Stock Volume Dependency of Forest Drought Responses in Yunnan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Materials
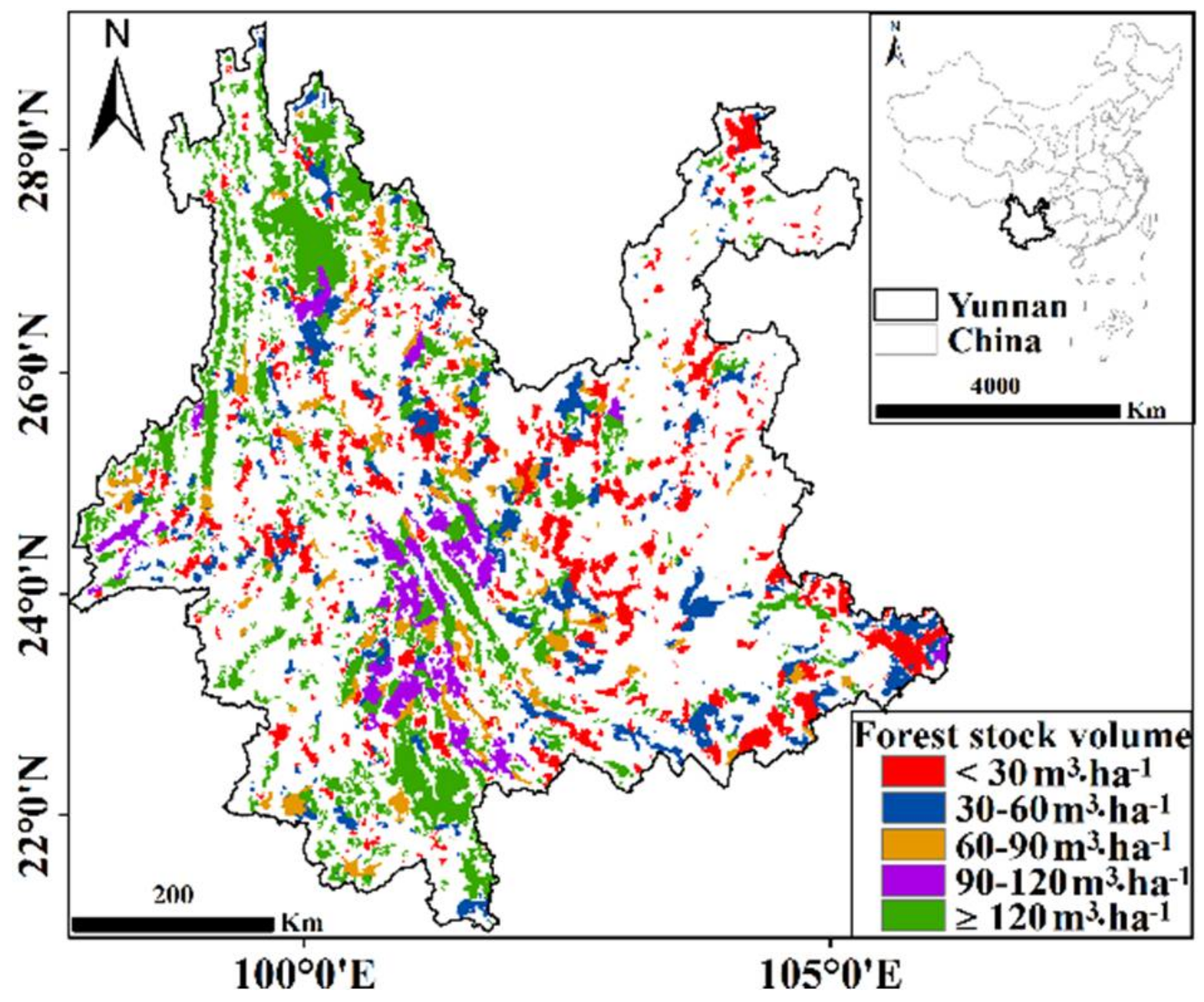
2.2.1. Distribution Map of Forest Stock Volume
2.2.2. MODIS-Enhanced Vegetation Index
2.2.3. Meteorological Drought Index
2.3. Methods
2.3.1. Optimal Time Scale for SPEI
2.3.2. Response to Drought as Affected by Different Forest Stock Volumes
3. Results
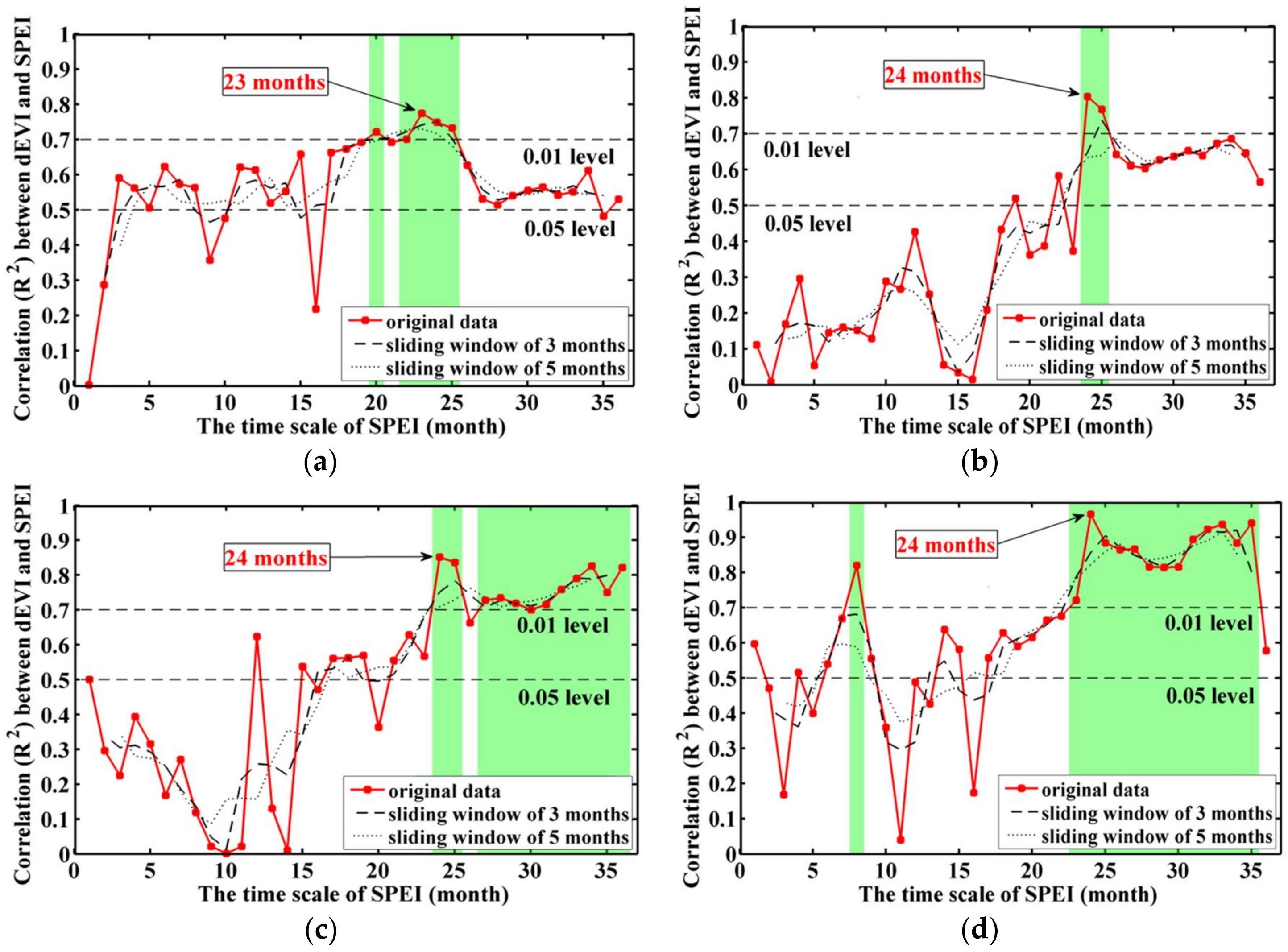
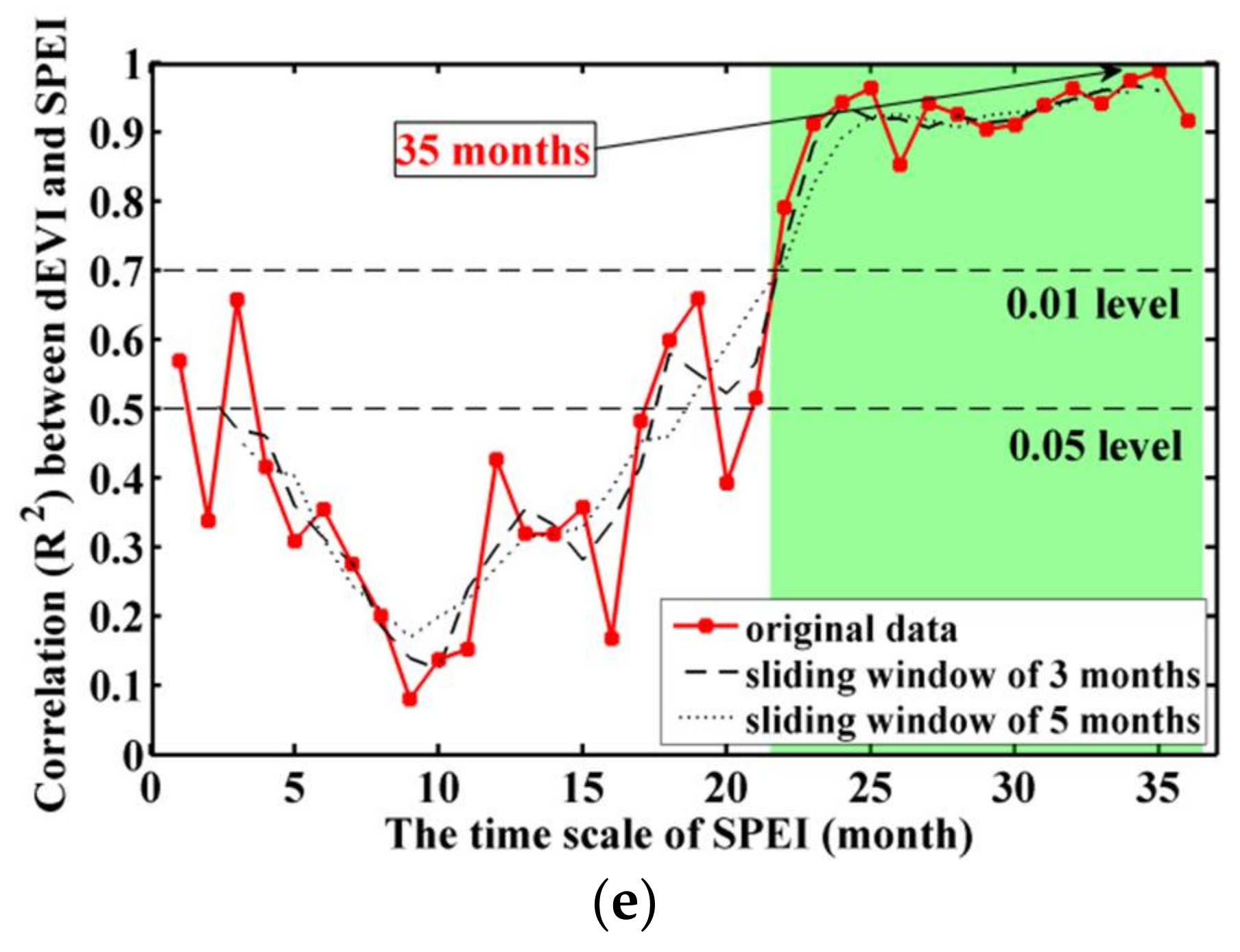
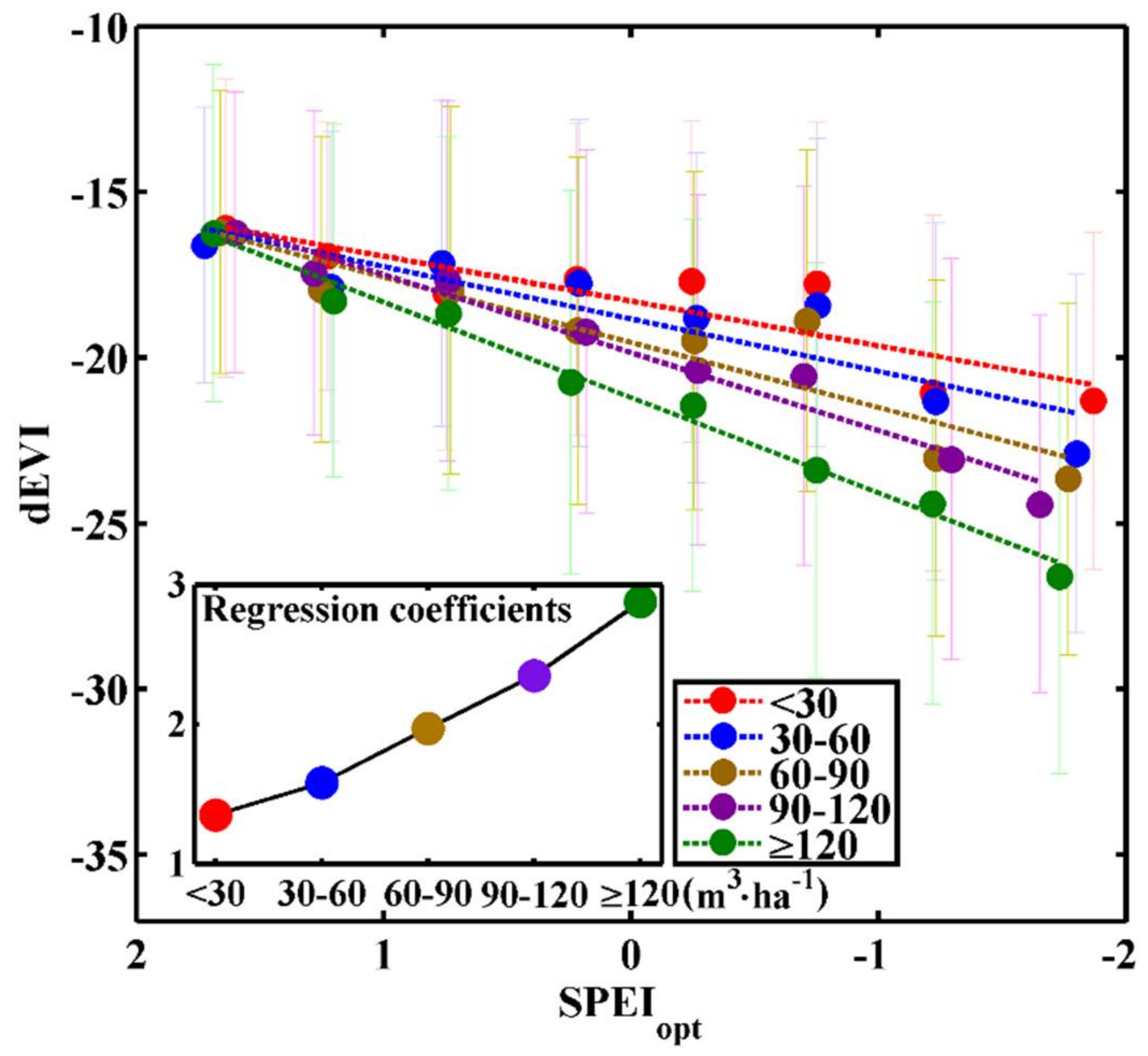
3.1. Stock-Volume-Dependent Time Scales of SPEI
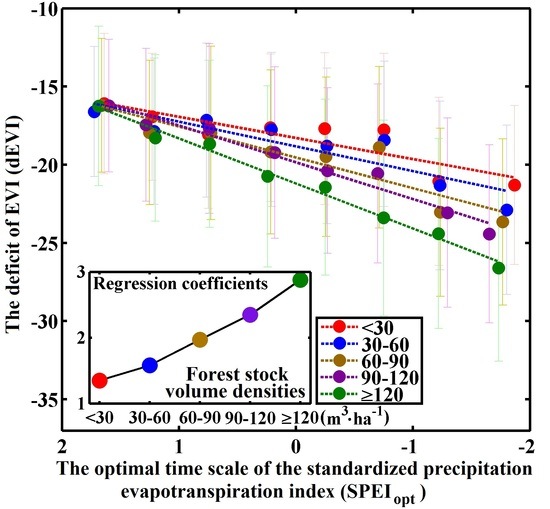
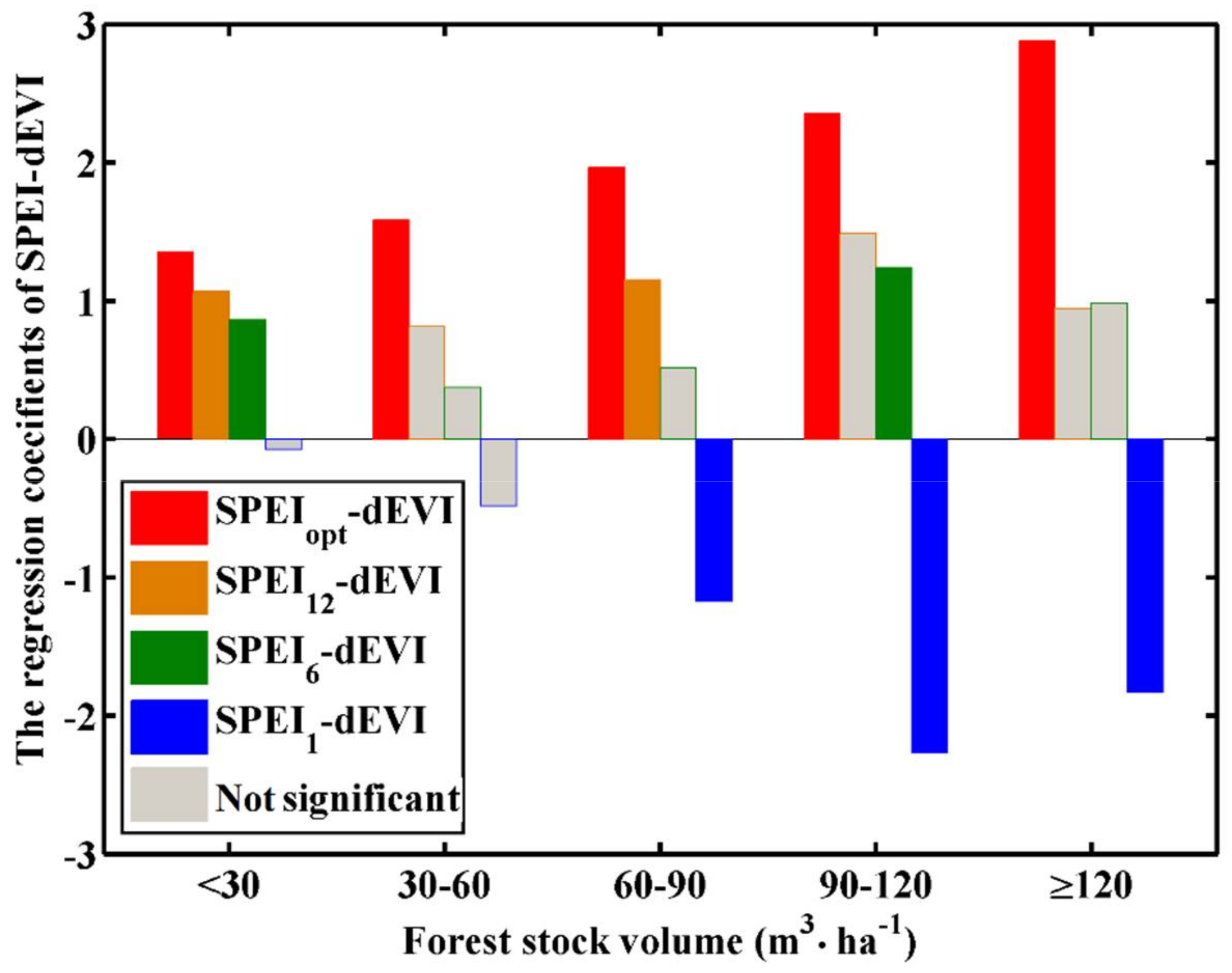
3.2. Differences in Sensitivity to Drought between Different Forest Stock Volumes
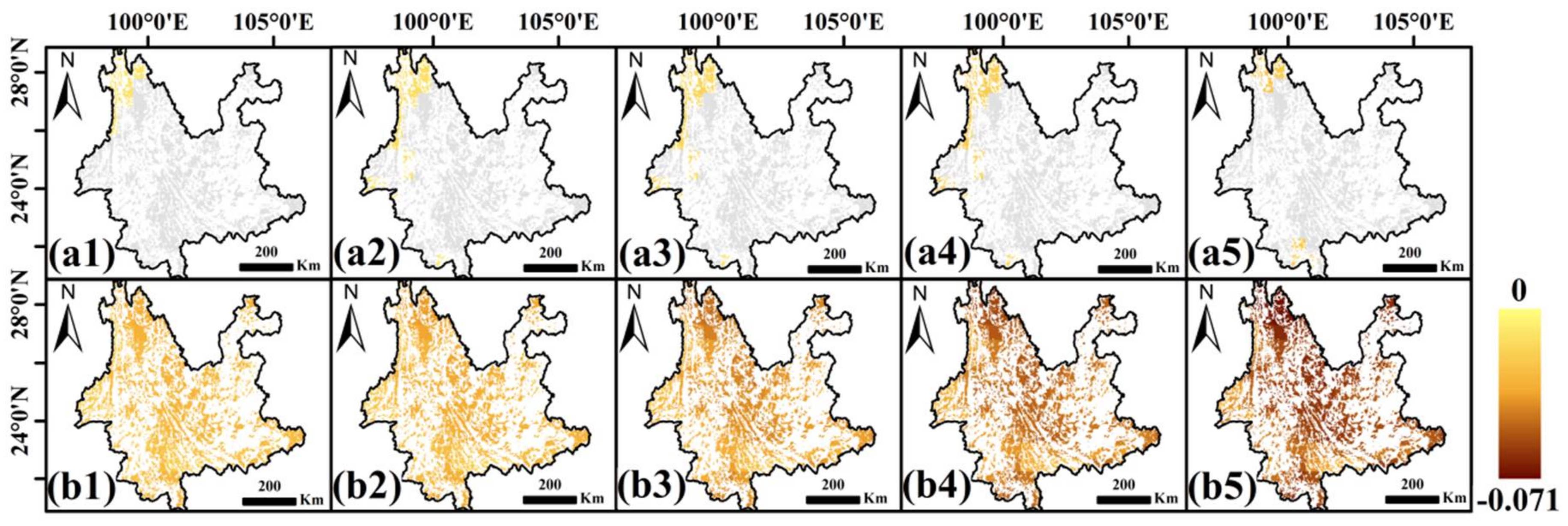
3.3. Error Caused by Stock-Volume-Independent Time Scale of SPEI
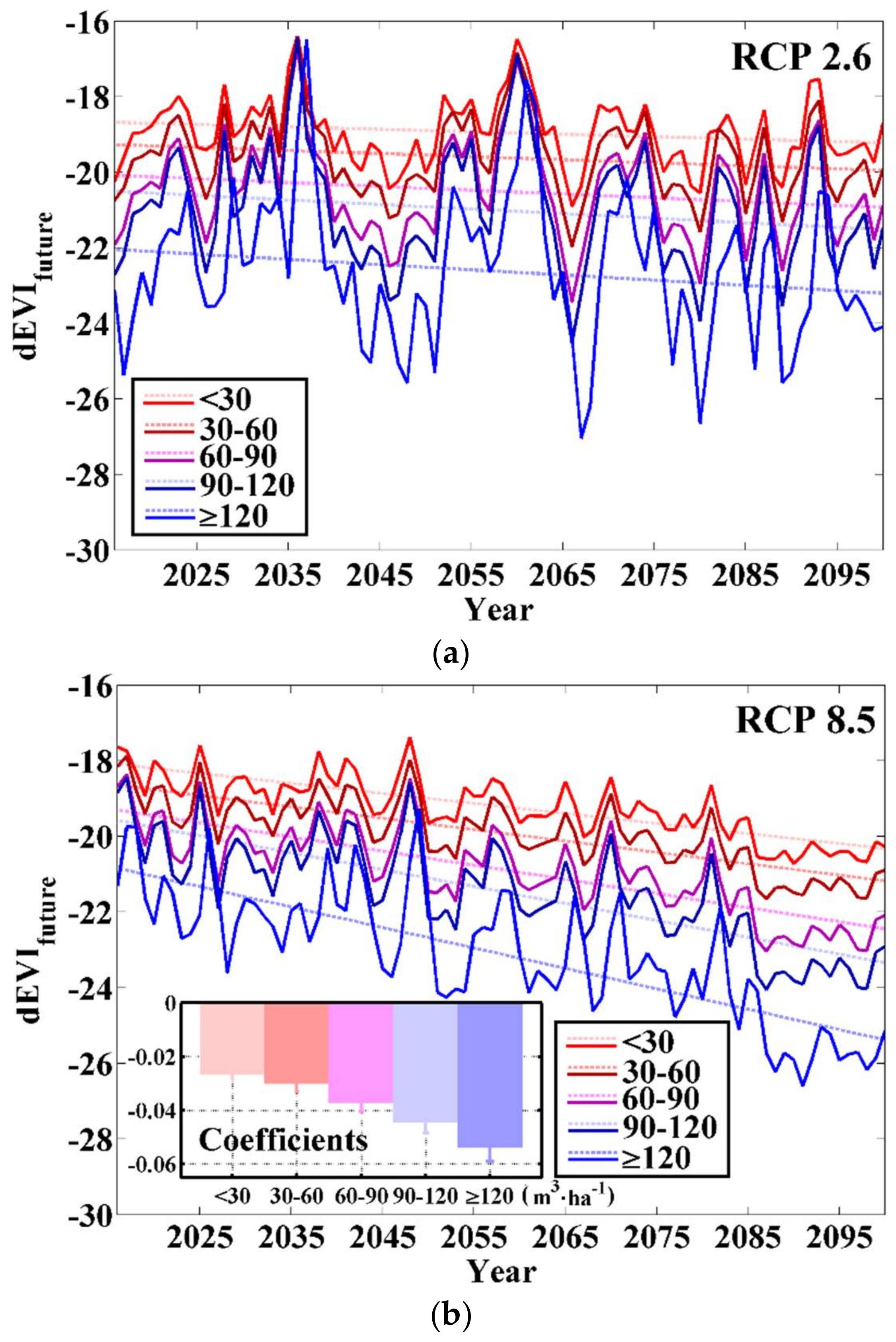
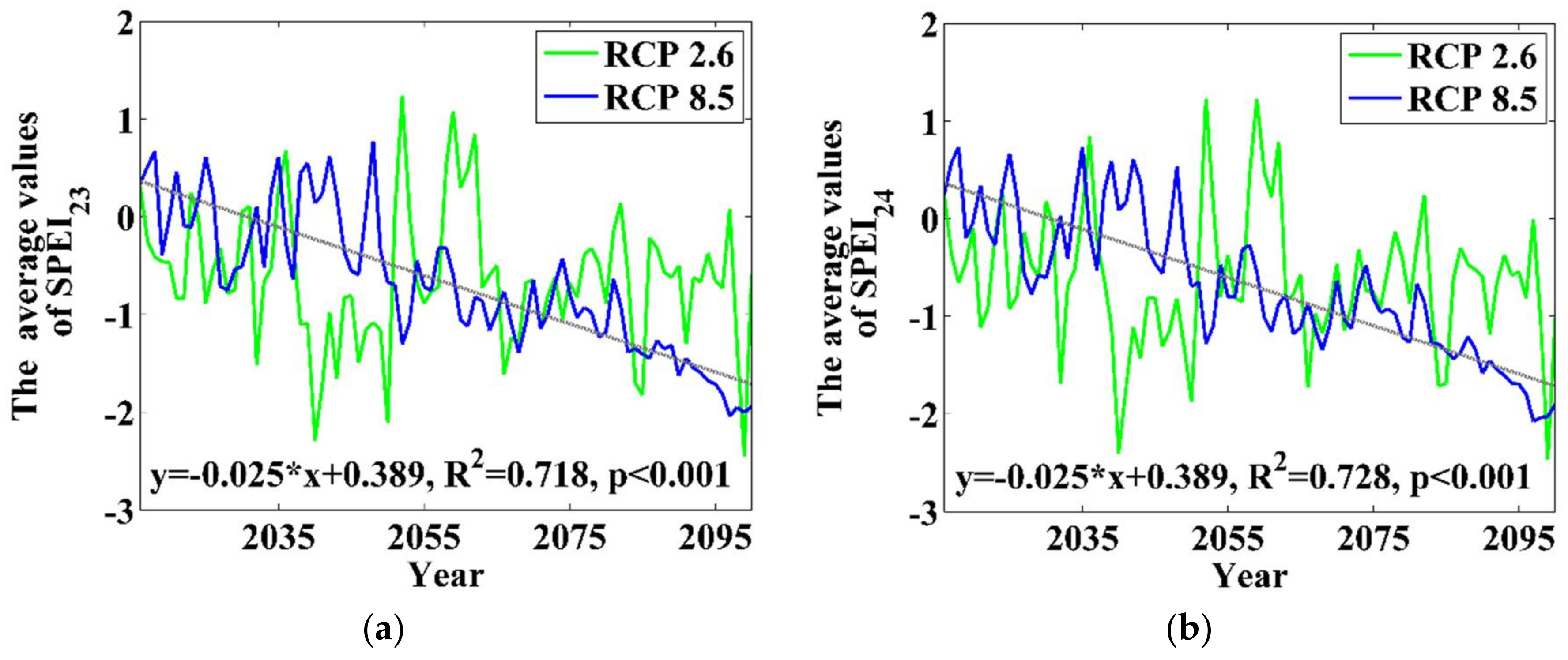
3.4. Stock-Volume-Related Drought Risk in Future
4. Discussion
4.1. Importance of Optimal Time Scale for SPEI
4.2. Differences in Response Due to Differences in Stock Volume Density
4.3. Difference in Response to Future Climate Change
4.4. Uncertainty
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Jobbagy, E.G.; Avissar, R.; Roy, S.B.; Barrett, D.J.; Cook, C.W.; Farley, K.A.; le Maitre, D.C.; McCarl, B.A.; Murray, B.C. Trading water for carbon with biological sequestration. Science 2005, 310, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Huang, S.; Krysanova, V.; Hattermann, F. Projections of climate change impacts on floods and droughts in Germany using an ensemble of climate change scenarios. Reg. Environ. Chang. 2015, 15, 461–473. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; Anenkhonov, O.A.; Korolyuk, A.Y.; Sandanov, D.V.; Balsanova, L.D.; Naidanov, B.B.; Wu, X. Long-term forest resilience to climate change indicated by mortality, regeneration, and growth in semiarid southern siberia. Glob. Chang. Biol. 2017, 23, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, T.; Wu, H.; Zhao, X.; Wang, Q.; Gao, S.; Li, Z. Contrasting responses of planted and natural forests to drought intensity in Yunnan, China. Remote Sens. 2016, 8, 635. [Google Scholar] [CrossRef]
- Goetz, R.; Cañizares, C.; Pujol, J.; Xabadia, A. Forest management and biodiversity in size-structured forests under climate change. In Dynamic Optimization in Environmental Economics; Springer: Berlin/Heidelberg, Germany, 2014; Volume 15, pp. 265–286. [Google Scholar]
- Walsh, D.; Rossi, S.; Lord, D. Size and age: Intrinsic confounding factors affecting the responses to a water deficit in black spruce seedlings. iFor. Biogeosci. For. 2015, 8, 401–409. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Folk, R.S. Stock quality assessment: Forecasting survival or performance on a reforestation site. Tree Plant. Notes 1993, 44, 113–121. [Google Scholar]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Midgley, J.J. Is bigger better in plants? The hydraulic costs of increasing size in trees. Trends Ecol. Evol. 2003, 18, 5–6. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, T.; Pons, T.L.; Bongers, F. The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Yoder, B.J.; Ryan, M.G.; Waring, R.H.; Schoettle, A.W.; Kaufmann, M.R. Evidence of reduced photosynthetic rates in old trees. For. Sci. 1994, 40, 513–527. [Google Scholar]
- Hubbard, R.M.; Bond, B.J.; Ryan, M.G. Evidence that hydraulic conductance limits photosynthesis in old pinus ponderosa trees. Tree Physiol. 1999, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Carlos Linares, J.; Taiqui, L.; Sangueesa-Barreda, G.; Ignacio Seco, J.; Julio Camarero, J. Age-related drought sensitivity of atlas cedar (Cedrus atlantica) in the moroccan middle atlas forests. Dendrochronologia 2013, 31, 88–96. [Google Scholar] [CrossRef]
- Bond, B.J. Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag effects of global vegetation responses to climate change. Glob. Chang. Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Li, X.; Ciais, P.; Babst, F.; Guo, W.; Zhang, C.; Magliulo, V.; Pavelka, M.; Liu, S.; et al. Differentiating drought legacy effects on vegetation growth over the temperate northern hemisphere. Glob. Chang. Biol. 2017, 24, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yi, C.; Wu, D.; Zhou, T.; Zhao, X.; Blanford, W.J.; Wei, S.; Wu, H.; Ling, D.; Li, Z. Tipping point of a conifer forest ecosystem under severe drought. Environ. Res. Lett. 2015, 10, 024011. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhao, X.; Huang, K.; Gao, S.; Wu, H.; Luo, H. Assessments of drought impacts on vegetation in china with the optimal time scales of the climatic drought index. Int. J. Environ. Res. Public Health 2015, 12, 7615–7634. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W. Meteorological Drought; US Department of Commerce Weather Bureau: Washington, DC, USA, 1965.
- Mckee, T.B.; Doesken, N.J.; Kleist, J. The relationship of drought frequency and duration to time scales. In Proceedings of the 8th Conference on Applied Climatology, Anaheim, CA, USA, 17–22 January 1993. [Google Scholar]
- Vicente-Serrano, S.M.; Begueria, S.; Lopez-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the modis vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Potop, V.; Mozny, M.; Soukup, J. Drought evolution at various time scales in the lowland regions and their impact on vegetable crops in the Czech Republic. Agric. For. Meteorol. 2012, 156, 121–133. [Google Scholar] [CrossRef]
- Mavromatis, T. Drought index evaluation for assessing future wheat production in Greece. Int. J. Climatol. 2007, 27, 911–924. [Google Scholar] [CrossRef]
- Feng, S.; Trnka, M.; Hayes, M.; Zhang, Y. Why do different drought indices show distinct future drought risk outcomes in the US Great Plains? J. Clim. 2017, 30, 265–278. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Begueria, S.; Lopez-Moreno, J.I. Comment on “characteristics and trends in various forms of the palmer drought severity index (PDSI) during 1900–2008” by Aiguo Dai. J. Geophys. Res.-Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, H.; Liang, S.; Zhou, T.; He, B.; Tang, B.; Wu, D. Responses of natural vegetation to different stages of extreme drought during 2009–2010 in southwestern china. Remote Sens. 2015, 7, 14039–14054. [Google Scholar] [CrossRef]
- State Forestry Administration of the People’s Republic of China. The Main Results at Eighth National Forest Resources Inventory (2009–2013). 2015. Available online: http://www.forestry.gov.cn/main/65/content-659670.html (accessed on 4 April 2018).
- The State Forestry Bureau. Atlas of Forest Resoureces of China; China Forestry Publishing House: Beijing, China, 2010. [Google Scholar]
- Chen, H.-P.; Jia, G.-S.; Feng, J.-M.; Dong, Y.-S. Increased browning of woody vegetation due to continuous seasonal droughts in Yunnan province, China. Atmos. Ocean. Sci. Lett. 2014, 7, 120–125. [Google Scholar]
- Yan, Z.; Zhang, Y.; Zhou, Z.; Han, N. The spatio-temporal variability of droughts using the standardized precipitation index in Yunnan, China. Nat. Hazards 2017, 88, 1023–1042. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Qamer, F.M.; Xu, J. Characterization of drought development through remote sensing: A case study in central Yunnan, China. Remote Sens. 2014, 6, 4998–5018. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, T.; Zhao, X.; Luo, H.; Gao, S.; Li, Z.; Cao, L. Diverse responses of different structured forest to drought in southwest china through remotely sensed data. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 217–225. [Google Scholar] [CrossRef]
- The Centre for Environmental Data Analysis. Available online: http://data.ceda.ac.uk/badc/cru/data/cru_ts/ (accessed on 4 April 2018).
- The Climatic Research Unit of the University of East Anglia. Available online: http://digital.csic.es/handle/10261/128892 (accessed on 4 April 2018).
- Guo, Z.D.; Hu, H.F.; Li, P.; Li, N.Y.; Fang, J.Y. Spatio-temporal changes in biomass carbon sinks in china’s forests from 1977 to 2008. Sci. Chin. Life Sci. 2013, 7, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Pilli, R.; Grassi, G.; Kurz, W.A.; Smyth, C.E.; Blujdea, V. Application of the cbm-cfs3 model to estimate Italy’s forest carbon budget, 1995–2020. Ecol. Model. 2013, 266, 144–171. [Google Scholar] [CrossRef]
- King, D.A. The adaptive significance of tree height. Am. Nat. 1990, 135, 809–828. [Google Scholar] [CrossRef]
- Forest Resources Management Department of the State Forestry Administration. Forest Resources Statistics of China—The Seventh National Forest Resources Inventory China; Forest Resources Management Department of the State Forestry Administration: Beijing, China, 2010.
- Maeda, E.E.; Heiskanen, J.; Aragao, L.E.O.C.; Rinne, J. Can modis evi monitor ecosystem productivity in the amazon rainforest? Geophys. Res. Lett. 2014, 41, 7176–7183. [Google Scholar] [CrossRef]
- The Land Processes Distributed Active Archive Center. Available online: http://reverb.echo.nasa.gov/reverb/#utf8=%E2%9C%93spatial_type=rectangle (accessed on 4 April 2018).
- Holben, B.N. Characteristics of maximum-value composite images from temporal avhrr data. Int. J. Remote Sens. 1986, 7, 1417–1434. [Google Scholar] [CrossRef]
- Garroutte, E.L.; Hansen, A.J.; Lawrence, R.L. Using ndvi and evi to map spatiotemporal variation in the biomass and quality of forage for migratory elk in the greater yellowstone ecosystem. Remote Sens. 2016, 8, 404. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Julio Camarero, J.; Begueria, S.; Trigo, R.; Lopez-Moreno, J.I.; Azorin-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Guttman, N.B. Comparing the palmer drought index and the standardized precipitation index. J. Am. Water Resour. Assoc. 1998, 34, 113–121. [Google Scholar] [CrossRef]
- Dunne, J.P.; John, J.G.; Shevliakova, E.; Stouffer, R.J.; Krasting, J.P.; Malyshev, S.L.; Milly, P.C.D.; Sentman, L.T.; Adcroft, A.J.; Cooke, W.; et al. GFDL’S ESM2 global coupled climate-carbon earth system models. Part II: Carbon system formulation and baseline simulation characteristics. J. Clim. 2013, 26, 2247–2267. [Google Scholar] [CrossRef]
- Dufresne, J.L.; Foujols, M.A.; Denvil, S.; Caubel, A.; Marti, O.; Aumont, O.; Balkanski, Y.; Bekki, S.; Bellenger, H.; Benshila, R.; et al. Climate change projections using the IPSL-CM5 earth system model: From CMIP3 to CMIP5. Clim. Dyn. 2013, 40, 2123–2165. [Google Scholar] [CrossRef]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845–872. [Google Scholar] [CrossRef]
- Bentsen, M.; Bethke, I.; Debernard, J.B.; Iversen, T.; Kirkevag, A.; Seland, O.; Drange, H.; Roelandt, C.; Seierstad, I.A.; Hoose, C.; et al. The Norwegian earth system model, NorESM1-M—Part 1: Description and basic evaluation of the physical climate. Geosci. Model Dev. 2013, 6, 687–720. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J. Changes in drought characteristics over china using the standardized precipitation evapotranspiration index. J. Clim. 2015, 28, 5430–5447. [Google Scholar] [CrossRef]
- The SPEI Calculation Procedure. Available online: http://digital.csic.es/handle/10261/10002 (accessed on 14 April 2018).
- Bellassen, V.; Luyssaert, S. Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.A.; Rosa, R.D.; Pereira, L.S. Climate trends and behaviour of drought indices based on precipitation and evapotranspiration in Portugal. Nat. Hazards Earth Syst. Sci. 2012, 12, 1481–1491. [Google Scholar] [CrossRef]
- Orwig, D.A.; Abrams, M.D. Variation in radial growth responses to drought among species, site, and canopy strata. Trees-Struct. Funct. 1997, 11, 474–484. [Google Scholar] [CrossRef]
- Gazis, C.; Feng, X.H. A stable isotope study of soil water: Evidence for mixing and preferential flow paths. Geoderma 2004, 119, 97–111. [Google Scholar] [CrossRef]
- Mahmood, T.H.; Vivoni, E.R. Forest ecohydrological response to bimodal precipitation during contrasting winter to summer transitions. Ecohydrology 2014, 7, 998–1013. [Google Scholar] [CrossRef]
- Sala, O.E.; Lauenroth, W.K.; Parton, W.J. Long-term soil-water dynamics in the shortgrass steppe. Ecology 1992, 73, 1175–1181. [Google Scholar] [CrossRef]
- Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Managing drought-sensitive forests under global Change. Low competition enhances long-term growth and water uptake in abies pinsapo. For. Ecol. Manag. 2017, 406, 72–82. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, W.; Chen, J.M.; Wang, X.; Yang, L.; Zheng, G. Disturbance-induced reduction of biomass carbon sinks of china’s forests in recent years. Environ. Res. Lett. 2015, 11, 114021. [Google Scholar] [CrossRef]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2001; China Forestry Publishing House: Beijing, China, 2002. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2002; China Forestry Publishing House: Beijing, China, 2003. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2003; China Forestry Publishing House: Beijing, China, 2004. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2004; China Forestry Publishing House: Beijing, China, 2005. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2005; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2006; China Forestry Publishing House: Beijing, China, 2007. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2007; China Forestry Publishing House: Beijing, China, 2008. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2008; China Forestry Publishing House: Beijing, China, 2009. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2009; China Forestry Publishing House: Beijing, China, 2010. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2010; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2011; China Forestry Publishing House: Beijing, China, 2012. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2012; China Forestry Publishing House: Beijing, China, 2013. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2013; China Forestry Publishing House: Beijing, China, 2014. [Google Scholar]
- The State Forestry Bureau. China Forestry Statistial Yearbook 2014; China Forestry Publishing House: Beijing, China, 2015. [Google Scholar]



| Drought Stress Intensity | SPEI |
|---|---|
| Severe drought | ≤−1.5 |
| Moderate drought | −1.5 to ≤−1 |
| Mild drought | −1 to ≤−0.5 |
| Normal (−) | −0.5 to ≤0 |
| Normal (+) | 0 to ≤0.5 |
| Mild wet | 0.5 to ≤1 |
| Moderate wet | 1 to ≤1.5 |
| Severe wet | >1.5 |
| Forest Stock Volume Density (m3·ha−1) | <30 | 30–60 | 60–90 | 90–120 | ≥120 |
|---|---|---|---|---|---|
| The optimal time scale of SPEI (month) | 23 | 24 | 24 | 24 | 35 |
| Regression coefficient | 1.35 | 1.58 | 1.97 | 2.35 | 2.88 |
| constant | −18.28 | −18.82 | −19.52 | −19.84 | −21.19 |
| R2max | 0.77 | 0.80 | 0.85 | 0.96 | 0.99 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Zhou, T.; Yi, C.; Xu, P.; Zhao, X.; Gao, S.; Liu, X. Stock Volume Dependency of Forest Drought Responses in Yunnan, China. Forests 2018, 9, 209. https://doi.org/10.3390/f9040209
Luo H, Zhou T, Yi C, Xu P, Zhao X, Gao S, Liu X. Stock Volume Dependency of Forest Drought Responses in Yunnan, China. Forests. 2018; 9(4):209. https://doi.org/10.3390/f9040209
Chicago/Turabian StyleLuo, Hui, Tao Zhou, Chuixiang Yi, Peipei Xu, Xiang Zhao, Shan Gao, and Xia Liu. 2018. "Stock Volume Dependency of Forest Drought Responses in Yunnan, China" Forests 9, no. 4: 209. https://doi.org/10.3390/f9040209
APA StyleLuo, H., Zhou, T., Yi, C., Xu, P., Zhao, X., Gao, S., & Liu, X. (2018). Stock Volume Dependency of Forest Drought Responses in Yunnan, China. Forests, 9(4), 209. https://doi.org/10.3390/f9040209