The Short-Term Effects of Experimental Forestry Treatments on Site Conditions in an Oak–Hornbeam Forest
Abstract
:1. Introduction
2. Materials and Methods
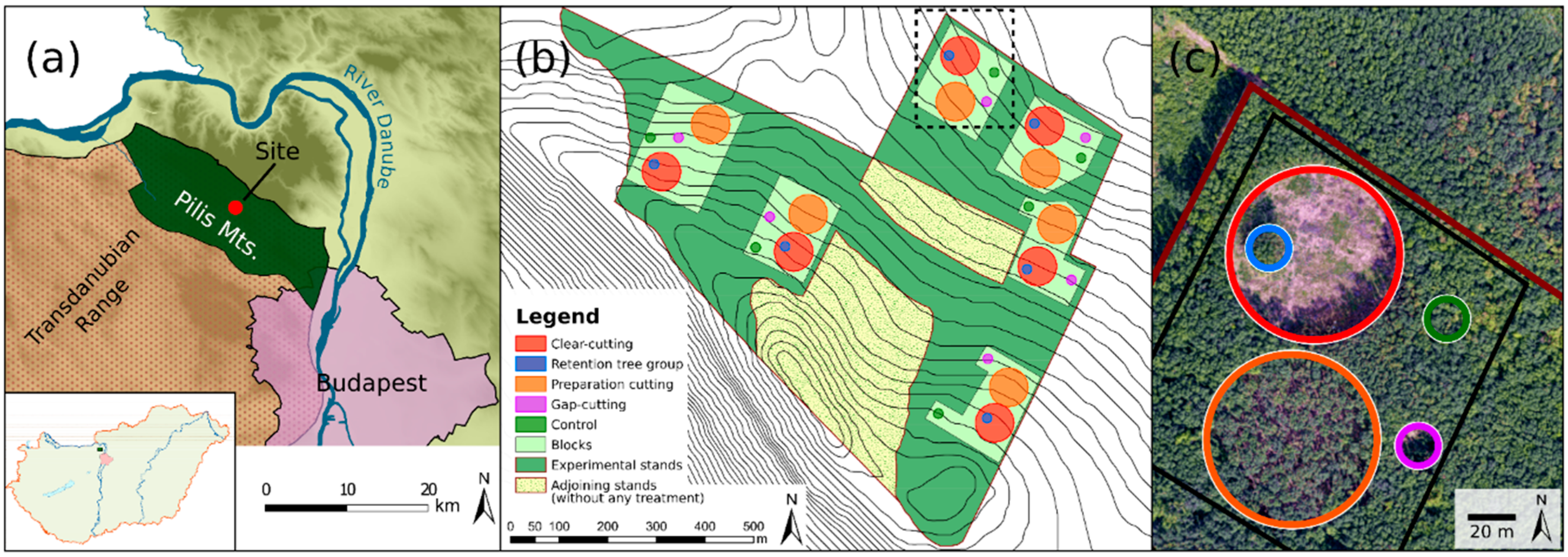
2.1. Study Area
2.2. Study Design
- Control (C): The original stand characteristics remained unaltered.
- Clear-cutting (CC): Approximately 0.5 ha sized circular clear-cuts were formed, surrounded by a closed-canopy stand. The area of the treatment was designated as the area surrounded by the trunks of the peripheral dominant forest trees, the applied diameter was 80 m. Within the clear-cuts, every tree individual (DBH ≥5 cm and/or height ≥2 m) was cut.
- Gap-cutting (G): Circular artificial gaps were established in the closed stand by the elimination of all of the tree individuals within a diameter of 20 m (~0.03 ha). Gap size was defined as expanded gaps [54] (i.e., by measuring the base of surrounding canopy trees). The chosen 1:1 gap diameter/intact canopy height ratio is widely used in Central Europe for transition system applying gap-cutting, and it also fits well with the records of gap area in oak forests [55,56].
- Preparation cutting (P): Uniform partial cutting was applied within a circle with a diameter of 80 m, and 30% of the initial total basal area of the upper canopy layer was cut in a spatially even arrangement. Furthermore, the complete subcanopy- and shrub-layer were also removed.
- Retention tree group (R): All of the tree and shrub individuals were retained within a 0.03 ha sized circular plot (diameter = 20 m) in the clear-cuts, which resulted a small patch of the remained stand with approximately 8–12 trees of the former upper layer.
2.3. Data Collection
2.4. Data Analysis
3. Results
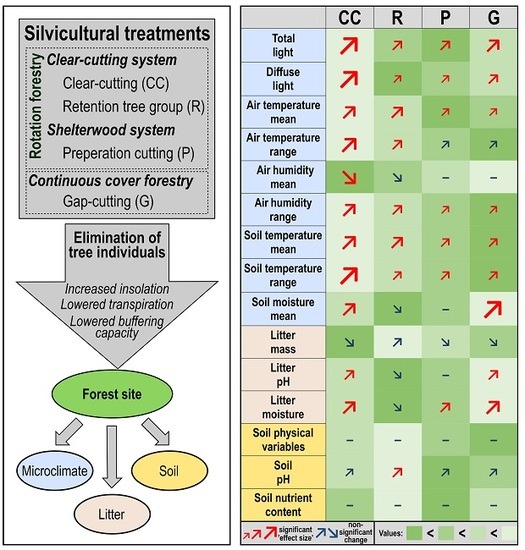
3.1. The Effects of Experimental Treatments on Site Condition Variables
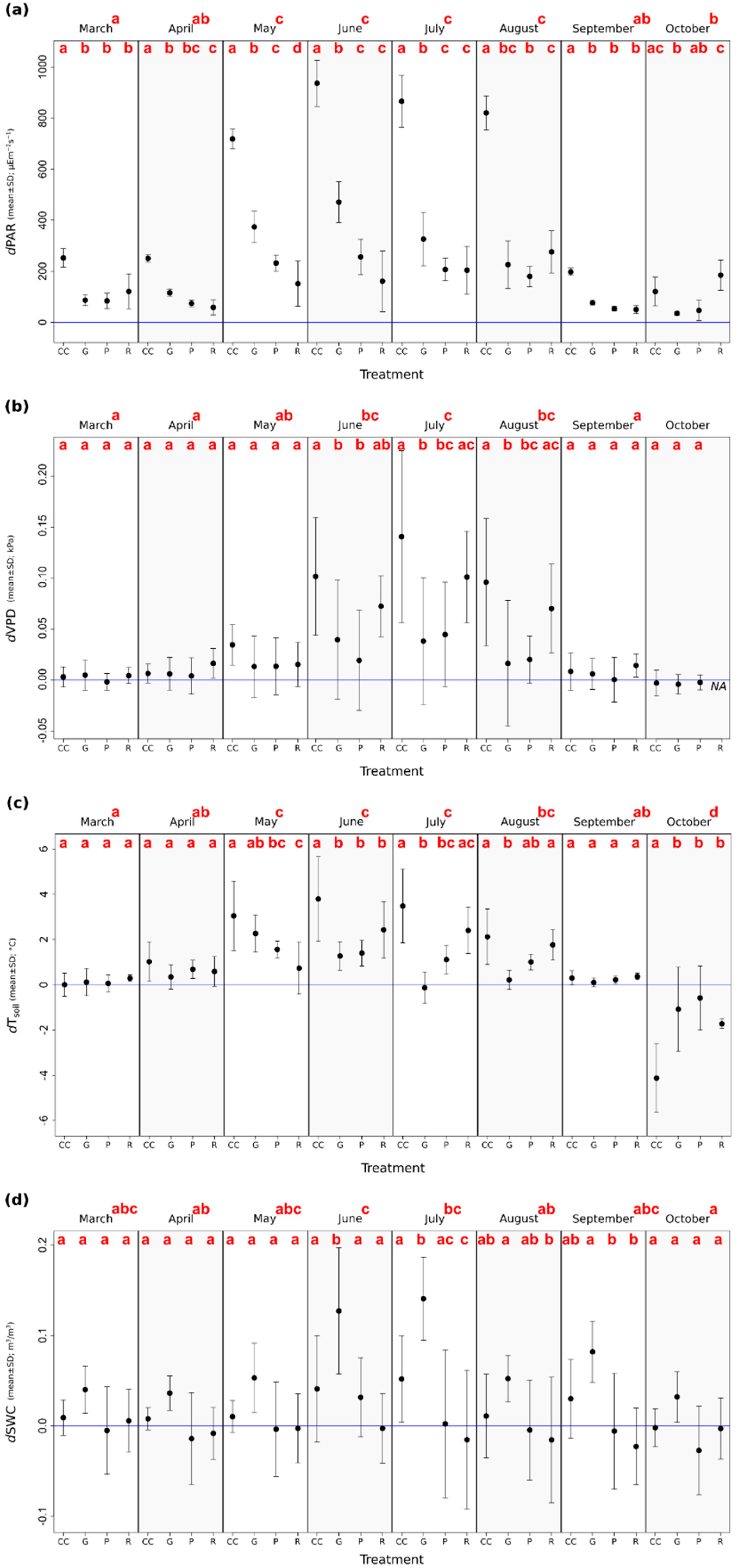
3.2. Temporal Differences among Treatments through the Growing Season
3.3. Diurnal Pattern of Microclimate Variables among the Treatments
4. Discussion
4.1. Rapid Changes in Microclimate and Litter Variables, but Not in Soil Properties
4.1.1. Light Variables
4.1.2. Air Variables
4.1.3. Soil Temperature
4.1.4. Soil Moisture
4.1.5. Litter Variables
4.1.6. Soil Chemical Variables
4.2. Distinct Temporal Patterns over the First Growing Season
4.3. Diurnal Patterns across Treatments Differed More during the Vegetation Peak
5. Conclusions and Management Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edwards, D.P.; Tobias, J.A.; Sheil, D.; Meijaard, E.; Laurance, W.F. Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 2014, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jackson, R.B. Biophysical forcings of land-use changes from potential forestry activities in North America. Ecol. Monogr. 2014, 84, 329–353. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.; Burivalova, Z.; Koh, L.P.; Hellweg, S. Impact of Forest Management on Species Richness: Global Meta-Analysis and Economic Trade-Offs. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, A.; Lõhmus, P. Epiphyte communities on the trunks of retention trees stabilise in 5 years after timber harvesting, but remain threatened due to tree loss. Biol. Conserv. 2010, 143, 891–898. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of dead-wood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Ibarra, J.T.; Martin, M.; Cockle, K.L.; Martin, K. Maintaining ecosystem resilience: Functional responses of tree cavity nesters to logging in temperate forests of the Americas. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, J.; Song, B.; Xu, M.; Sneed, P.; Jensen, R. Effects of silvicultural treatments on summer forest microclimate in southeastern Missouri Ozarks. Clim. Res. 2000, 15, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2005, 81, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Thiffault, E.; Hannam, K.D.; Paré, D.; Titus, B.D.; Hazlett, P.W.; Maynard, D.G.; Brais, S. Effects of forest biomass harvesting on soil productivity in boreal and temperate forests—A review. Environ. Rev. 2011, 19, 278–309. [Google Scholar] [CrossRef]
- Kishchuk, B.E.; Quideau, S.; Wang, Y.; Prescott, C. Long-term soil response to variable-retention harvesting in the EMEND (Ecosystem Management Emulating Natural Disturbance) experiment, northwestern Alberta. Can. J. Soil Sci. 2014, 94, 263–279. [Google Scholar] [CrossRef]
- Frey, S.J.K.; Hadley, A.S.; Johnson, S.L.; Schulze, M.; Jones, J.A.; Betts, M.G. Spatial models reveal the microclimatic buffering capacity of old-growth forests. Sci. Adv. 2016, 2, e1501392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibodeau, L.; Raymond, P.; Camiré, C.; Munson, A.D. Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics, microbial biomass, decomposition, and foliar nutrition. Can. J. For. Res. 2000, 30, 229–238. [Google Scholar] [CrossRef]
- Knapp, B.O.; Olson, M.G.; Larsen, D.R.; Kabrick, J.M.; Jensen, R.G. Missouri Ozark Forest Ecosystem Project: A Long-Term, Landscape-Scale, Collaborative Forest Management Research Project. J. For. 2014, 112, 513–524. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Gillingham, P.K.; Hill, J.K.; Huntley, B.; Kunin, W.E.; Roy, D.B.; Thomas, C.D. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 2011, 120, 1–8. [Google Scholar] [CrossRef]
- Latimer, C.E.; Zuckerberg, B. Forest fragmentation alters winter microclimates and microrefugia in human-modified landscapes. Ecography 2017, 40, 158–170. [Google Scholar] [CrossRef]
- Lenoir, J.; Hattab, T.; Pierre, G. Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 2017, 40, 253–266. [Google Scholar] [CrossRef]
- Greiser, C.; Meineri, E.; Luoto, M.; Ehrlén, J.; Hylander, K. Monthly microclimate models in a managed boreal forest landscape. Agric. For. Meteorol. 2018, 250–251, 147–158. [Google Scholar] [CrossRef]
- Geiger, R.; Aron, R.H.; Todhunter, P. The Climate near the Ground; Vieweg+Teubner Verlag: Wiesbaden, Germany, 1995; ISBN 978-3-322-86584-7. [Google Scholar]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in Forest Ecosystem and Landscape Ecology. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Von Arx, G.; Dobbertin, M.; Rebetez, M. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agric. For. Meteorol. 2012, 166–167, 144–155. [Google Scholar] [CrossRef]
- Hardwick, S.R.; Toumi, R.; Pfeifer, M.; Turner, E.C.; Nilus, R.; Ewers, R.M. The relationship between leaf area index and microclimate in tropical forest and oil palm plantation: Forest disturbance drives changes in microclimate. Agric. For. Meteorol. 2015, 201, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, T.; Horna, V.; Leuschner, C. Canopy transpiration of pure and mixed forest stands with variable abundance of European beech. J. Hydrol. 2012, 442–443, 2–14. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Schall, P.; Ammer, C.; Seidel, D. Quantifying stand structural complexity and its relationship with forest management, tree species diversity and microclimate. Agric. For. Meteorol. 2017, 242, 1–9. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Song, Q.; Fu, P.; Cleverly, J.; Magliulo, V.; Law, B.E.; Gough, C.M.; Hörtnagl, L.; Di Gennaro, F.; et al. Quantifying deforestation and forest degradation with thermal response. Sci. Total Environ. 2017, 607–608, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, G.; Graf Pannatier, E.; Thimonier, A.; Rebetez, M. Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J. Ecol. 2013, 101, 1201–1213. [Google Scholar] [CrossRef]
- Kovács, B.; Tinya, F.; Ódor, P. Stand structural drivers of microclimate in mature temperate mixed forests. Agric. For. Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef]
- Ogée, J.; Brunet, Y. A forest floor model for heat and moisture including a litter layer. J. Hydrol. 2002, 255, 212–233. [Google Scholar] [CrossRef]
- Bicknell, J.E.; Struebig, M.J.; Edwards, D.P.; Davies, Z.G. Improved timber harvest techniques maintain biodiversity in tropical forests. Curr. Biol. 2014, 24, R1119–R1120. [Google Scholar] [CrossRef] [PubMed]
- Dieler, J.; Uhl, E.; Biber, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. Eur. J. For. Res. 2017, 136, 739–766. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Keenan, R.J.; Kimmins, J.P. The ecological effects of clear-cutting. Environ. Rev. 1993, 1, 121–144. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Marshall, V.G. Impacts of forest harvesting on biological processes in northern forest soils. For. Ecol. Manag. 2000, 133, 43–60. [Google Scholar] [CrossRef]
- Carlson, D.W.; Groot, A. Microclimate of clear-cut, forest interior, and small openings in trembling aspen forest. Agric. For. Meteorol. 1997, 87, 313–329. [Google Scholar] [CrossRef]
- Heithecker, T.D.; Halpern, C.B. Variation in microclimate associated with dispersed-retention harvests in coniferous forests of western Washington. For. Ecol. Manag. 2006, 226, 60–71. [Google Scholar] [CrossRef]
- Ryu, S.-R.; Concilio, A.; Chen, J.; North, M.; Ma, S. Prescribed burning and mechanical thinning effects on belowground conditions and soil respiration in a mixed-conifer forest, California. For. Ecol. Manag. 2009, 257, 1324–1332. [Google Scholar] [CrossRef]
- Bigelow, S.W.; North, M.P. Microclimate effects of fuels-reduction and group-selection silviculture: Implications for fire behavior in Sierran mixed-conifer forests. For. Ecol. Manag. 2012, 264, 51–59. [Google Scholar] [CrossRef]
- Coulombe, D.; Sirois, L.; Paré, D. Effect of harvest gap formation and thinning on soil nitrogen cycling at the boreal–temperate interface. Can. J. For. Res. 2017, 47, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Bernes, C.; Jonsson, B.G.; Junninen, K.; Lõhmus, A.; Macdonald, E.; Müller, J.; Sandström, J. What is the impact of active management on biodiversity in boreal and temperate forests set aside for conservation or restoration? A systematic map. Environ. Evid. 2015, 4. [Google Scholar] [CrossRef]
- Gálhidy, L.; Mihók, B.; Hagyó, A.; Rajkai, K.; Standovár, T. Effects of gap size and associated changes in light and soil moisture on the understorey vegetation of a Hungarian beech forest. Plant Ecol. 2006, 183, 133–145. [Google Scholar] [CrossRef]
- Weng, S.-H.; Kuo, S.-R.; Guan, B.T.; Chang, T.-Y.; Hsu, H.-W.; Shen, C.-W. Microclimatic responses to different thinning intensities in a Japanese cedar plantation of northern Taiwan. For. Ecol. Manag. 2007, 241, 91–100. [Google Scholar] [CrossRef]
- Rambo, T.R.; North, M.P. Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest. For. Ecol. Manag. 2009, 257, 435–442. [Google Scholar] [CrossRef]
- Ma, S.; Concilio, A.; Oakley, B.; North, M.; Chen, J. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For. Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Jerabkova, L.; Prescott, C.E.; Titus, B.D.; Hope, G.D.; Walters, M.B. A meta-analysis of the effects of clearcut and variable-retention harvesting on soil nitrogen fluxes in boreal and temperate forests. Can. J. For. Res. 2011, 41, 1852–1870. [Google Scholar] [CrossRef]
- Matthews, J.D. Silvicultural Systems; Oxford Science Publications; Clarendon Press: Oxford, UK, 1991; ISBN 978-0-19-854670-2. [Google Scholar]
- Effect of Forestry Treatments on Forest Site, Regeneration and Biodiversity. An Experimental Study. (Project Website). Available online: http://piliskiserlet.okologia.mta.hu/en (accessed on 4 July 2018).
- Bölöni, J.; Molnár, Z.; Biró, M.; Horváth, F. Distribution of the (semi-)natural habitats in Hungary II. Woodlands and shrublands. Acta Bot. Hung. 2008, 50, 107–148. [Google Scholar] [CrossRef] [Green Version]
- Brus, D.J.; Hengeveld, G.M.; Walvoort, D.J.J.; Goedhart, P.W.; Heidema, A.H.; Nabuurs, G.J.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2012, 131, 145–157. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef] [PubMed]
- Dövényi, Z. (Ed.) Magyarország Kistájainak Katasztere [Cadastre of Hungarian Regions]; MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; ISBN 978-963-9545-29-8. [Google Scholar]
- Krasilnikov, P.; Marti, J.-J.I.; Arnold, R.; Shoba, S. (Eds.) A Handbook of Soil Terminology, Correlation and Classification; Earthscan: London, UK; Sterling, VA, USA, 2009; ISBN 978-1-84977-435-2. [Google Scholar]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. 1992, L206, 7–50. [Google Scholar]
- Runkle, J.R. Thirty-two years of change in an old-growth Ohio beech-maple forest. Ecology 2013, 94, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Wijdeven, S.; van Hees, A. Natural gaps in beech forests in The Netherlands, North-West Germany, Belgium and France. In Natural Canopy Gap Characteristics in European Beech Forests; Mountford, P., Ed.; Nat-Man Working Report 2; Forest & Landscape Denmark: Copenhagen, Denmark, 2001; p. 29. [Google Scholar]
- Dey, D.C. The ecological basis for oak silviculture in eastern North America. In Oak Forest Ecosystems: Ecology and Management for Wildlife; McShea, W.J., Healy, W.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 60–79. ISBN 978-0-8018-6745-3. [Google Scholar]
- Évi LVI. törvény az erdőről, az erdő védelméről és az erdőgazdálkodásról szóló 2009. évi XXXVII. törvény és egyéb kapcsolódó törvények módosításáról [Act No. LVI of 2017 on forests, on the protection and management of forests]. Magy. Közlöny 2017, 75, 7752–7796. Available online: http://www.kozlonyok.hu/nkonline/MKPDF/hiteles/MK17075.pdf (accessed on 5 June 2018).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and Drainage Paper No. 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Anderson, D.B. Relative Humidity or Vapor Pressure Deficit. Ecology 1936, 17, 277–282. [Google Scholar] [CrossRef]
- Tinya, F.; Mihók, B.; Márialigeti, S.; Mag, Z.; Ódor, P. A comparison of three indirect methods for estimating understory light at different spatial scales in temperate mixed forests. Community Ecol. 2009, 10, 81–90. [Google Scholar] [CrossRef]
- LI-COR Inc. LAI-2000 Plant Canopy Analyzer: Instruction Manual; LI-COR Inc.: Lincoln, NE, USA, 1992. [Google Scholar]
- MSZ-08-0205:1978. A Talaj Fizikai és Vízgazdálkodási Tulajdonságainak Vizsgálata. [Determination of Physical and Hydrophysical Properties of Soils]; Hungarian Standards Institution (MSZT): Budapest, Hangary, 1978. [Google Scholar]
- Verstraeten, L.M.J.; Livens, J. Hygroscopicity as a valuable complement in soil analysis, 1. Characterization of the hygroscopic constant. Geoderma 1971, 6, 255–262. [Google Scholar] [CrossRef]
- ISO 10694:1995. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis); International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- ISO 13878:1998. Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis); International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- MSZ 20135:1999. A Talaj Oldható Tápelemtartalmának Meghatározása. [Determination of the Soluble Nutrient Element Content of the Soil]; Hungarian Standards Institution (MSZT): Budapest, Hungary, 1999. [Google Scholar]
- Furieri, A. SpatiaLite Version 4.3.0a. Available online: https://www.gaia-gis.it/fossil/libspatialite/index (accessed on 5 June 2018).
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006; ISBN 978-1-58488-424-8. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference, R Package Version 1.15.6. Available online: https://github.com/cran/MuMIn/releases/tag/1.15.6 (accessed on 5 June 2018).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-130. Available online: https://mran.microsoft.com/snapshot/2017-02-04/web/packages/nlme/index.html (accessed on 5 June 2018).
- Reiczigel, J.; Harnos, A.; Solymosi, N. Biostatisztika Nem Statisztikusoknak; Pars Kft.: Nagykovácsi, Hungary, 2014; ISBN 978-963-06-3736-7. [Google Scholar]
- Bristow, K.L.; Campbell, G.S. On the relationship between incoming solar radiation and daily maximum and minimum temperature. Agric. For. Meteorol. 1984, 31, 159–166. [Google Scholar] [CrossRef]
- Wood, P.J.; Hannah, D.M.; Sadler, J.P. (Eds.) Hydroecology and Ecohydrology: Past, Present and Future; Wiley: Chichester, UK; Hoboken, NJ, USA, 2007; ISBN 978-0-470-01017-4. [Google Scholar]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Matlack, G.R. Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv. 1993, 66, 185–194. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimatic and soil moisture responses to gap formation in coastal Douglas-fir forests. Can. J. For. Res. 2002, 32, 332–343. [Google Scholar] [CrossRef]
- Ritter, E.; Dalsgaard, L.; Einhorn, K.S. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Abd Latif, Z.; Blackburn, G.A. The effects of gap size on some microclimate variables during late summer and autumn in a temperate broadleaved deciduous forest. Int. J. Biometeorol. 2010, 54, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Brose, P.H. A comparison of the effects of different shelterwood harvest methods on the survival and growth of acorn-origin oak seedlings. Can. J. For. Res. 2011, 41, 2359–2374. [Google Scholar] [CrossRef]
- Grayson, S.F.; Buckley, D.S.; Henning, J.G.; Schweitzer, C.J.; Gottschalk, K.W.; Loftis, D.L. Understory light regimes following silvicultural treatments in central hardwood forests in Kentucky, USA. For. Ecol. Manag. 2012, 279, 66–76. [Google Scholar] [CrossRef]
- Musselman, K.N.; Pomeroy, J.W.; Link, T.E. Variability in shortwave irradiance caused by forest gaps: Measurements, modelling, and implications for snow energetics. Agric. For. Meteorol. 2015, 207, 69–82. [Google Scholar] [CrossRef]
- Dovčiak, M.; Brown, J. Secondary edge effects in regenerating forest landscapes: Vegetation and microclimate patterns and their implications for management and conservation. New For. 2014, 45, 733–744. [Google Scholar] [CrossRef]
- Baker, T.P.; Jordan, G.J.; Steel, E.A.; Fountain-Jones, N.M.; Wardlaw, T.J.; Baker, S.C. Microclimate through space and time: Microclimatic variation at the edge of regeneration forests over daily, yearly and decadal time scales. For. Ecol. Manag. 2014, 334, 174–184. [Google Scholar] [CrossRef]
- Brooks, R.T.; Kyker-Snowman, T.D. Forest floor temperature and relative humidity following timber harvesting in southern New England, USA. For. Ecol. Manag. 2008, 254, 65–73. [Google Scholar] [CrossRef]
- Chen, J.; Franklin, J.F.; Spies, T.A. Growing-Season Microclimatic Gradients from Clearcut Edges into Old-Growth Douglas-Fir Forests. Ecol. Appl. 1995, 5, 74–86. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Payne, G.W.; van Elswijk, M. Microclimate gradients across a forest edge. N. Z. J. Ecol. 2000, 24, 111–121. [Google Scholar]
- Baker, T.P.; Jordan, G.J.; Baker, S.C. Microclimatic edge effects in a recently harvested forest: Do remnant forest patches create the same impact as large forest areas? For. Ecol. Manag. 2016, 365, 128–136. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Saldaña-Vázquez, R.A.; Fahrig, L.; Santos, B.A. Does forest fragmentation cause an increase in forest temperature? Ecol. Res. 2017, 32, 81–88. [Google Scholar] [CrossRef]
- Robson, T.M.; Rodriguez-Calcerrada, J.; Sanchez-Gomez, D.; Aranda, I. Summer drought impedes beech seedling performance more in a sub-Mediterranean forest understory than in small gaps. Tree Physiol. 2008, 29, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, R.M.; Banks-Leite, C. Fragmentation Impairs the Microclimate Buffering Effect of Tropical Forests. PLoS ONE 2013, 8, e58093. [Google Scholar] [CrossRef] [PubMed]
- Heithecker, T.D.; Halpern, C.B. Edge-related gradients in microclimate in forest aggregates following structural retention harvests in western Washington. For. Ecol. Manag. 2007, 248, 163–173. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Oh, S.; Lee, D. Predicting spatial and temporal patterns of soil temperature based on topography, surface cover and air temperature. For. Ecol. Manag. 2000, 136, 173–184. [Google Scholar] [CrossRef]
- Ritter, E.; Starr, M.; Vesterdal, L. Losses of nitrate from gaps of different sizes in a managed beech (Fagus sylvatica) forest. Can. J. For. Res. 2005, 35, 308–319. [Google Scholar] [CrossRef]
- Williams-Linera, G.; Dominguez-Gastelu, V.; Garcia-Zurita, M.E. Microenvironment and Floristics of Different Edges in a Fragmented Tropical Rainforest. Conserv. Biol. 1998, 12, 1091–1102. [Google Scholar] [CrossRef]
- Aussenac, G.; Granier, A. Effects of thinning on water stress and growth in Douglas-fir. Can. J. For. Res. 1988, 18, 100–105. [Google Scholar] [CrossRef]
- Adams, P.W.; Flint, A.L.; Fredriksen, R.L. Long-term patterns in soil moisture and revegetation after a clearcut of a Douglas-fir forest in Oregon. For. Ecol. Manag. 1991, 41, 249–263. [Google Scholar] [CrossRef]
- Lewandowski, T.E.; Forrester, J.A.; Mladenoff, D.J.; Stoffel, J.L.; Gower, S.T.; D’Amato, A.W.; Balser, T.C. Soil microbial community response and recovery following group selection harvest: Temporal patterns from an experimental harvest in a US northern hardwood forest. For. Ecol. Manag. 2015, 340, 82–94. [Google Scholar] [CrossRef]
- Chase, C.W.; Kimsey, M.J.; Shaw, T.M.; Coleman, M.D. The response of light, water, and nutrient availability to pre-commercial thinning in dry inland Douglas-fir forests. For. Ecol. Manag. 2016, 363, 98–109. [Google Scholar] [CrossRef]
- Tinya, F.; (MTA Centre for Ecological Research). Personal communication, 2016.
- Finzi, A.C.; Canham, C.D.; Van Breemen, N. Canopy tree–soil interactions within temperate forests: Species effects on ph and cations. Ecol. Appl. 1998, 8, 447–454. [Google Scholar] [CrossRef]
- Nykvist, N.; Rosén, K. Effect of clear-felling and slash removal on the acidity of Northern coniferous soils. For. Ecol. Manag. 1985, 11, 157–169. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils, 4th ed.; Wiley-Blackwell: Chichester, UK; Hoboken, NJ, USA, 2013; ISBN 978-0-470-97946-4. [Google Scholar]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Lindo, Z.; Visser, S. Microbial biomass, nitrogen and phosphorus mineralization, and mesofauna in boreal conifer and deciduous forest floors following partial and clear-cut harvesting. Can. J. For. Res. 2003, 33, 1610–1620. [Google Scholar] [CrossRef]
- Ji, C.-J.; Yang, Y.-H.; Han, W.-X.; He, Y.-F.; Smith, J.; Smith, P. Climatic and Edaphic Controls on Soil pH in Alpine Grasslands on the Tibetan Plateau, China: A Quantitative Analysis. Pedosphere 2014, 24, 39–44. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; Gollan, J.R. Moisture, thermal inertia, and the spatial distributions of near-surface soil and air temperatures: Understanding factors that promote microrefugia. Agric. For. Meteorol. 2013, 176, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, M. The soil fauna of a beech forest on limestone: Trophic structure and energy budget. Oecologia 1990, 82, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Holst, T.; Mayer, H.; Schindler, D. Microclimate within beech stands—Part II: Thermal conditions. Eur. J. For. Res. 2004, 123, 13–28. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lõhmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]



| Treatment | Pre-Treatment (2014) | Post-Treatment (2015) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DBH | Height | Basal Area | Canopy Closure | Basal Area | Canopy Closure | |||||
| U | S | U | S | U | S | U | S | |||
| C | 28.0 ± 5.8 | 11.9 ± 3.8 | 20.9 ± 1.5 | 10.8 ± 3.5 | 29.32 ± 0.12 | 8.83 ± 0.10 | 89.8 ± 2.6 | 29.32 ± 0.12 | 8.83 ± 0.10 | 93.5 ± 3.9 |
| CC | 28.0 ± 5.7 | 11.8 ± 4.2 | 21.6 ± 1.6 | 10.4 ± 3.8 | 29.58 ± 6.47 | 9.98 ± 4.66 | 87.9 ± 3.6 | 0.00 | 0.00 | 2.5 ± 2.1 |
| G | 27.3 ± 5.3 | 12.5 ± 2.8 | 20.5 ± 1.1 | 11.2 ± 2.9 | 29.53 ± 9.03 | 9.33 ± 4.51 | 88.4 ± 4.4 | 0.00 | 0.00 | 44.8 ± 10.4 |
| P | 27.2 ± 5.3 | 10.9 ± 4.1 | 21.2 ± 1.4 | 10.0 ± 3.5 | 28.07 ± 2.10 | 8.03 ± 1.33 | 89.4 ± 4.4 | 19.67 ± 1.48 | 0.00 | 70.2 ± 6.9 |
| R | 27.3 ± 5.8 | 11.1 ± 3.4 | 20.4 ± 1.9 | 11.8 ± 3.9 | 30.47 ± 3.73 | 8.17 ± 2.35 | 88.7 ± 3.2 | 30.47 ± 3.73 | 8.17 ± 2.35 | 81.9 ± 9.2 |
| Dependent Variable | Model | Treatment | Time | Treatment: Time | |||||
|---|---|---|---|---|---|---|---|---|---|
| Chi2 | p | R2LR | F | p | F | p | F | p | |
| dPAR mean | 454.711 | <0.0001 | 0.922 | 225.579 | <0.0001 | 133.928 | <0.0001 | 8.941 | <0.0001 |
| dPAR IQR | 343.698 | <0.0001 | 0.852 | 114.259 | <0.0001 | 57.575 | <0.0001 | 6.292 | <0.0001 |
| dDIFN | 29.086 | <0.0001 | 0.766 | 21.699 | <0.0001 | - | - | - | - |
| dTair mean | 273.305 | <0.0001 | 0.781 | 21.888 | <0.0001 | 54.082 | <0.0001 | 4.903 | <0.0001 |
| dTair IQR | 265.160 | <0.0001 | 0.771 | 44.487 | <0.0001 | 47.139 | <0.0001 | 2.016 | 0.0086 |
| dRH mean | 46.096 | <0.0001 | 0.434 | 5.177 | 0.0021 | 2.939 | 0.0105 | 0.609 | 0.8866 |
| dRH IQR | 125.451 | <0.0001 | 0.569 | 14.054 | <0.0001 | 16.694 | <0.0001 | 1.275 | 0.2173 |
| dVPD mean | 122.668 | <0.0001 | 0.595 | 13.2782 | <0.0001 | 13.9286 | <0.0001 | 1.8528 | 0.0267 |
| dVPD IQR | 259.555 | <0.0001 | 0.823 | 37.279 | <0.0001 | 63.435 | <0.0001 | 5.491 | <0.0001 |
| dTsoil mean | 261.975 | <0.0001 | 0.768 | 9.107 | <0.0001 | 44.611 | <0.0001 | 7.368 | <0.0001 |
| dTsoil IQR | 201.537 | <0.0001 | 0.674 | 24.397 | <0.0001 | 24.166 | <0.0001 | 3.248 | <0.0001 |
| dSWC mean | 109.965 | <0.0001 | 0.534 | 29.145 | <0.0001 | 2.3129 | 0.0292 | 1.089 | 0.3666 |
| dLitter mass | 21.338 | 0.0033 | 0.424 | 2.164 | 0.1097 | 10.812 | 0.0057 | 1.955 | 0.1387 |
| dLitter pH | 35.390 | <0.0001 | 0.524 | 8.888 | 0.0002 | 8.685 | 0.0057 | 3.646 | 0.0218 |
| dLitter moisture | 47.003 | <0.0001 | 0.624 | 9.318 | 0.0001 | 16.478 | 0.0003 | 7.355 | 0.0009 |
| dSoil pH | 23.863 | 0.0012 | 0.544 | 3.633 | 0.0221 | 15.754 | 0.0003 | 0.041 | 0.9889 |
| dhy | 10.428 | 0.1656 | 0.219 | 2.824 | 0.0528 | 0.115 | 0.7369 | 0.426 | 0.7358 |
| d[SOC] | 5.008 | 0.6590 | 0.352 | 1.202 | 0.3242 | 0.159 | 0.6930 | 0.223 | 0.8799 |
| d[N] | 3.415 | 0.8442 | 0.357 | 0.912 | 0.4451 | 0.008 | 0.9316 | 0.074 | 0.9738 |
| d[PAL] | 10.308 | 0.1718 | 0.388 | 1.936 | 0.1418 | 1.034 | 0.3163 | 0.965 | 0.4200 |
| d[KAL] | 12.735 | 0.0788 | 0.299 | 1.641 | 0.1821 | 6.956 | 0.0124 | 0.173 | 0.9143 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, B.; Tinya, F.; Guba, E.; Németh, C.; Sass, V.; Bidló, A.; Ódor, P. The Short-Term Effects of Experimental Forestry Treatments on Site Conditions in an Oak–Hornbeam Forest. Forests 2018, 9, 406. https://doi.org/10.3390/f9070406
Kovács B, Tinya F, Guba E, Németh C, Sass V, Bidló A, Ódor P. The Short-Term Effects of Experimental Forestry Treatments on Site Conditions in an Oak–Hornbeam Forest. Forests. 2018; 9(7):406. https://doi.org/10.3390/f9070406
Chicago/Turabian StyleKovács, Bence, Flóra Tinya, Erika Guba, Csaba Németh, Vivien Sass, András Bidló, and Péter Ódor. 2018. "The Short-Term Effects of Experimental Forestry Treatments on Site Conditions in an Oak–Hornbeam Forest" Forests 9, no. 7: 406. https://doi.org/10.3390/f9070406