Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014
Abstract
:1. Introduction
2. Materials and Methods
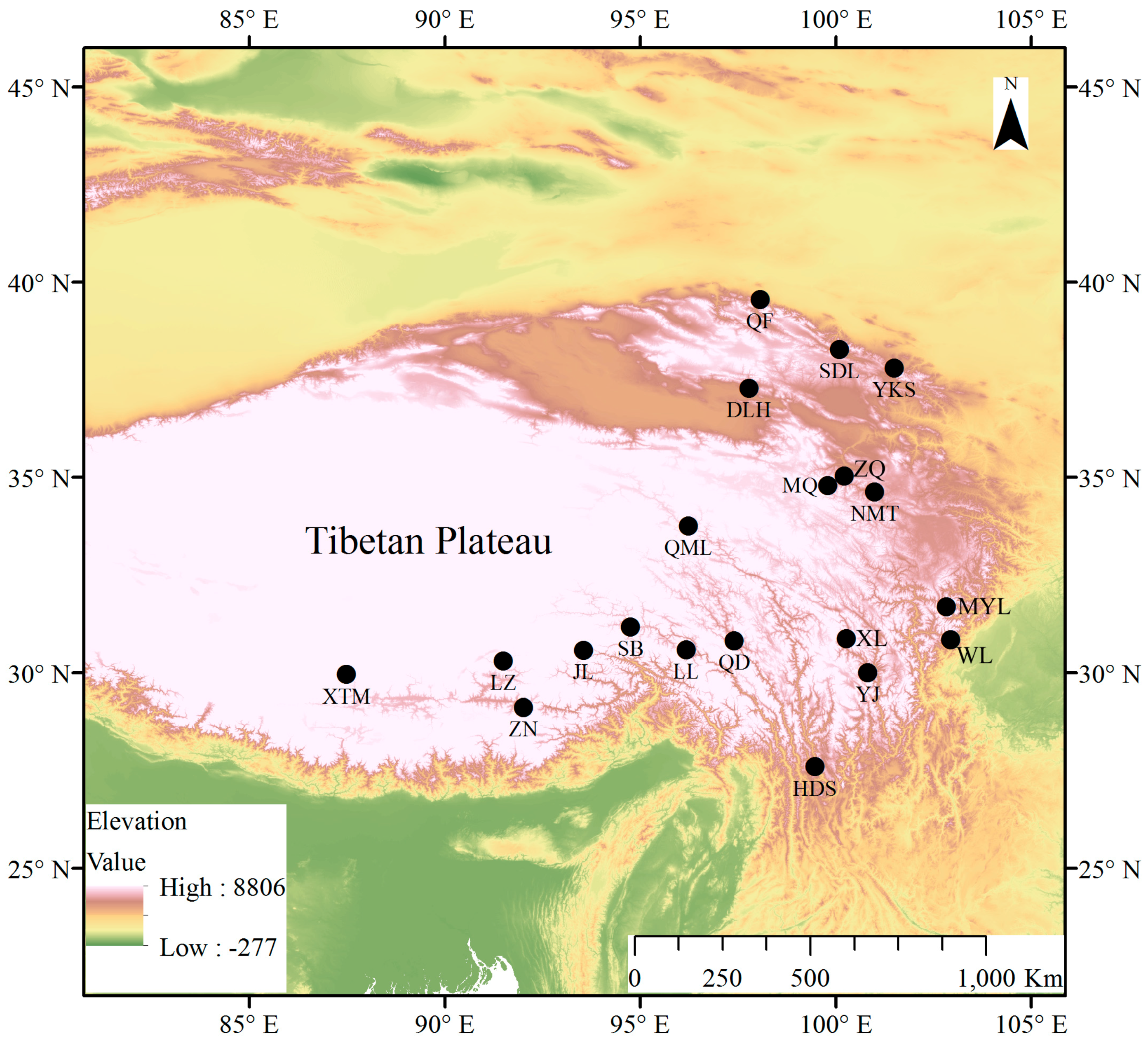
2.1. Study Region
2.2. Tree-Ring Width Chronology
2.3. Timings of Cambium Phenology
2.4. Statistical Analyses
3. Results
3.1. Tree-Ring Width Chronologies
3.2. Timings of Cambium Phenology
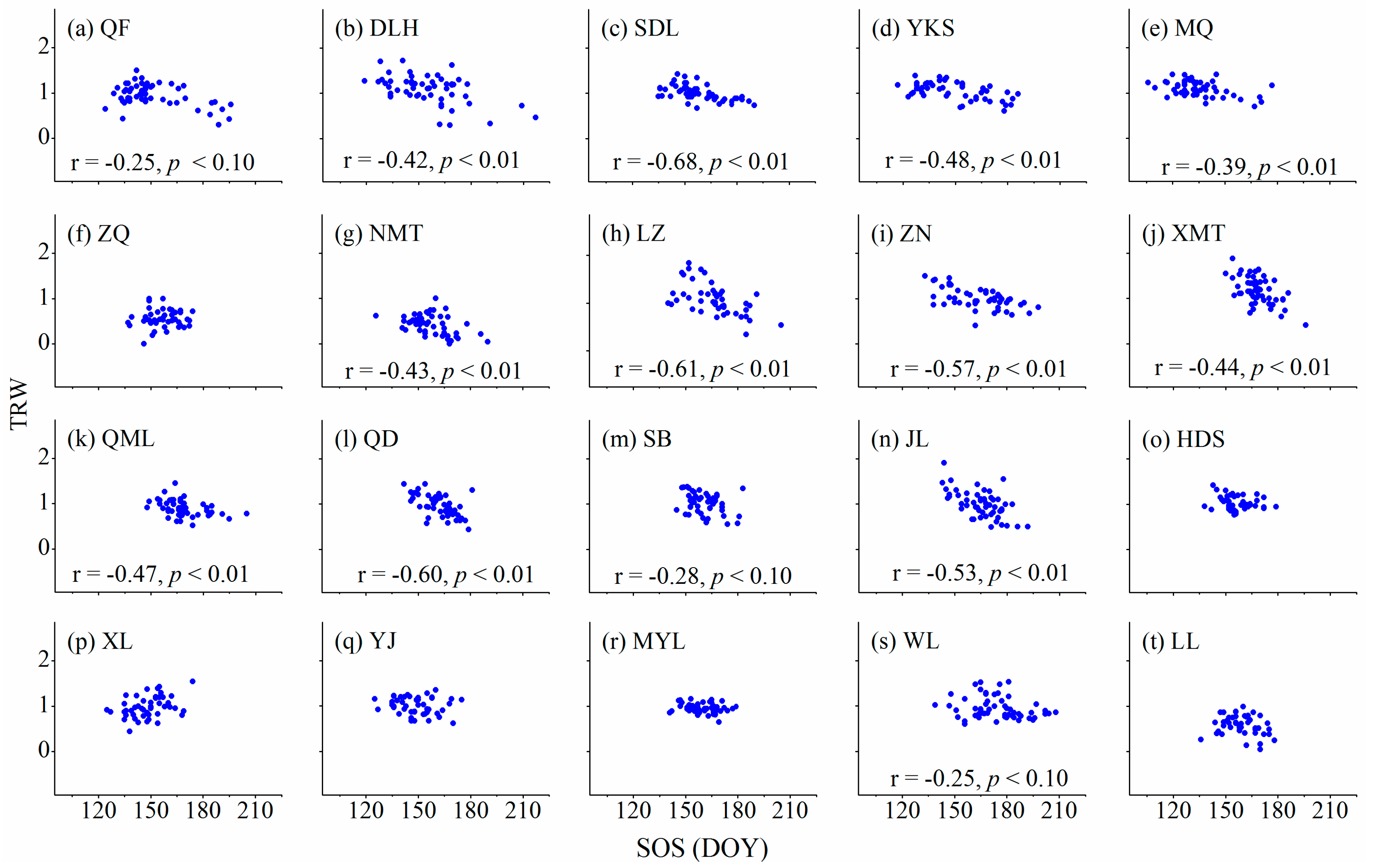
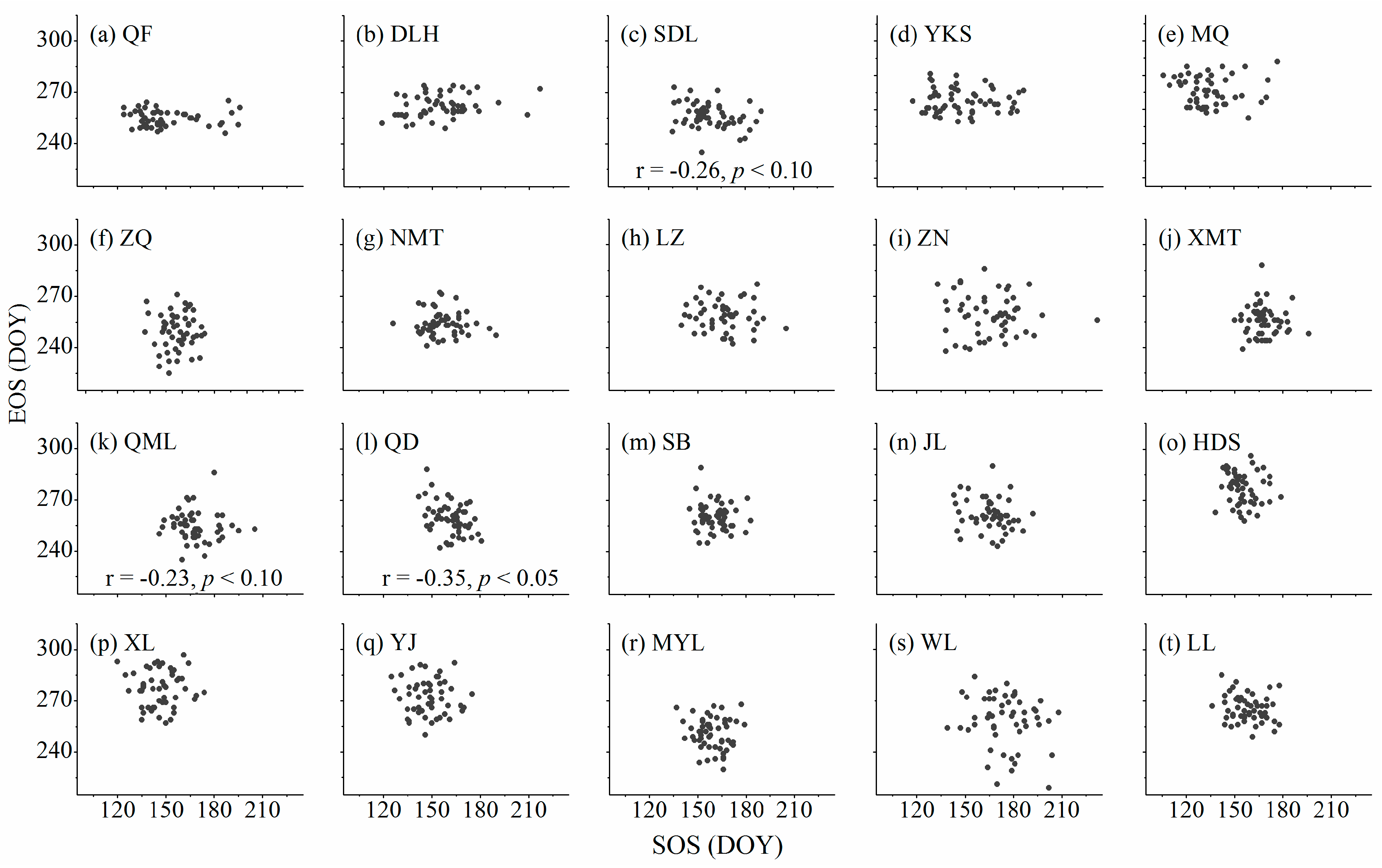
3.3. Relationships between Cambium Phenology and Wood Formation
4. Discussion
4.1. Relationships between Wood Formation and Start (Ending) of the Growing Season
4.2. Relationships between Wood Formation and Length of Growing Season
4.3. Relationships between Start and Ending of the Growing Season
4.4. Other Factors Influencing Wood Formation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Sass-Klaassen, U. Tree physiology: Tracking tree carbon gain. Nat. Plants 2015, 1, 15175. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Eugster, W.; Etzold, S.; Dobbertin, M.; Buchmann, N.; Hasler, R. Link between continuous stem radius changes and net ecosystem productivity of a subalpine Norway spruce forest in the Swiss Alps. New Phytol. 2010, 187, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.E.; Rathgeber, C.B.; Frank, D.; Fonti, P.; Makinen, H.; Prislan, P.; Rossi, S.; Del Castillo, E.M.; Campelo, F.; Vavrcik, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.M.S.; Winston, G.C.; Goulden, M.L. Age-dependent response of boreal forest to temperature and rainfall variability. Glob. Chang. Biol. 2008, 14, 1904–1916. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; King, G.M.; et al. A meta-analysis of cambium phenology and growth: Linear and non-linear patterns in conifers of the northern hemisphere. Ann. Bot. 2013, 112, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Morin, H.; Deslauriers, A.; Rossi, S. Xylogenesis in black spruce: Does soil temperature matter? Tree Physiol. 2012, 32, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Palombo, C.; Tognetti, R.; La Porta, N.; Anichini, M.; Giovannelli, A.; Emiliani, G. Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol. 2016, 36, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Girard, M.J.; Morin, H. Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob. Chang. Biol. 2014, 20, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Morin, H.; Deslauriers, A.; Rossi, S. Xylem phenology and wood production: Resolving the chicken-or-egg dilemma. Plant Cell Environ. 2010, 33, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Rathgeber, C.B.; Rossi, S.; Bontemps, J.D. Cambial activity related to tree size in a mature silver-fir plantation. Ann. Bot. 2011, 108, 429–438. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yang, B.; Wang, Z.; Bräuning, A.; Pourtahmasi, K.; Oladi, R. Climatic forcing of xylem formation in Qilian juniper on the northeastern Tibetan Plateau. Trees 2016, 30, 923–933. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Gričar, J.; Liang, E.; Čufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Vaganov, E.; Hughes, M.; Shashkin, A. Modeling external influence on xylem development. In Growth Dynamics of Conifer Tree Rings; Ecological Studies (Analysis and Synthesis); Springer: Berlin/Heidelberg, Germany, 2006; Volume 183. [Google Scholar]
- Shishov, V.V.; Tychkov, I.I.; Popkova, M.I.; Ilyin, V.A.; Bryukhanova, M.V.; Kirdyanov, A.V. VS-oscilloscope: A new tool to parameterize tree radial growth based on climate conditions. Dendrochronologia 2016, 39, 42–50. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganove, E.; Rossi, S.; Ljungqvist, F.C.; Bräuning, A.; Grießinger, J. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Sun, G.; Zhu-Barker, X.; Liang, Q.L.; Wu, N.; Horwath, W.R. Tree growth acceleration and expansion of alpine forests: The synergistic effect of atmospheric and edaphic change. Sci. Adv. 2016, 2, e1501302. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, H.; Liu, X.; Liang, E.; Grießinger, J.; Wu, G.; Li, X.; Bräuning, A. Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural alpine timberline on the southeastern Tibetan Plateau? Glob. Planet. Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Shao, X.; Qin, D.; Xu, G.; Wang, B.; Zeng, X.; Wu, G.; Zhang, X. Differential response of Qilian juniper radial growth to climate variations in the middle of Qilian Mountains and the northeastern Qaidam Basin. Clim. Chang. 2015, 133, 237–251. [Google Scholar] [CrossRef]
- Fu, Y.S.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Richardson, A.D. The timing of autumn senescence is affected by the timing of spring phenology: Implications for predictive models. Glob. Chang. Biol. 2015, 21, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, Y.H.; Zhu, Z.; Liu, Y.; Liu, Z.; Huang, M.; Janssens, I.A.; Piao, S. Delayed autumn phenology in the Northern Hemisphere is related to change in both climate and spring phenology. Glob. Chang. Biol. 2016, 22, 3702–3711. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Masson-Delmotte, V.; Gao, J.; Yu, W.; Yang, X.; Risi, C.; Sturm, C.; Werner, M.; Zhao, H.; He, Y.; et al. A review of climatic controls on δ18O in precipitation over the Tibetan Plateau: Observations and simulations. Rev. Geophys. 2013, 51, 525–548. [Google Scholar] [CrossRef] [Green Version]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; The University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN, a Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling with Interactive Graphics; Tree-Ring Laboratory Lamont Doherty Earth Observatory of Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Cook, E.R.; Peters, K. Calculating unbiased tree-ring indices for the study of climatic and environmental change. Holocene 1997, 7, 361–370. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology; Kluwer Academic Press: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Fisher, R.A. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 1915, 10, 507–521. [Google Scholar] [CrossRef]
- Gruber, A.; Zimmermann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.K.; Tardif, J.C.; Bergeron, Y. Xylem production in six tree species growing on an island in the boreal forest region of western Quebec, Canada. Can. J. Bot. 2007, 85, 518–525. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, E.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol. 2013, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Deslauriers, A.; Bräuning, A. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 2014, 29, 25–34. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Zhang, Y.X.; Lu, M.; Xu, X.Y.; Zhang, F.; Liu, W.H.; Gao, L.L. Forward modeling analyses of Qilian Juniper (Sabina przewalskii) growth in response to climate factors in different regions of the Qilian Mountains, northwestern China. Trees 2015, 30, 175–188. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Gričar, J.; Zupančič, M.; Čufar, K.; Oven, P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Technol. 2007, 41, 463–475. [Google Scholar] [CrossRef]
- Cong, N.; Shen, M.; Piao, S. Spatial variations in responses of vegetation autumn phenology to climate change on the Tibetan Plateau. J. Plant Ecol. 2016, 10, 744–752. [Google Scholar] [CrossRef]
- Huang, J.G.; Deslauriers, A.; Rossi, S. Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol. 2014, 203, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, J.X.; Dang, Q.L.; Apps, M.J.; Jiang, H. TRIPLEX: A generic hybrid model for predicting forest growth and carbon and nitrogen dynamics. Ecol. Model. 2002, 153, 109–130. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Anchukaitis, K.J.; Evans, M.N. How well understood are the processes that create dendroclimatic records? A mechanistic model of the climatic control on conifer tree-ring growth dynamics. In Dendroclimatology; Hughes, M.K., Swetnam, T.W., Diaz, H.F., Eds.; Developments in Paleoenvironmental Research; Springer: Berlin, Germany; Dordrecht, The Netherlands, 2011; pp. 37–75. [Google Scholar]
- Henttonen, H.M.; Mäkinen, H.; Nöjd, P. Seasonal dynamics of the radial increment of Scots pine and Norway spruce in the southern and middle boreal zones in Finland. Can. J. For. Res. 2009, 39, 606–618. [Google Scholar] [CrossRef]
- De Micco, V.; Campelo, F.; Cherubini, P.; Battipaglia, G.; Bräuning, A.; Grabner, M.; De Luis, M. Intra-annual density fluctuations in tree rings: How, when, where, and why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- Gower, S.T.; Krankina, O.; Olson, R.J.; Apps, M.; Linder, S.; Wang, C. Net primary production and carbon allocation patterns of boreal forest ecosystems. Ecol. Appl. 2001, 11, 1395–1411. [Google Scholar] [CrossRef]
- Franke, J.; Frank, D.; Raible, C.C.; Esper, J.; Brönnimann, S. Spectral biases in tree-ring climate proxies. Nat. Clim. Chang. 2013, 3, 360–364. [Google Scholar] [CrossRef]
- Esper, J.; Schneider, L.; Smerdon, J.E.; Schöne, B.R.; Büntgen, U. Signals and memory in tree-ring width and density data. Dendrochronologia 2015, 35, 62–70. [Google Scholar] [CrossRef]
- Büntgen, U.; Trnka, M.; Krusic, P.J.; Kyncl, T.; Kyncl, J.; Luterbacher, J.; Zorita, E.; Ljungqvist, F.C.; Auer, I.; Konter, O.; et al. Tree-Ring amplification of the early nineteenth-century summer cooling in central Europe. J. Clim. 2015, 28, 5272–5288. [Google Scholar] [CrossRef]

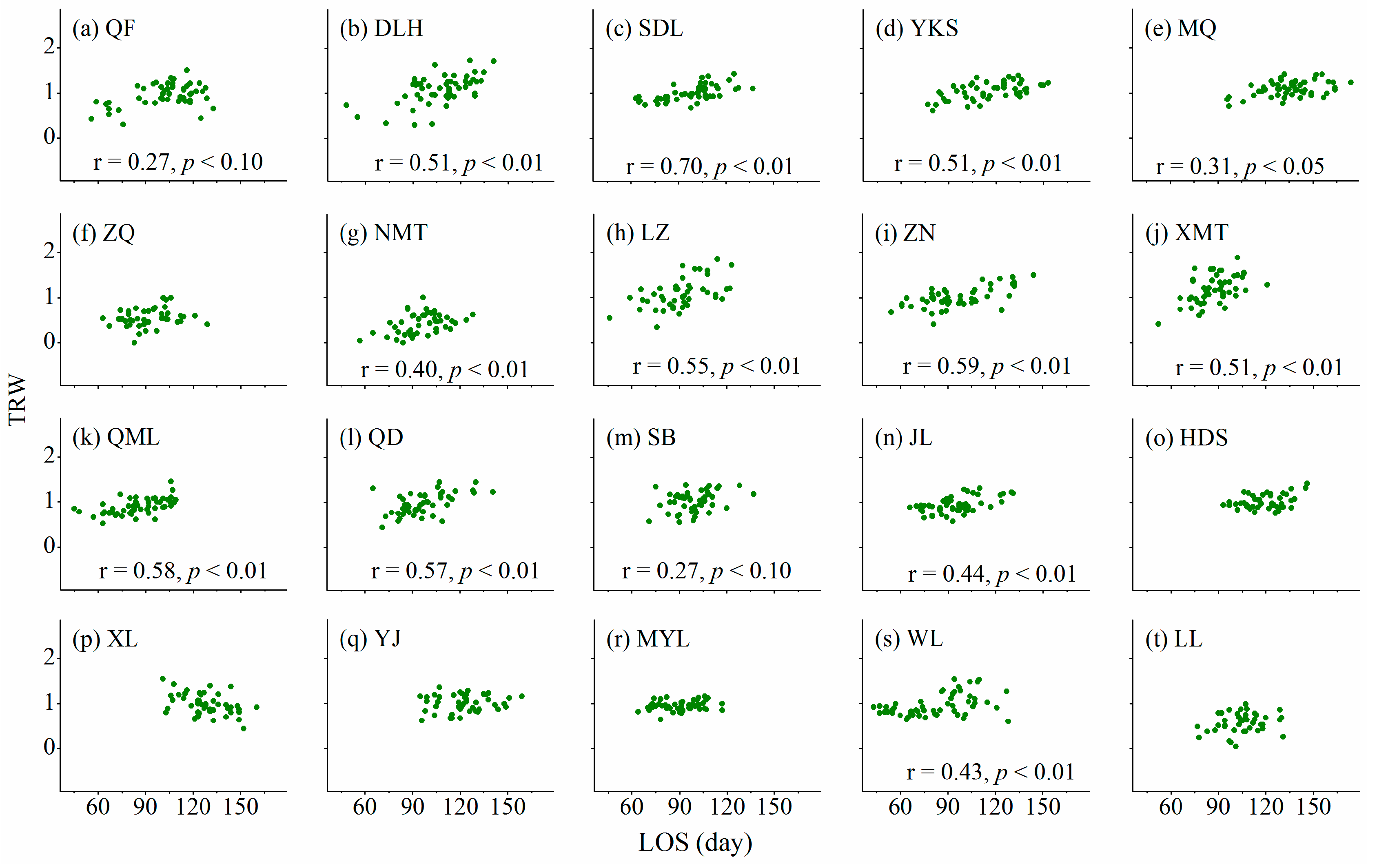
| TRW–SOS | TRW–EOS | TRW–LOS | SOS–EOS | |
|---|---|---|---|---|
| Mean value | −0.36 | 0.18 | 0.38 | −0.09 |
| 95% | −0.55 | 0.43 | 0.57 | −0.34 |
| 5% | −0.08 | −0.09 | 0.11 | 0.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Yang, B.; Shishov, V.; Rossi, S.; Bräuning, A.; Ljungqvist, F.C.; Grießinger, J. Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014. Forests 2018, 9, 86. https://doi.org/10.3390/f9020086
He M, Yang B, Shishov V, Rossi S, Bräuning A, Ljungqvist FC, Grießinger J. Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014. Forests. 2018; 9(2):86. https://doi.org/10.3390/f9020086
Chicago/Turabian StyleHe, Minhui, Bao Yang, Vladimir Shishov, Sergio Rossi, Achim Bräuning, Fredrik Charpentier Ljungqvist, and Jussi Grießinger. 2018. "Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014" Forests 9, no. 2: 86. https://doi.org/10.3390/f9020086
APA StyleHe, M., Yang, B., Shishov, V., Rossi, S., Bräuning, A., Ljungqvist, F. C., & Grießinger, J. (2018). Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014. Forests, 9(2), 86. https://doi.org/10.3390/f9020086










