Charcoal Increases Microbial Activity in Eastern Sierra Nevada Forest Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Soil Collection and Charcoal Production
2.3. Soil and Charcoal Characterization
2.4. Polyphenol Adsorption
2.5. Microbial Respiration
2.6. Potential Nitrification Rate
2.7. Data Analysis
3. Results
3.1. Soil and Charcoal Characteristics
3.2. Polyphenol Adsorption
3.3. Microbial Respiration
3.4. Potential Nitrification
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stephens, S.L.; Collins, B.M. Fire regimes of mixed-conifer forests in the north-central Sierra Nevada at multiple spatial scales. Northwest Sci. 2004, 78, 12–23. [Google Scholar]
- Moghaddas, E.E.Y.; Stephens, S.L. Thinning, burning, and thin-burn fuel treatment effects on soil properties in a Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2007, 250, 156–166. [Google Scholar] [CrossRef]
- Jiménez Esquilín, A.E.; Stromberger, M.E.; Massman, W.J.; Frank, J.M.; Shepperd, W.D. Microbial community structure and activity in a Colorado Rocky Mountain forest soil scarred by slash pile burning. Soil Biol. Biochem. 2007, 39, 1111–1120. [Google Scholar] [CrossRef]
- Johnson, D.W.; Miller, W.W.; Susfalk, R.B.; Murphy, J.D.; Dahlgren, R.A.; Glass, D.W. Biogeochemical cycling in forest soils of the eastern Sierra Nevada Mountains, USA. For. Ecol. Manag. 2009, 258, 2249–2260. [Google Scholar] [CrossRef]
- Prieto-Fernández, Á.; Carballas, M.; Carballas, T. Inorganic and organic N pools in soils burned or heated: Immediate alterations and evolution after forest wildfires. Geoderma 2004, 121, 291–306. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choromanska, U.; DeLuca, T.H. Prescribed fire alters the impact of wildfire on soil biochemical properties in a Ponderosa pine forest. Soil Sci. Soc. Am. J. 2001, 65, 232–238. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.R.; Rogers, B.M.; Treseder, K.R.; Randerson, J.T. Fire severity influences the response of soil microbes to a boreal forest fire. Environ. Res. Lett. 2016, 11, 035004. [Google Scholar] [CrossRef]
- Knelman, J.E.; Graham, E.B.; Trahan, N.A.; Schmidt, S.K.; Nemergut, D.R. Fire severity shapes plant colonization effects on bacterial community structure, microbial biomass, and soil enzyme activity in secondary succession of a burned forest. Soil Biol. Biochem. 2015, 90, 161–168. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Science 2008, 320, 629. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, O.; Nilsson, M.C.; Wardle, D.A. Key ecological function of charcoal from wildfire in the Boreal forest. Oikos 1996, 77, 10–19. [Google Scholar] [CrossRef]
- Gibson, C.; Berry, T.D.; Wang, R.; Spencer, J.A.; Johnston, C.T.; Jiang, Y.; Bird, J.A.; Filley, T.R. Weathering of pyrogenic organic matter induces fungal oxidative enzyme response in single culture inoculation experiments. Org. Geochem. 2016, 92, 32–41. [Google Scholar] [CrossRef]
- Alexander, M. Introduction to Soil Microbiology, 2nd ed.; Wiley: New York, NY, USA, 1977; 467p. [Google Scholar]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H. Charcoal and carbon storage in forest soils of the Rocky Mountain West. Front. Ecol. Environ. 2008, 6, 18–24. [Google Scholar] [CrossRef]
- Keech, O.; Carcaillet, C.; Nilsson, M.C. Adsorption of allelopathic compounds by wood-derived charcoal: The role of wood porosity. Plant Soil. 2005, 272, 291–300. [Google Scholar] [CrossRef]
- Hatton, P.J.; Chatterjee, S.; Filley, T.; Dastmalchi, K.; Plante, A.F.; Abiven, S.; Ga, X.; Masiello, C.A.; Leavitt, S.W.; Nadelhoffer, K.J.; et al. Tree taxa and pyrolysis temperature interact to control the efficacy of pyrogenic organic matter formation. Biogeochemistry 2016, 130, 103–116. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Berglund, L.M.; DeLuca, T.H.; Zackrisson, O. Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol. Biochem. 2004, 36, 2067–2073. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For. Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture-National Resources Conservation Service: Washington, DC, USA, 2014; pp. 1–360.
- Johnson, B.G.; Johnson, D.W.; Miller, W.W.; Carroll-Moore, E.M.; Board, D.I. The effects of slash pile burning on soil and water macronutrients. Soil Sci. 2011, 176, 413–425. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Water Works Association: Washington, DC, USA, 2012. [Google Scholar]
- Belser, L.W.; Mays, E.L. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl. Environ. Microb. 1980, 39, 505–510. [Google Scholar]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization, and nitrification. In Methods of Soil Analysis: II. Microbiological and Biochemical Properties; Weaver, R.W., Ed.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 985–1018. [Google Scholar]
- Sullivan, B.W.; Selmants, P.C.; Hart, S.C. New evidence that high potential nitrification rates occur in soils during dry sesasons: Are microbial communities metabolically active during dry seasons? Soil Biol. Biochem. 2012, 53, 28–31. [Google Scholar] [CrossRef]
- Wang, R.; Gibson, C.D.; Berry, T.D.; Jiang, Y.; Bird, J.A.; Filley, T.R. Photooxidation of pyrogenic organic matter (PyOM) reduces the reactive, labile C pool and the apparent soil oxidative microbial enzyme response. Geoderma 2017, 293, 10–18. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas-fir ecosystem. Biol. Fertil. Soils 2007, 43, 303–311. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Nilsson, M.C.; Zackrisson, O. Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 2002, 133, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Zackrisson, O.; Nilsson, M.C. The charcoal effect in Boreal forests: Mechanisms and ecological consequences. Oecologia 1998, 115, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ball, P.N.; MacKenzie, M.D.; DeLuca, T.H.; Holben, W.E. Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J. Environ. Qual. 2010, 39, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.E.; Kulmatiski, A.; Beard, K.H.; Norton, J.M. Activated carbon decreases invasive plant growth by mediating plant–microbe interactions. AoB Plants 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
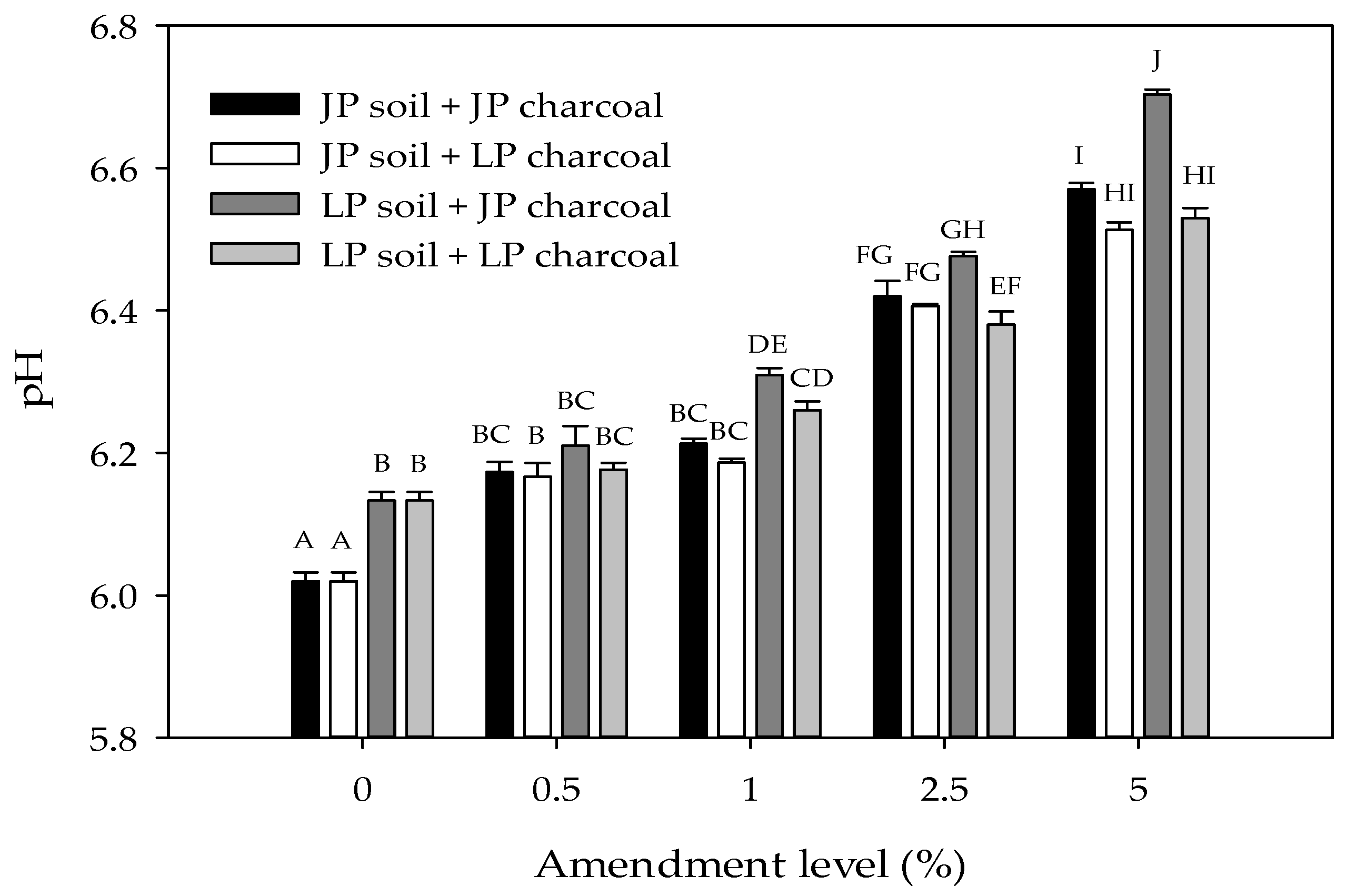
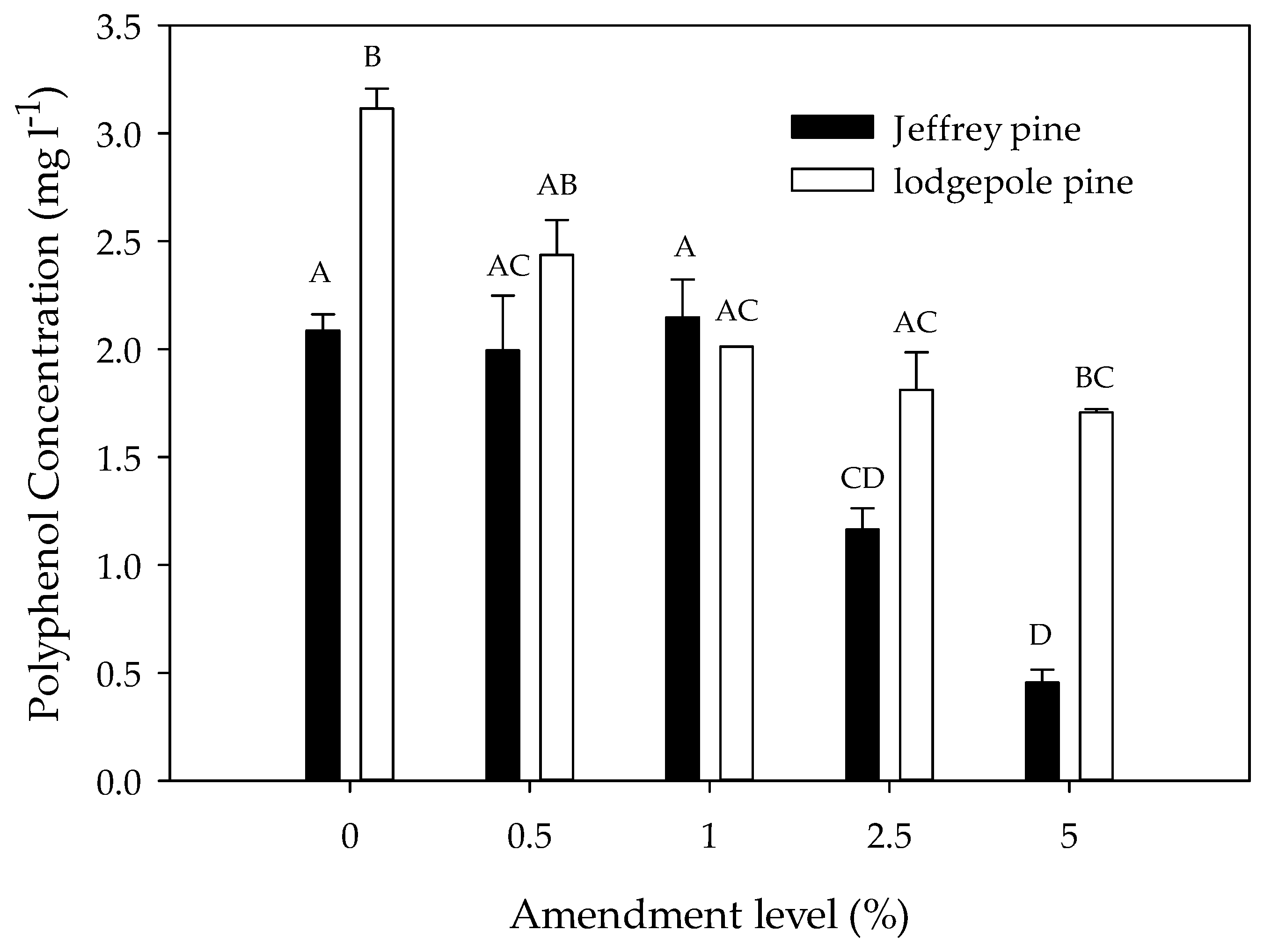

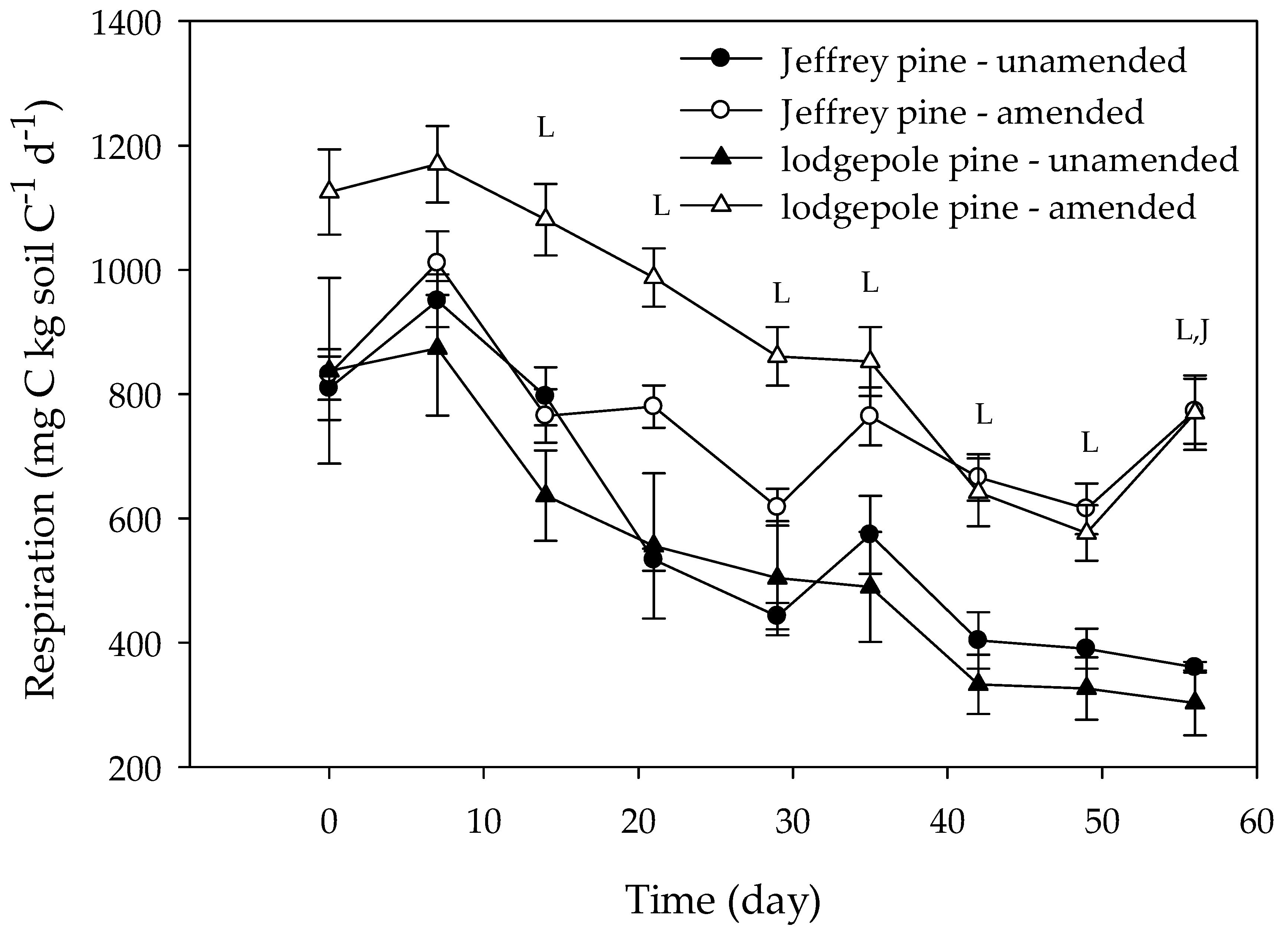
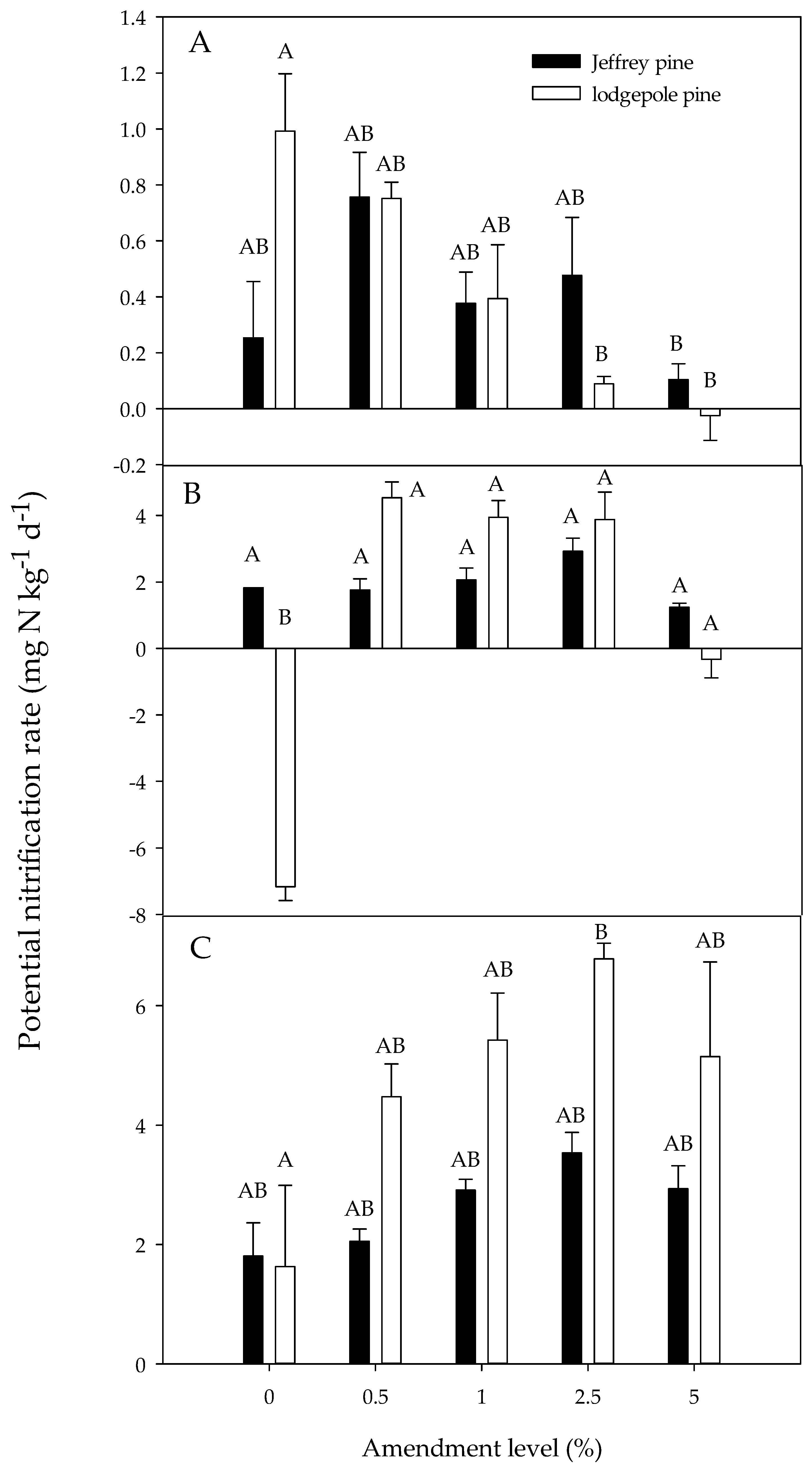
| Site | pH | Total C | Total N | CEC | Surface Area |
|---|---|---|---|---|---|
| (%) | (%) | meq 100 g−1 | m2 g−1 | ||
| JP Soil | 6.02 ± 0.02 | 1.59 ± 0.02 | 0.06 ± 0.002 | 9.8 | - |
| LP Soil | 6.13 ± 0.01 | 2.24 ± 0.21 | 0.08 ± 0.006 | 11.2 | - |
| JP charcoal | - | 93.0 ± 0.2 | - | 26.5 | 468.5 ± 14.5 |
| LP charcoal | - | 92.9 ± 5.7 | - | 27.7 | 449.1 ± 7.0 |
| pH | Polyphenol | Respiration | Respiration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per g Soil C | Per g Total C | |||||||||||
| df | F | p | df | F | p | df | F | p | df | F | p | |
| Amendment Level (AL) | 4 | 574.997 | <0.001 | 4 | 21.845 | <0.001 | 4 | 6.997 | <0.001 | 4 | 24.021 | <0.001 |
| Charcoal Type (CT) | 1 | 37.358 | <0.001 | - | - | - | 1 | <0.001 | 0.980 | 4 | 0.030 | 0.863 |
| Soil Type (ST) | 1 | 69.938 | <0.001 | 1 | 32.040 | <0.001 | 1 | 4.772 | 0.035 | 1 | 6.630 | 0.014 |
| Time (T) | - | - | - | - | - | - | 8 | 66.556 | <0.001 | 8 | 79.612 | <0.001 |
| AL × CT | 4 | 6.923 | <0.001 | - | - | - | 4 | 0.264 | 0.990 | 4 | 0.191 | 0.942 |
| AL × ST | 4 | 6.333 | <0.001 | 4 | 4.107 | 0.016 | 4 | 3.618 | 0.013 | 4 | 3.035 | 0.028 |
| AL × T | - | - | - | - | - | - | 32 | 2.109 | 0.002 | 32 | 4.215 | <0.001 |
| CT × ST | 1 | 11.25 | 0.002 | - | - | - | 1 | 0.021 | 0.886 | 1 | 0.080 | 0.780 |
| CT × T | - | - | - | - | - | - | 8 | 1.461 | 0.209 | 8 | 1.313 | 0.271 |
| T × ST | - | - | - | - | - | - | 8 | 5.880 | <0.001 | 8 | 5.702 | <0.001 |
| AL × CT × ST | 4 | 2.095 | 0.100 | - | - | - | 32 | 0.925 | 0.459 | 32 | 0.341 | 0.848 |
| AL × CT × T | - | - | - | - | - | - | 32 | 1.344 | 0.128 | 32 | 0.974 | 0.516 |
| AL × ST × T | - | - | - | - | - | - | 32 | 1.656 | 0.027 | 32 | 2.160 | <0.001 |
| CT × ST × T | - | - | - | - | - | - | 8 | 3.292 | <0.001 | 8 | 1.611 | 0.160 |
| CT × T × ST × AL | - | - | - | - | - | - | 32 | 1.735 | 0.017 | 32 | 1.097 | 0.349 |
| Amendment | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 29 | 35 | 42 | 49 | 56 | ||
| 0% | A | A | A | A | A | A | A | A | A | |
| 0.5% | AB | AB | AB | B | AB | AB | B | B | B | |
| Per g soil C | 1% | B | B | B | B | B | B | B | B | B |
| 2.5% | AB | AB | AB | B | B | B | B | B | B | |
| 5% | A | A | AB | B | AB | AB | AB | B | B | |
| 0% | A | A | A | AB | AB | AB | AB | AB | CD | |
| 0.5% | A | A | A | A | A | A | A | A | AB | |
| Per g total C | 1% | A | A | A | A | A | A | AB | A | A |
| 2.5% | B | B | B | BC | B | BC | B | BC | BC | |
| 5% | B | C | B | C | C | C | C | C | D |
| Day 0 | Day 30 | Day 60 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| Amendment Level | 4 | 5.941 | 0.026 | 4 | 7.569 | 0.001 | 4 | 3.444 | 0.028 |
| Soil Type | 1 | 0.177 | 0.679 | 1 | 8.414 | 0.010 | 1 | 8.478 | 0.009 |
| Amend. Level × Soil Type | 4 | 3.224 | 0.034 | 4 | 7.230 | 0.002 | 4 | 0.838 | 0.518 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, Z.W.; Sullivan, B.W.; Qualls, R.G.; Blank, R.R.; Schmidt, C.A.; Verburg, P.S.J. Charcoal Increases Microbial Activity in Eastern Sierra Nevada Forest Soils. Forests 2018, 9, 93. https://doi.org/10.3390/f9020093
Carter ZW, Sullivan BW, Qualls RG, Blank RR, Schmidt CA, Verburg PSJ. Charcoal Increases Microbial Activity in Eastern Sierra Nevada Forest Soils. Forests. 2018; 9(2):93. https://doi.org/10.3390/f9020093
Chicago/Turabian StyleCarter, Zachary W., Benjamin W. Sullivan, Robert G. Qualls, Robert R. Blank, Casey A. Schmidt, and Paul S.J. Verburg. 2018. "Charcoal Increases Microbial Activity in Eastern Sierra Nevada Forest Soils" Forests 9, no. 2: 93. https://doi.org/10.3390/f9020093




