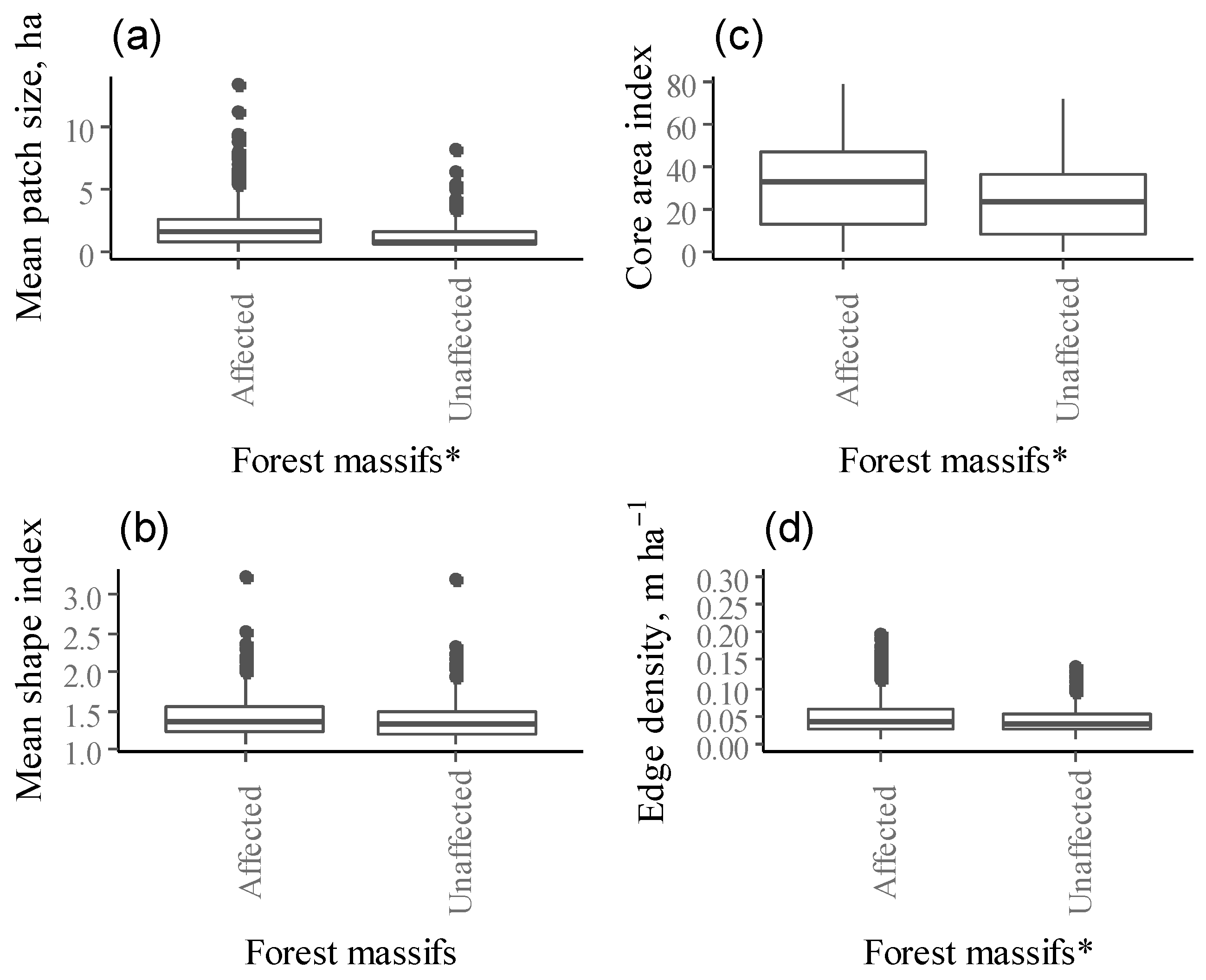
3.2. Habitat Characterisation with Landscape Metrics at Class Level
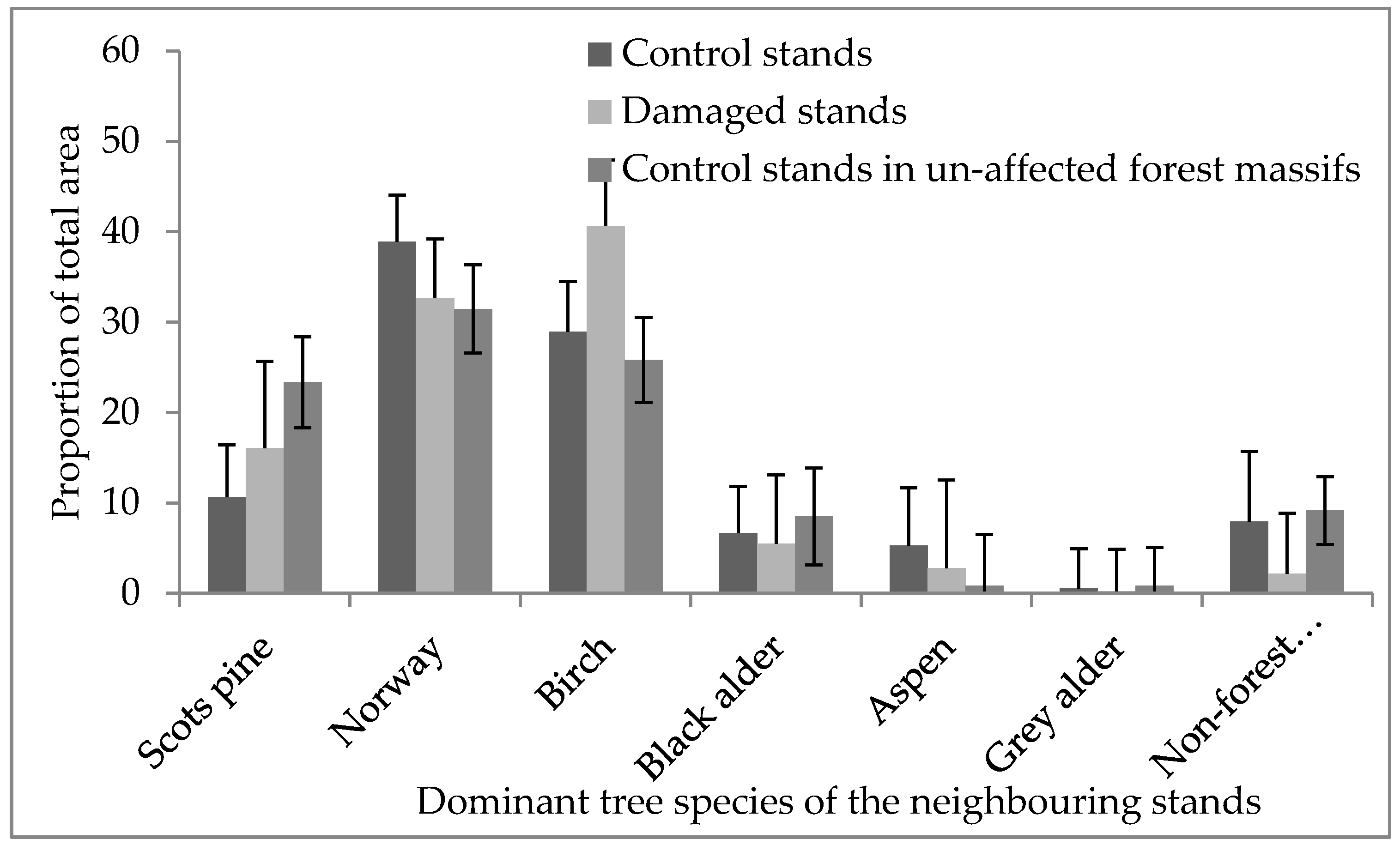
3.3. Spatial Heterogeneity of Pest-Damaged Stands
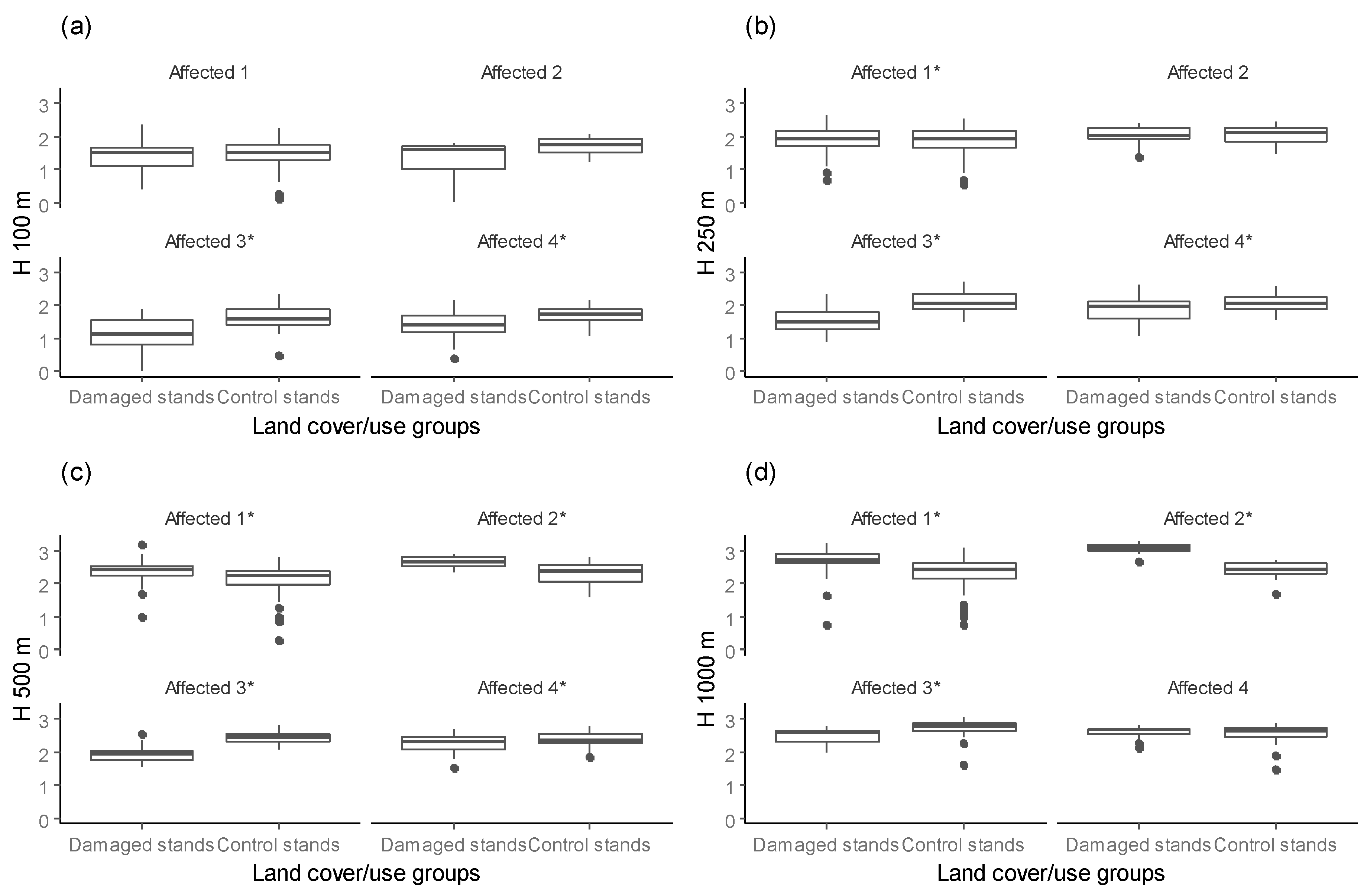
3.4. Habitat Modelling of Spatial Distribution of Predicted Spruce Stand Damage by Spruce Bud Scale
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schelhaas, M.J.; Nabuurs, G.J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Jager, D.; Lexer, M.J. Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. For. Ecol. Manag. 2008, 256, 209–220. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Nöjd, P.; Mielikäinen, K. Climatic signal in annual growth variation in damaged and healthy stands of Norway spruce [Picea abies (L.) Karst.] in Southern Finland. Trees 2001, 15, 177–185. [Google Scholar] [CrossRef]
- Linnakoski, R.; de Beer, Z.W.; Niemelä, P.; Wingfield, M.J. Associations of Conifer-Infesting Bark Beetles and Fungi in Fennoscandia. Insects 2012, 3, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Wermelinger, B. Ecology and management of the spruce bark beetle, Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Hanewinkel, M. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Pouttu, A.; Roininen, H. The influence of windthrow area and timber characteristics on colonization of wind-felled spruces by Ips typographus (L.). For. Ecol. Manag. 2005, 216, 105–116. [Google Scholar] [CrossRef]
- Långström, B.; Lindelöw, Å.; Schroeder, M.; Björklund, N.; Öhrn, P. The spruce bark beetle outbreak in Sweden following the January-storms in 2005 and 2007. In Proceedings of the IUFRO Forest Insect and Disease Survey in Central Europe, Štrbské Pleso, Slovakia, 15–19 September 2008; pp. 13–19. [Google Scholar]
- Gertsson, C.A. A zoogeographical analysis of the scale insect (Hemiptera, Coccoidea) fauna of Fennoscandia and Denmark. Nor. J. Entomol. 2013, 60, 81–89. [Google Scholar]
- Gertsson, C.A.; Isacsson, G. The Hungarian spruce Scale, Physokermes inopinatus Danzig & Kozar (Hemiptera: Coccoidea: Coccidae) in Sweden. Acta Zool. Bulg. 2014, 66, 83–86. [Google Scholar]
- Pellizzari, G.; Dalla Montá, L. 1945–1995: Fifty years of incidental insect pest introductions to Italy. Acta Phytopathol. Entomol. Hung. 1997, 32, 171–183. [Google Scholar]
- Kozár, F. Geographical segregation of scale—Insects (Homoptera: Coccoidea) on fruit trees and the role of host plant ranges. Acta Zool. Acad. Sci. Hung. 1995, 41, 315–325. [Google Scholar]
- Malumphy, C.; Ostrauskas, H.; Pye, D. A provisional Catalogue of scale insects (Hemiptera, Coccoidea) of Lithuania. Acta Zool. Litu. 2008, 18, 108–121. [Google Scholar] [CrossRef]
- Łagowska, B. Scale insects (Homoptera, Coccinea) of Roztocze and the Lublin Upland. Bull. Entomol. Pol. 1986, 56, 475–478. [Google Scholar]
- Graora, D.; Spasić, R.; Mihajlović, L. Bionomy of spruce bud scale, Physokermes piceae (Schrank.) (Hemiptera: Coccidae) in the Belgrade area, Serbia. Arch. Biol. Sci. 2012, 64, 337–343. [Google Scholar] [CrossRef]
- Olsson, P.O.; Jönsson, A.M.; Eklundh, L. A new invasive insect in Sweden—Physokermes inopinatus: Tracing forest damage with satellite based remote sensing. For. Ecol. Manag. 2012, 285, 29–37. [Google Scholar] [CrossRef]
- Winde, I.; Anderbrant, O.; Jönsson, A.M. Tree recovery during the aftermath of an outbreak episode of the Hungarian spruce scale in southern Sweden. Scand. J. For. Res. 2018, 33, 313–319. [Google Scholar] [CrossRef]
- Lazdiņš, A.; Miezite, O.; Bārdule, A. Characterizations of severe damages of spruce (Picea abies Karst.) stands in relation to soil properties. In Proceedings of the Research for Rural Development 2011. Annual 17th International Scientific Conference, Jelgava, LLU, Latvia, May 2011; pp. 22–28. Available online: http://llufb.llu.lv/conference/Research-for-Rural-Development/2011/LatviaResearchRuralDevel17th_volume2.pdf (accessed on 4 September 2018).
- Miezite, O.; Okmanis, M.; Indriksons, A.; Ruba, J.; Polmanis, K.; Freimane, L. Assessment of sanitary condition in stands of Norway spruce (Picea abies Karst.) damaged by spruce bud scale (Physokermes piceae Schrnk.). iForest 2013, 6, 73–78. [Google Scholar] [CrossRef]
- Gedminas, A.; Lynikienë, J.; Marčiulynas, A.; Povilaitienë, A. Effect of Physokermes piceae Schrank. on Shoot and Needle Growth in Norway Spruce stands in Lithuania. Balt. For. 2015, 21, 162–169. [Google Scholar]
- Menkis, A.; Marčiulynas, A.; Gedminas, A.; Lynikienė, J.; Povilaitienė, A. High-Throughput Sequencing Reveals Drastic Changes in Fungal Communities in the Phyllosphere of Norway Spruce (Picea abies) Following Invasion of the spruce Bud Scale (Physokermes piceae). Microb. Ecol. 2015, 70, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Gertsson, C.A.; Isacsson, G. Spruce-bud Scales (Hemiptera, Coccoidea, genus Physokermes) in south Sweden. Entomol. Tidskr. 2012, 133, 121–128, (In Swedish, English summary). [Google Scholar]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, E.J. Quantifying landscape spatial pattern: What is the state of the art? Ecosystems 1998, 1, 143–156. [Google Scholar] [CrossRef]
- Turner, M.G.; Gardner, R.H.; O’Neill, R.V. Landscape disturbance dynamics. In Landscape Ecology in Theory and Practice; Springer: New York, NY, USA, 2001; pp. 157–199. ISBN 978-0-387-21694-2. [Google Scholar]
- Kuuluvainen, T. Natural variability of forests as a reference for restoring and managing biological diversity in boreal Fennoscandia. Silva Fenn. 2002, 36, 97–125. [Google Scholar] [CrossRef]
- Turner, M.G.; O’Neill, R.V.; Gardner, R.H.; Milne, B.T. Effects of changing spatial scale on the analysis of landscape pattern. Landsc. Ecol. 1989, 3, 153–162. [Google Scholar] [CrossRef]
- Peterson, C.J.; Pickett, S.T. Patch type influences on regeneration in a western Pennsylvania, USA, catastrophic windthrow. Oikos 2000, 90, 489–500. [Google Scholar] [CrossRef]
- Hunter, M.D. Landscape structure, habitat fragmentation, and the ecology of insects. Agric. For. Entomol. 2002, 4, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stohlgren, T.J.; Chong, G.W. Spatial heterogeneity influences native and nonnative plant species richness. Ecology 2006, 87, 3186–3199. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Analysis Program for Quantifying Landscape Structure; USDA Technical Report PNWGTR-351 for Forest Service General; USDA Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995. [Google Scholar]
- Hernandez-Stefanoni, J.L. Relationships between landscape patterns and species richness of trees shrubs and vines in a tropical forest. Plant Ecol. 2005, 179, 53–65. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E.; Duelli, P. A test of the biodiversity-stability theory: Meta-analysis of tree species diversity effects on insect pest infestations, re-examination of responsible factors. In Forest Diversity and Function: Temperate and Boreal Systems; Scherer-Lorenzen, M., Körner, C., Schulze, E.D., Eds.; Springer: Berlin, Germany, 2005; pp. 235–262. ISBN 978-3-540-22191-3. [Google Scholar]
- Johnstone, J.F.; McIntire, E.J.B.; Pedersen, E.J.; King, G.; Pisaric, M.J.F. A sensitive slope: Estimating landscape patterns of forest resilience in a changing climate. Ecosphere 2010, 1, 1–21. [Google Scholar] [CrossRef]
- Su, Q.; McLean, D.A.; Needham, T.D. The influence of hardwood content on balsam fir defoliation by spruce budworm. Can. J. For. Res. 1996, 26, 1620–1628. [Google Scholar] [CrossRef]
- Jäkel, A.; Roth, M. Conversion of single-layered Scots pine monocultures into close-to-nature mixed hardwood forests: Effects on parasitoid wasps as pest antagonists. Eur. J. For. Res. 2004, 123, 203–212. [Google Scholar] [CrossRef]
- Candau, J.N.; Fleming, R.A. Landscape-scale spatial distribution of spruce budworm defoliation in relation to bioclimatic conditions. Can. J. For. Res. 2005, 35, 2218–2232. [Google Scholar] [CrossRef]
- Lizuma, L.; Kļaviņš, M.; Briede, A.; Rodinovs, V. Long-term changes of air temperature in Latvia. In Climate Change in Latvia; Kļaviņš, M., Ed.; UL Publishing House: Riga, Latvia, 2007; pp. 11–20. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; 97p, ISBN 92-5-105521-1. [Google Scholar]
- Smits, A.; (Latvian State Forest Research Institue “Silava”, Salaspils, Latvia). Personal communication, 2018.
- Buja, K.; Menza, C. Sampling Design Tool for ArcGIS; National Oceanic and Atmospheric Administration Biogeography Branch, Silver Spring: Montgomery County, MD, USA, 2013. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2014. [Google Scholar]
- Rempel, R.S.; Carr, A.; Elkie, P. Patch Analyst and Patch Analyst (Grid) Function Reference; Centre for Northern Forest Ecosystem Research, Ontario Ministry of Natural Resources, Lakehead University: Thunder Bay, ON, Canada, 1999. [Google Scholar]
- Radeloff, V.C.; Mladenoff, D.J.; Boyce, M.S. The changing relation of landscape patterns and jack pine budworm populations during an outbreak. Oikos 2000, 90, 417–430. [Google Scholar] [CrossRef]
- Uuemaa, E.; Antrop, M.; Roosaare, J.; Marja, R.; Mander, Ü. Landscape metrics and indices: An overview of their use in landscape research. Living Rev. Landsc. Res. 2009, 3, 1–28. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume, F.; Kindt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L. Vegan: Community Ecology Package Version 1.17-6, 2011. Available online: http://CRAN.R-project.org/package=vegan (accessed on 4 September 2018).
- Mitchell, A. The ESRI Guide to GIS Analysis, 2nd ed.; ESRI Press: Redlands, CA, USA, 2005; p. 252. ISBN 9781589481169. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bürkner, P.-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Szaro, R.C.; Johnson, D.W. Biodiversity in Managed Landscapes: Theory and Practice; Oxford University Press: New York, NY, USA, 1996; ISBN 978-0195079586. [Google Scholar]
- Pasitschniak alk-Arts, M.; Clark, R.G.; Messier, F. Duck nesting success in a fragmented prairie landscape: Is edge effect important? Biol. Conserv. 1998, 85, 55–62. [Google Scholar] [CrossRef]
- Kindlmann, P.; Burel, F. Connectivity measures: A review. Landsc. Ecol. 2008, 23, 879–890. [Google Scholar] [CrossRef]
- Marčiulynas, A. Biology and Significance of the spruce Bud Scale (Physokermes Piceae Schrank.) to Sanitary Condition of Norway Spruce (Picea Abies (L.) H. Karst.) in Lithuania. Ph.D. Thesis, Alexander Stulginski University, Kaunas, Lithuania, March 2016. [Google Scholar]
- Cappuccino, N.; Lavertu, D.; Bergeron, Y.; Regniere, J. Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 1998, 114, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Turguter, S.; Ülgentürk, S. Physokermes piceae (Schrank) (Yumrulu Ladin Koşnili) (Hemiptera: Coccidae)’nin Biyolojik Özellikleri. Tarim Bilim. Derg. 2006, 12, 44–50. [Google Scholar] [CrossRef]
- Filgueiras, B.K.C.; Tabarelli, M.; Leal, I.R.; Vaz-de-Mello, F.Z.; Peres, C.A.; Iannuzzi, L. Spatial replacement of dung beetles in edge-affected habitats: Biotic homogenization or divergence in fragmented tropical forest landscapes? Divers. Distrib. 2016, 22, 400–409. [Google Scholar] [CrossRef]
- Berry, J.K. Map Analysis: Understanding Spatial Patterns and Relationships; GeoTec Media: San Francisco, CA, USA, 2007; 224p, ISBN 9780974861319. [Google Scholar]
- Li, H.; Reynolds, J.F. A Simulation Experiment to Quantify Spatial Heterogeneity in Categorical Maps Habin. Ecology 1994, 75, 2446–2455. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; W.H. Freeman and Company: New York, NY, USA, 1983; 468p, ISBN 9780716711865. [Google Scholar]
- Setiawan, N.N.; Vanhellemont, M.; Baeten, L.; Dillen, M.; Verheyen, K. The effects of local neighbourhood diversity on pest and disease damage of trees in a young experimental forest. For. Ecol. Manag. 2014, 334, 1–9. [Google Scholar] [CrossRef]
- LaGro, J., Jr. Assessing patch shape in landscape mosaics. Photogramm. Eng. Remote Sens. 1991, 57, 285–293. [Google Scholar]
- Wiens, J.A.; Schooely, R.L.; Weeks, R.D., Jr. Patchy landscapes and animal movements: Do beetles per- colate? Oikos 1997, 78, 257–264. [Google Scholar] [CrossRef]
- Bergeron, Y.; Leduc, A.; Morin, H.; Joyal, C. Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can. J. For. Res. 1995, 25, 1375–1384. [Google Scholar] [CrossRef]
- Kosztarab, M.; Kozár, F. Introduction of Anthribus nebulosus (Coleoptera: Anthribidae) in Virginia control of scale insects. Va. J. Sci. 1983, 34, 223–236. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995; 436p, ISBN 9780521499521. [Google Scholar]
- Oxbrough, A.; Irwin, S.; Kelly, T.C.; O’Halloran, J. Ground-dwelling invertebrates in reforested conifer plantations. For. Ecol. Manag. 2010, 259, 2111–2121. [Google Scholar] [CrossRef] [Green Version]



| Defined Forest Land Cover/Use Categories | Description |
|---|---|
| Damaged stands | Norway spruce stands where sanitary clear-cut occurred after SBS infestation in 2010 |
| Scots pine pure stands 1 | Scots pine pure stands |
| Scots pine mixed woodland 2 | Scots pine mixed with Norway spruce, birch or aspen |
| Norway spruce control stands 3 | Norway spruce pure stands from 40 to 70 years in age |
| Norway spruce mixed woodland | Norway spruce mixed with scots pine, birch, black alder, grey alder, aspen or ash |
| Norway spruce pure stands | Norway spruce pure stands younger than 40 years or older than 70 years |
| Non-forest land | Agricultural land, farmland, the buffer area outside the boundaries of the forest massif |
| Soft broadleaves pure stands | Pure birch, black alder, grey alder or aspen stands |
| Soft broadleaves mixed woodland | Mixed birch, black alder, grey alder or aspen stands |
| Hard broadleaves stands | Common oak, ash, wych elm, linden or maple stands |
| Other damages | Any species stands damaged by windthrows, snow damages, other insect outbreaks or browsing damages |
| Infrastructure | Infrastructure such as roads, forest roads, power lines, buildings or crossrides |
| Glade | A forest opening of less than 0.2 ha |
| Wetlands | Includes mangroves, rivers, lakes, ditches or seasonally inundated areas |
| Clear-cut | Any species stands felled in a clear-cut |
| Bogs | Low, transition or high swamps |
| Row Labels | Affected 1 | Affected 2 | Affected 3 | Affected 4 | Unaffected 1 | Unaffected 2 |
|---|---|---|---|---|---|---|
| Other damages | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Bogs | 0.1 | 2.4 | 0.5 | 11.3 | 2.0 | 2.9 |
| Wetlands | 0.4 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 |
| Infrastructure | 0.4 | 0.8 | 0.6 | 0.6 | 1.5 | 2.6 |
| Hard broadleaves | 0.4 | 0.1 | 0.9 | 0.5 | 0.3 | 0.1 |
| Selected Norway spruce control stands * | 1.0 | 0.2 | 0.5 | 1.0 | 1.6 | 1.6 |
| Damaged stands ** | 1.4 | 0.2 | 0.7 | 1.3 | 0.0 | 0.0 |
| Glade | 1.5 | 1.3 | 1.1 | 0.9 | 0.8 | 1.2 |
| Non-forest | 1.9 | 5.2 | 2.6 | 2.8 | 1.9 | 2.2 |
| Scots pine mixed woodland | 2.4 | 3.1 | 1.8 | 2.6 | 4.2 | 4.9 |
| Clear-cut | 4.5 | 4.3 | 5.0 | 2.6 | 3.0 | 4.7 |
| Norway spruce mixed woodland | 5.1 | 5.8 | 4.1 | 3.7 | 4.4 | 3.9 |
| Norway spruce Target stands | 7.3 | 9.1 | 13.0 | 6.8 | 9.9 | 8.5 |
| Norway spruce stands (Non-target stands) | 8.1 | 9.7 | 6.4 | 9.4 | 4.7 | 4.5 |
| Soft broadleaves | 9.6 | 9.7 | 13.9 | 10.5 | 10.4 | 7.1 |
| Scots pine pure stands | 18.5 | 10.6 | 12.0 | 16.7 | 28.4 | 29.0 |
| Soft broadleaves mixed woodland | 37.4 | 37.4 | 36.6 | 28.9 | 26.6 | 26.7 |
| Parameter | Estimate | Est. Error | l-95% CI | u-95% CI |
|---|---|---|---|---|
| (Intercept) | 2.14 | 5.81 | –9.09 | 14.19 |
| H 100 | −4.24 | 1.58 | –7.86 | −1.72 |
| H 1000 | 5.82 | 2.09 | 2.15 | 10.32 |
| MSI | −0.57 | 1.10 | –2.80 | 1.64 |
| MPS | −0.03 | 1.33 | –2.83 | 2.50 |
| Core area | 2.53 | 1.63 | −0.24 | 6.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bāders, E.; Jansons, Ā.; Matisons, R.; Elferts, D.; Desaine, I. Landscape Diversity for Reduced Risk of Insect Damage: A Case Study of Spruce Bud Scale in Latvia. Forests 2018, 9, 545. https://doi.org/10.3390/f9090545
Bāders E, Jansons Ā, Matisons R, Elferts D, Desaine I. Landscape Diversity for Reduced Risk of Insect Damage: A Case Study of Spruce Bud Scale in Latvia. Forests. 2018; 9(9):545. https://doi.org/10.3390/f9090545
Chicago/Turabian StyleBāders, Endijs, Āris Jansons, Roberts Matisons, Didzis Elferts, and Iveta Desaine. 2018. "Landscape Diversity for Reduced Risk of Insect Damage: A Case Study of Spruce Bud Scale in Latvia" Forests 9, no. 9: 545. https://doi.org/10.3390/f9090545







