Temporal Variations in Soil Profile Carbon and Nitrogen during Three Consecutive Years of 15N Deposition in Temperate Oak and Pine Forest Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Soil Description
2.2. Natural Field 15N Experiments
2.3. Sampling and Chemical Analyses
2.4. Calculation and Statistical Analysis
3. Results
3.1. Initial Chemical Properties of Two Different Forest Floor Soils
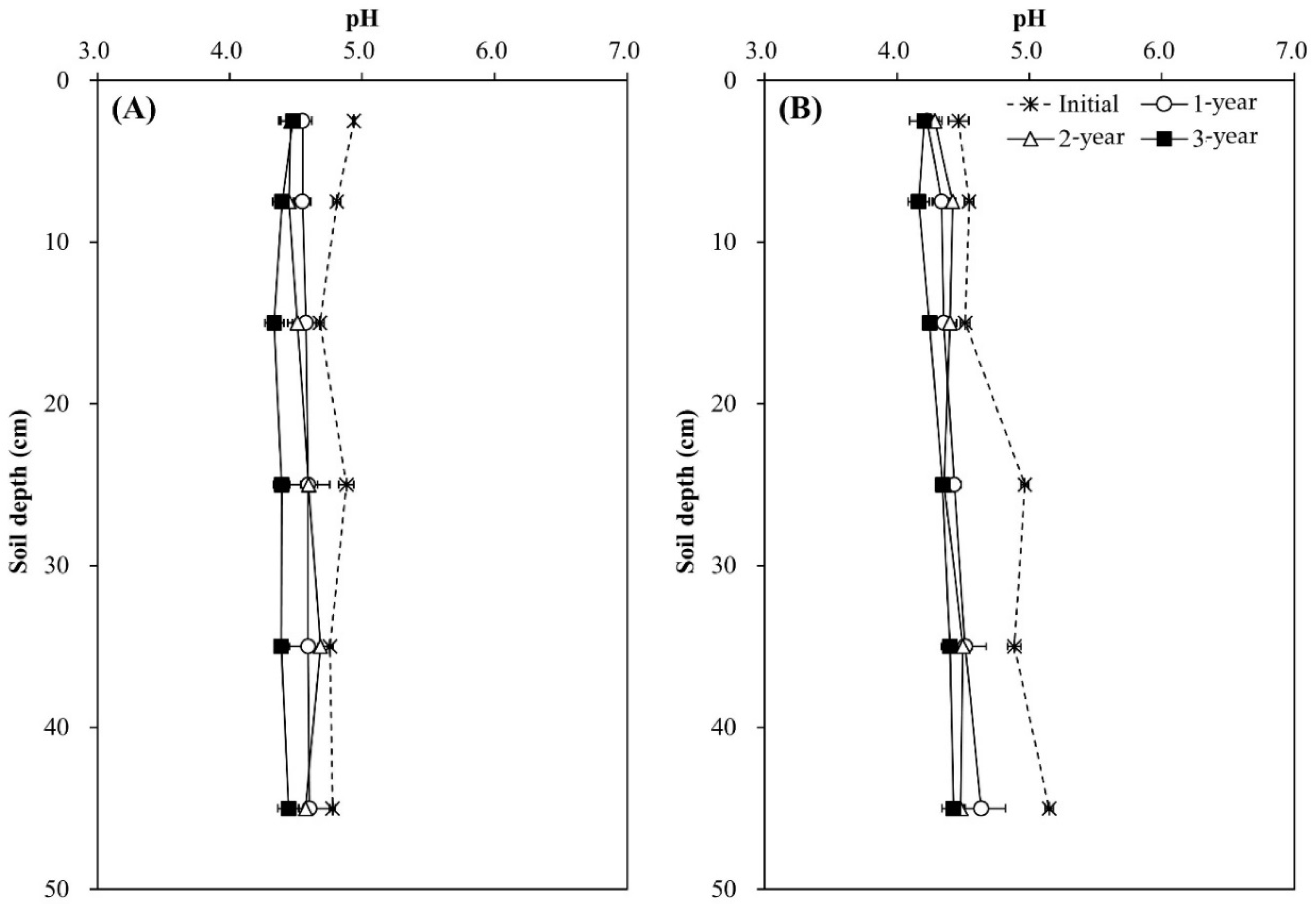
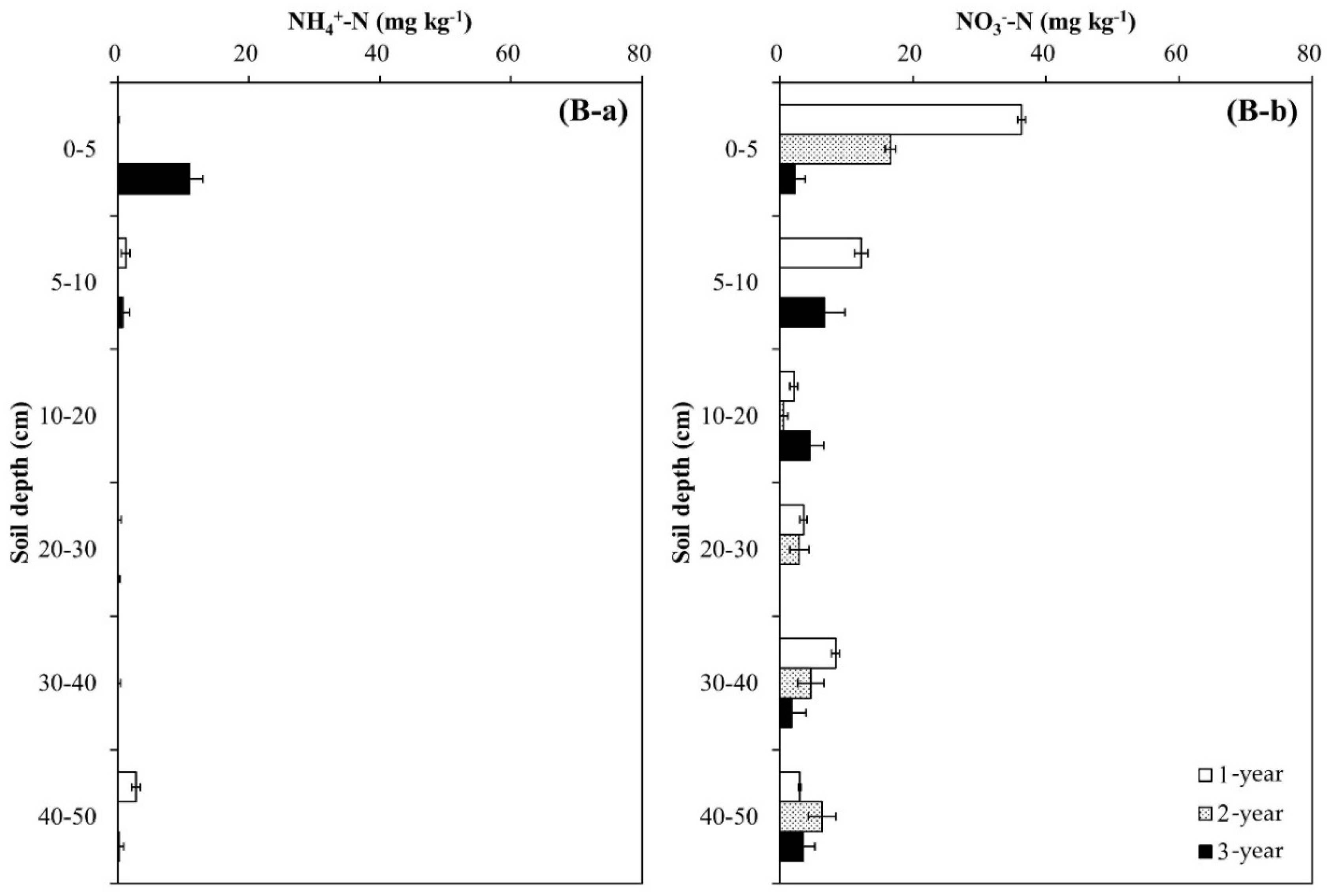
3.2. Soil pH and Mineral N Contents
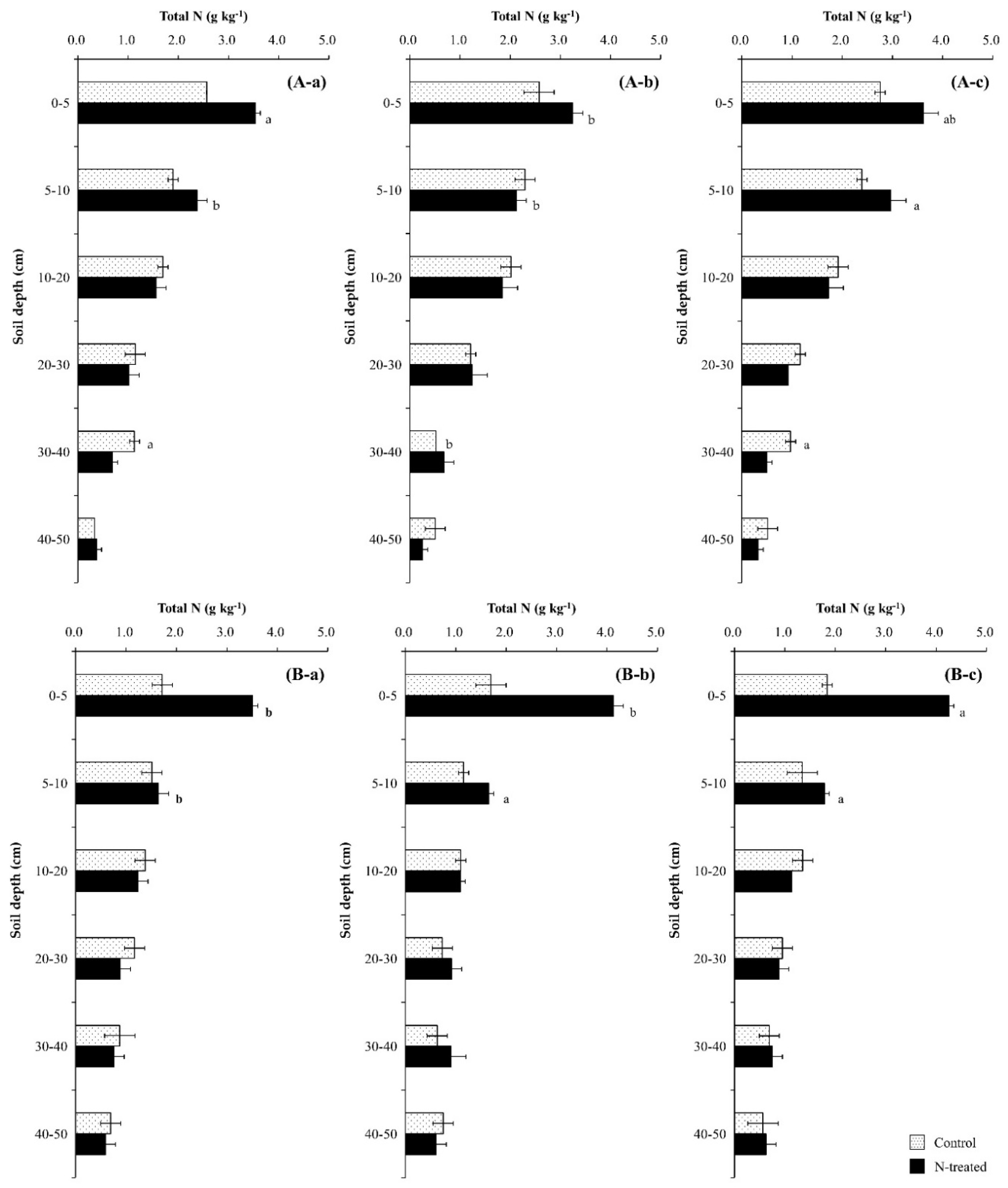
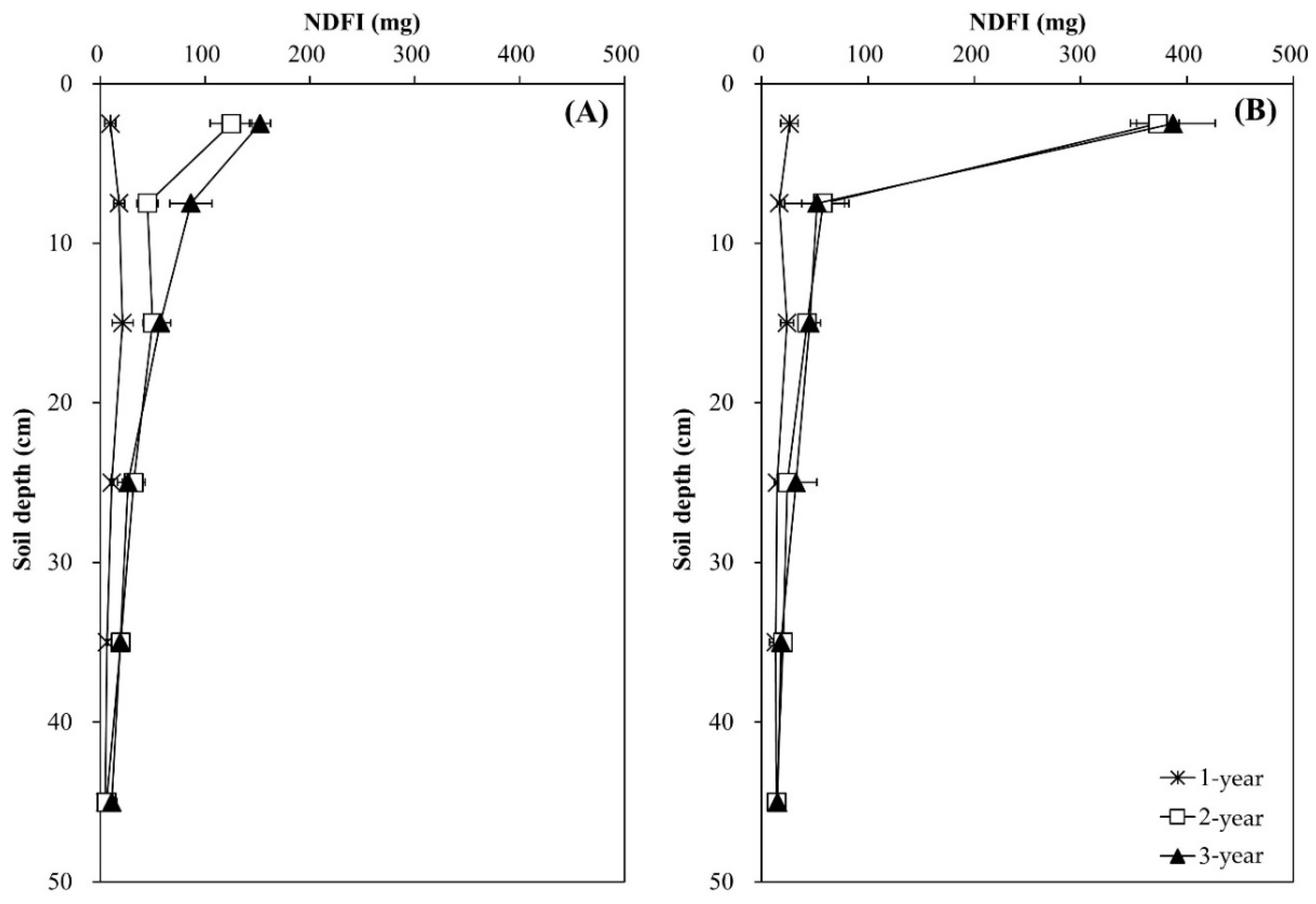
3.3. Soil Total N Content, NDFI, and 15N Recovery
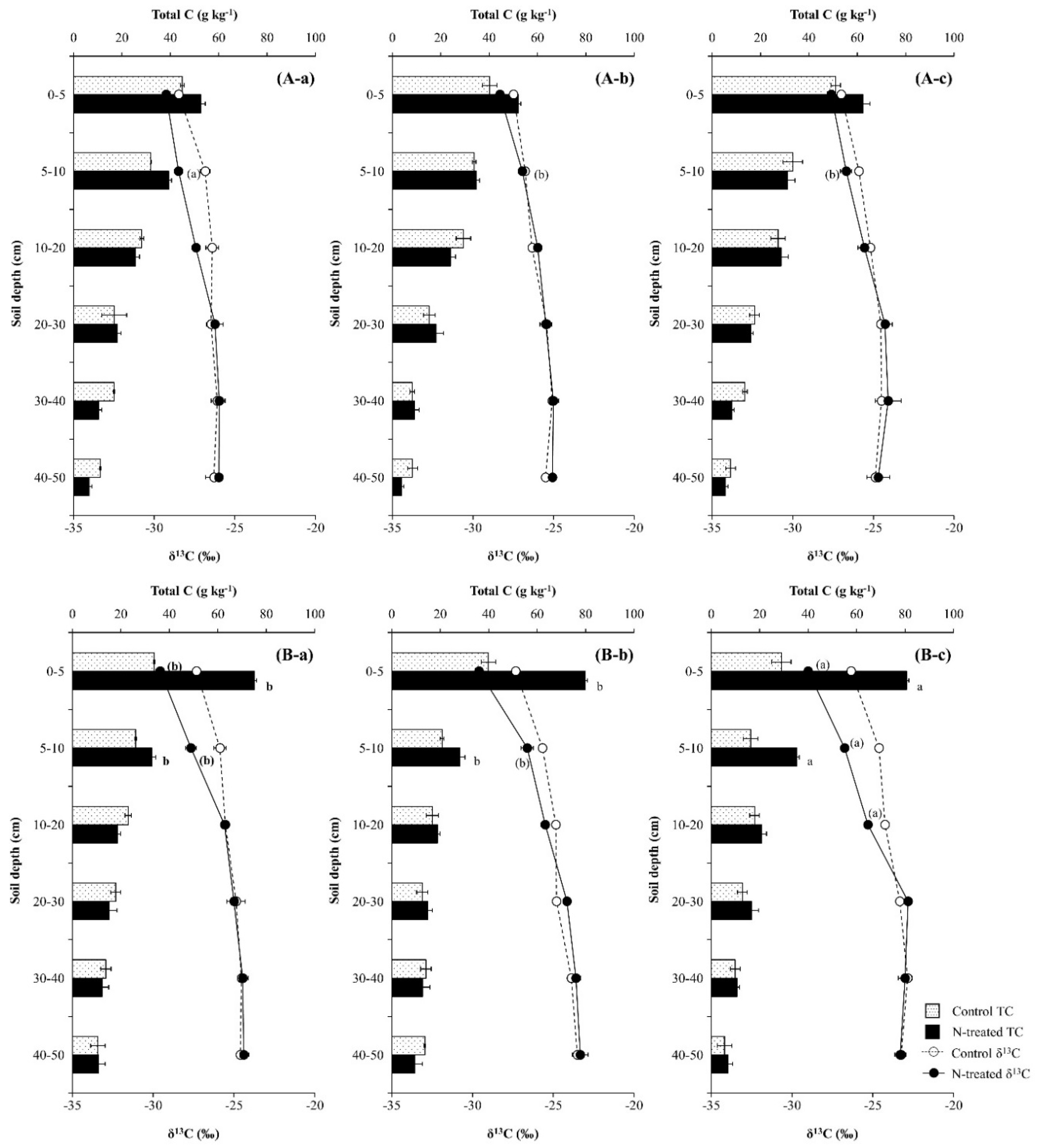
3.4. Soil Total C Content and δ13C
4. Discussion
4.1. Differences in Initial Chemical Properties of Two Different Forest Soils
4.2. Effect of N Treatment on Soil pH and Mineral N
4.3. Effect of N Treatment on Soil Total N Contents
4.4. Effect of N Treatment on Soil Total C Contents and δ13C Values
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; pp. 1–151. [Google Scholar]
- Tietema, A.; Wessel, W.W. Gross nitrogen transformations in the organic layer of acid forest ecosystems subjected to increased atmospheric nitrogen input. Soil Biol. Biochem. 1992, 24, 943–950. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen nutrition of trees in temperate forests—The significance of nitrogen availability in the pedosphere and atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Christ, M.; Zhang, Y.; Likens, G.E.; Driscoll, C.T. Nitrogen retention capacity of a northern hardwood forest soil under ammonium sulfate additions. Ecol. Appl. 1995, 5, 802–812. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Zhang, J.; Wang, Q.; Zhao, N.; Jia, Y.; Ge, J.; Yu, G. Estimation of carbon sequestration in China’s forests induced by atmospheric wet nitrogen deposition using the principles of ecological stoichiometry. Environ. Res. Lett. 2017, 12, 114038. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, N.S.; Trumbore, S.E.; Schuur, E.A.; Mack, M.C.; Shaver, G.R. Nutrient addition prompts rapid destabilization of organic matter in an arctic tundra ecosystem. Ecosystems 2008, 11, 16–25. [Google Scholar] [CrossRef]
- Fog, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Kim, H.; Kang, H. The impacts of excessive nitrogen additions on enzyme activities and nutrient leaching in two contrasting forest soils. J. Microbiol. 2011, 49, 369–375. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.; Du, E.; Butterbach-Bahl, K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr. Opin. Environ. Sustain. 2014, 9, 90–104. [Google Scholar] [CrossRef]
- Van Diepen, L.T.; Frey, S.D.; Sthultz, C.M.; Morrison, E.W.; Minocha, R.; Pringle, A. Changes in litter quality caused by simulated nitrogen deposition reinforce the N-induced suppression of litter decay. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Fierer, N.; Reynolds, J.F. Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct. Ecol. 2008, 22, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, S.E. Plant species effects on nutrient cycling: Revisiting litter feedbacks. Trends Ecol. Evol. 2015, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gartzia-Bengoetxea, N.; Kandeler, E.; de Arano, I.M.; Arias-González, A. Soil microbial functional activity is governed by a combination of tree species composition and soil properties in temperate forests. Appl. Soil Ecol. 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Huang, Z.; Wan, X.; He, Z.; Yu, Z.; Wang, M.; Hu, Z.; Yang, Y. Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol. Biochem. 2013, 62, 68–75. [Google Scholar] [CrossRef]
- Gregory, A.S.; Dungait, J.A.J.; Watts, C.W.; Bol, R.; Dixon, E.R.; White, R.P.; Whitmore, A.P. Long-term management changes topsoil and subsoil organic carbon and nitrogen dynamics in a temperate agricultural system. Eur. J. Soil Sci. 2016, 67, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Turner, G.L.; Bergersen, F.J.; Tantala, H. Natural enrichment of 15N during decomposition of plant material in soil. Soil Biol. Biochem. 1983, 15, 495–497. [Google Scholar] [CrossRef]
- Perakis, S.S.; Compton, J.E.; Hedin, L.O. Nitrogen retention across a gradient of 15N additions to an unpolluted temperate forest soil in Chile. Ecology 2005, 86, 96–105. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis; Arnold, K., Page, A.L., Eds.; Science Society of America Inc.: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic Forms. In Methods of Soil Analysis; Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Ro, H.M.; Kim, P.G.; Park, J.S.; Yun, S.I.; Han, J.H. Nitrogen removal through N cycling from sediments in a constructed coastal marsh as assessed by 15N-isotope dilution. Mar. Pollut. Bull. 2018, 129, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stocks of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, X.; He, X.; Song, F.; Ren, L.; Jiang, P. Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur. J. Soil Biol. 2008, 44, 392–399. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Schmidt, I.K.; Gundersen, P.; Vesterdal, L. Soil carbon accumulation and nitrogen retention traits of four tree species grown in common gardens. For. Ecol. Manag. 2013, 309, 47–57. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Gundersen, P.; Callesen, I.; Reinds, G.J. Throughfall nitrogen deposition has different impacts on soil solution nitrate concentration in European coniferous and deciduous forests. Ecosystems 2014, 7, 180–192. [Google Scholar] [CrossRef]
- Xing, S.; Chen, C.; Zhou, B.; Zhang, H.; Nang, Z.; Xu, Z. Soil soluble organic nitrogen and active microbial characteristics under adjacent coniferous and broadleaf plantation forests. J. Soils Sediment. 2010, 10, 748–757. [Google Scholar] [CrossRef]
- Boström, B.; Comstedt, D.; Ekblad, A. Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 2007, 153, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.; Mahieu, N.; Cadisch, G. Carbon isotopic fractionation during decomposition of plant materials of different quality. Glob. Biogeochem. Cycles 2003, 17, 1–11. [Google Scholar] [CrossRef]
- Kao, W.Y.; Tsai, H.C.; Shin, C.N.; Tsai, T.T.; Handley, L.L. Nutrient contents d13C and d15N during leaf senescence in the mangrove, Kandelia candel (L.) Druce. Bot. Bull. Acad. Sin. 2002, 43, 277–282. [Google Scholar]
- Acton, P.; Fox, J.; Campbell, E.; Rowe, H.; Wilkinson, M. Carbon isotope for estimating soil decomposition and physical mixing in well-drained forest soils. J. Geophys. Res. Biogeosci. 2013, 118, 1532–1545. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Fry, B. Controls on natural Nitrogen 15 and Carbon 13 abundances in forest soil organic matter. Soil Sci. Soc. Am. J. 1988, 52, 1633–1640. [Google Scholar] [CrossRef]
- Davidson, E.A.; Hart, S.C.; Firestone, M.K. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 1992, 73, 1148–1156. [Google Scholar] [CrossRef]
- Luo, X.; Yan, Q.; Wang, C.; Luo, C.; Zhou, N.; Jian, C. Treatment of ammonia nitrogen wastewater in low concentration by two-stage ozonization. Int. J. Envion. Res. Pubilc Health 2015, 12, 11975–11987. [Google Scholar] [CrossRef] [PubMed]
- Staelens, J.; Rütting, T.; Huygens, D.; De Schrijver, A.; Müller, C.; Verheyen, K.; Boeckx, P. In situ gross nitrogen transformations differ between temperate deciduous and coniferous forest soils. Biogeochemistry 2012, 108, 259–277. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Gundersen, P.; Callesen, I.; de Vries, W. Nitrate leaching in forest ecosystems is related to forest floor CN ratios. Environ. Pollut. 1998, 102, 403–407. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Tonitto, C.; Goodale, C.L.; Weiss, M.S.; Frey, S.D.; Ollinger, S.V. The effect of nitrogen addition on soil organic matter dynamics: A model analysis of the Harvard Forest Chronic Nitrogen Amendment Study and soil carbon response to anthropogenic N deposition. Biogeochemistry 2014, 117, 431–454. [Google Scholar] [CrossRef]
- Finn, D.; Page, K.; Catton, K.; Strounina, E.; Kienzle, M.; Robertson, F.; Armstrong, R.; Dalal, R. Effect of added nitrogen on plant litter decomposition depends on initial soil carbon and nitrogen stoichiometry. Soil Biol. Biochem. 2015, 91, 160–168. [Google Scholar] [CrossRef]
- Lettens, S.; Orshoven, J.O.S.; Wesemael, B.A.S.; Muys, B.; Perrin, D. Soil organic carbon changes in landscape units of Belgium between 1960 and 2000 with reference to 1990. Glob. Chang. Biol. 2005, 11, 2128–2140. [Google Scholar] [CrossRef]
- Kim, D.G.; Mu, S.; Kang, S.; Lee, D. Factors controlling soil CO2 effluxes and the effects of rewetting on effluxes in adjacent deciduous, coniferous, and mixed forests in Korea. Soil Biol. Biochem. 2010, 42, 576–585. [Google Scholar] [CrossRef]
- Laganière, J.; Pare, D.; Bradley, R.L. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Qualls, R.G. Long–Term (13 Years) Decomposition rates of forest floor organic matter on paired coniferous and deciduous watersheds with contrasting temperature regimes. Forests 2016, 7, 231. [Google Scholar] [CrossRef]
- Edmoned, R.L. Organic matter decomposition in western United States forests. USDA For. Ser. Gen. Tech. Rep. 1991, 280, 116–128. [Google Scholar]



| Soil Properties | Q. acutissima | P. koraiensis |
|---|---|---|
| pH (soil:water = 1:5) a | 4.8 ± 0.0 b | 4.6 ± 0.0 |
| Mineral N (mg kg−1) | 5.5 ± 1.1 | 12.1 ± 3.0 |
| Total C (g kg−1) | 39.4 ± 1.4 | 22.6 ± 0.8 |
| δ13C (‰) | −26.9 ± 0.1 | −24.9 ± 0.4 |
| Total N (g kg−1) | 2.8 ± 0.1 | 1.4 ± 0.1 |
| δ15N (‰) | 1.8 ± 0.1 | 2.1 ± 0.2 |
| C/N ratio | 14.1 ± 0.2 | 16.1 ± 0.3 |
| Factors | Mineral N | ||||||
|---|---|---|---|---|---|---|---|
| pH | NH4+ | NO3− | TN | NDFI | TC | δ13C | |
| Time (T) | * | n.s. | n.s. | n.s. | * | n.s. | *** |
| Treatment (N) | *** | * | *** | ** | *** | *** | * |
| Soil depth (D) | *** | *** | *** | *** | *** | *** | ** |
| Tree species (S) | *** | n.s. | * | n.s. | n.s. | ** | *** |
| T × N | * | *** | * | * | * | * | * |
| T × D | * | *** | n.s. | * | * | ** | * |
| T × S | * | * | * | n.s. | n.s. | n.s. | n.s. |
| N × D | *** | *** | *** | *** | *** | *** | ** |
| N × S | *** | ** | *** | * | * | *** | n.s. |
| D × S | *** | n.s. | * | n.s. | n.s. | ** | ** |
| T × N × D | n.s. | *** | n.s. | n.s. | ** | * | * |
| T × N × S | * | * | * | n.s. | * | * | * |
| T × D × S | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| N × D × S | ** | * | * | * | n.s. | ** | n.s. |
| T × N × D × S | * | n.s. | * | n.s. | * | n.s. | * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Ro, H. Temporal Variations in Soil Profile Carbon and Nitrogen during Three Consecutive Years of 15N Deposition in Temperate Oak and Pine Forest Stands. Forests 2018, 9, 338. https://doi.org/10.3390/f9060338
Park J, Ro H. Temporal Variations in Soil Profile Carbon and Nitrogen during Three Consecutive Years of 15N Deposition in Temperate Oak and Pine Forest Stands. Forests. 2018; 9(6):338. https://doi.org/10.3390/f9060338
Chicago/Turabian StylePark, Ji–Suk, and Hee–Myong Ro. 2018. "Temporal Variations in Soil Profile Carbon and Nitrogen during Three Consecutive Years of 15N Deposition in Temperate Oak and Pine Forest Stands" Forests 9, no. 6: 338. https://doi.org/10.3390/f9060338





