Long-Term Thinning Does not Significantly Affect Soil Water-Stable Aggregates and Diversity of Bacteria and Fungi in Chinese Fir (Cunninghamia lanceolata) Plantations in Eastern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Vegetation Measurements
2.4. Soil Sampling and Measurements
2.5. WSA Fractions Separation
2.6. Soil Microbial Community Measurements
2.7. Data Analyses
3. Results
3.1. Composition, SOC, and TN Concentration of WSA
3.2. Diversity of the Soil Bacterial and Fungal Communities
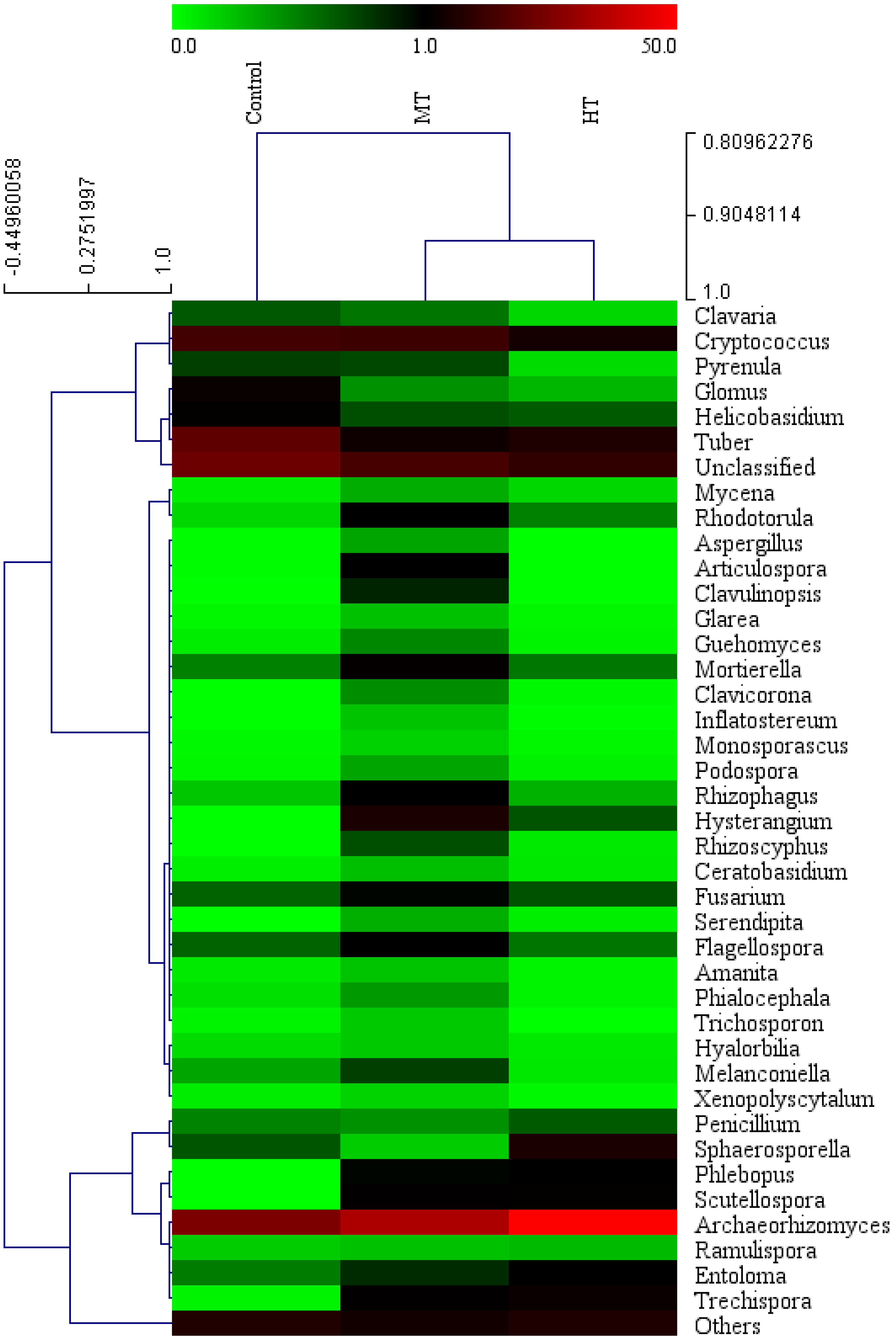
3.3. Composition of the Soil Bacterial and Fungal Community
4. Discussion
4.1. WSA Change
4.2. Microbial Diversity and Composition
4.3. WSA and Microbial Diversity And Composition
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| V | FFB | UVB | FTOC | FTN | SOC | STN | pH | MACC | MICC | CSC | MACN | MICN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFB | 0.012 | ||||||||||||
| UVB | −0.037 | 0.954 ** | |||||||||||
| FTOC | −0.775 * | 0.058 | −0.019 | ||||||||||
| FTN | −0.776 * | 0.271 | 0.381 | 0.698 * | |||||||||
| SOC | −0.484 | 0.043 | 0.095 | 0.009 | 0.230 | ||||||||
| STN | −0.244 | 0.709 * | 0.744 * | 0.283 | 0.372 | −0.218 | |||||||
| pH | 0.009 | 0.121 | 0.219 | −0.164 | 0.389 | 0.331 | −0.330 | ||||||
| MACC | −0.159 | −0.008 | 0.158 | −0.364 | 0.093 | 0.836 ** | −0.227 | 0.399 | |||||
| MICC | −0.623 | −0.013 | 0.183 | 0.191 | 0.586 | 0.709 * | 0.095 | 0.314 | 0.766 * | ||||
| CSC | −0.361 | −0.003 | 0.230 | 0.101 | 0.692 * | 0.243 | 0.077 | 0.671 * | 0.428 | 0.733 * | |||
| MACN | −0.551 | 0.620 | 0.677 * | 0.540 | 0.888 ** | 0.108 | 0.529 | 0.419 | −0.018 | 0.339 | 0.529 | ||
| MICN | −0.398 | 0.630 | 0.611 | 0.385 | 0.302 | −0.038 | 0.936 ** | −0.497 | −0.193 | 0.116 | −0.109 | 0.419 | |
| CSN | −0.085 | 0.309 | 0.241 | 0.047 | 0.274 | 0.285 | −0.277 | 0.646 | 0.090 | −0.111 | 0.064 | 0.488 | −0.290 |
| V | FFB | UVB | FTOC | FTN | SOC | STN | pH | MACC | MICC | CSC | MACN | MICN | CSN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OUT1 | −0.674 * | −0.333 | −0.251 | 0.487 | 0.311 | 0.283 | 0.232 | −0.421 | 0.149 | 0.558 | 0.180 | −0.061 | 0.389 | −0.631 |
| Chao1 | −0.705 * | −0.219 | −0.174 | 0.585 | 0.366 | 0.281 | 0.258 | −0.424 | 0.062 | 0.424 | 0.032 | 0.036 | 0.426 | −0.453 |
| Shannon1 | −0.349 | −0.262 | −0.212 | 0.350 | 0.153 | 0.128 | 0.185 | −0.300 | 0.060 | 0.394 | 0.149 | −0.193 | 0.261 | −0.710 * |
| Simpson1 | 0.118 | 0.289 | 0.175 | −0.187 | −0.119 | 0.080 | −0.161 | 0.245 | −0.089 | −0.391 | −0.283 | 0.210 | −0.111 | 0.800 ** |
| OTU2 | −0.391 | 0.044 | 0.056 | 0.496 | 0.421 | −0.180 | 0.429 | −0.015 | −0.392 | 0.085 | 0.270 | 0.269 | 0.411 | −0.294 |
| Chao2 | −0.516 | 0.129 | 0.170 | 0.554 | 0.590 | −0.008 | 0.447 | 0.132 | −0.219 | 0.292 | 0.441 | 0.425 | 0.421 | −0.205 |
| Shannon2 | 0.321 | −0.362 | −0.401 | −0.171 | −0.218 | −0.408 | −0.376 | 0.263 | −0.472 | −0.565 | −0.090 | −0.226 | −0.454 | 0.201 |
| Simpson2 | 0.209 | 0.138 | 0.212 | −0.240 | −0.136 | −0.041 | 0.180 | −0.238 | 0.308 | 0.271 | 0.096 | −0.074 | 0.113 | −0.357 |

References
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.A.; Bijlsma, R.J.; De Bruyn, L.U.; Fuhr, M.; Grandin, U.L.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Overby, S.T.; Owen, S.M.; Hart, S.C.; Neary, D.G.; Johnson, N.C. Soil microbial community resilience with tree thinning in a 40-year-old experimental ponderosa pine forest. Appl. Soil Ecol. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Owen, S.M.; Sieg, C.H.; Gehring, C.A.; Bowker, M.A. Above- and belowground responses to tree thinning depend on the treatment of tree debris. For. Ecol. Manag. 2009, 259, 71–80. [Google Scholar] [CrossRef]
- Pickles, B.J.; Genney, D.R.; Potts, J.M.; Lennon, J.J.; Anderson, I.C.; Alexander, I.J. Spatial and temporal ecology of Scots pine ectomycorrhizas. New Phytol. 2010, 186, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.; Gundersen, P.; Jönsson-Belyazid, U.; Kjønaas, O.J.; Persson, T.; Sigurdsson, B.D.; Stupak, I.; Vesterdal, L. Influence of different tree-harvesting intensities on forest soil carbon stocks in boreal and northern temperate forest ecosystems. For. Ecol. Manag. 2015, 351, 9–19. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; He, Z.; Wang, R.; Wu, P.; Ma, X. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. 2016, 23, 24135–24150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, Y.; Zhou, C.; Wu, Z.; Zheng, L.; Hu, X.; Chen, H.; Gan, J. Effects of cutting intensity on soil physical and chemical properties in a mixed natural forest in southeastern China. Forests 2015, 6, 4495–4509. [Google Scholar] [CrossRef]
- Fang, S.; Lin, D.; Tian, Y.; Hong, S. Thinning intensity affects soil-atmosphere fluxes of greenhouse gases and soil nitrogen mineralization in a lowland poplar plantation. Forests 2016, 7, 141. [Google Scholar] [CrossRef]
- Rab, M.A. Soil physical and hydrological properties following logging and slash burning in the Eucalyptus regnans forest of southeastern Australia. For. Ecol. Manag. 1996, 84, 159–176. [Google Scholar] [CrossRef]
- Lin, J.C.; Chiu, C.M.; Lin, Y.J.; Liu, W.Y. Thinning effects on biomass and carbon stock for Young Taiwania plantations. Sci. Rep. 2018, 8, 3070. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dighton, J.; Gray, D. The effects of thinning and soil disturbance on enzyme activities under pitch pine soil in New Jersey Pinelands. Appl. Soil Ecol. 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Ruiz-Peinado, R.; Modrego, P.; Alonso, R.; Montero, G. Forest thinning impact on carbon stock and soil condition in Southern European populations of P. sylvestris L. For. Ecol. Manag. 2015, 357, 259–267. [Google Scholar] [CrossRef]
- Zhang, J.; Webster, J.; Young, D.H.; Fiddler, G.O. Effect of thinning and soil treatments on Pinus ponderosa plantations: 15-year results. For. Ecol. Manag. 2016, 368, 123–132. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Wang, G.G. Effects of Thinning on Soil Organic Carbon Fractions and Soil Properties in Cunninghamia lanceolata Stands in Eastern China. Forests 2017, 8, 198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.L.; Dong, X.B. Grey clustering evaluation of soil nutrients of a low-quality forest in the Xiaoxing’an Mountain at different cutting intensities. J. Northeast For. Univ. 2014, 42, 21–24, (In Chinese with English Abstract). [Google Scholar]
- Le Bissonnais, Y. Aggregate stability and assessment of soil crustability and erodibility. 1. Theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Angers, D.A.; Caron, J. Plant-induced changes in soil structure: Processes and feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Six, J. Organic resource quality influences short-term aggregate dynamics and soil organic carbon and nitrogen accumulation. Soil Biol. Biochem. 2011, 43, 657–666. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D.; Bergeron, Y.; Chen, H.Y. Black spruce soils accumulate more uncomplexed organic matter than aspen soils. Soil Sci. Soc. Am. J. 2011, 75, 1125–1132. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Shukla, M.K.; Lal, R.; Ebinger, M. Soil quality indicators for reclaimed mine soils in southeastern Ohio. Soil Sci. 2004, 169, 133–142. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Wen, D. Change of soil carbon fractions and water-stable aggregates in a forest ecosystem succession in south China. Forests 2015, 6, 2703–2718. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Besnard, E.; Chenu, C.; Balesdent, J.; Puget, P.; Arrouaya, D. Fate of particulate organic matter in soil aggregates during cultivation. Eur. J. Soil Sci. 1996, 47, 495–503. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Shrestha, B.M. Stand age, fire and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests. Soil Biol. Biochem. 2012, 50, 149–157. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R.; Sitaula, B.K.; Lal, R.; Bajracharya, R.M. Soil aggregate-and particle-associated organic carbon under different land uses in Nepal. Soil Sci. Soc. Am. J. 2007, 71, 1194–1203. [Google Scholar] [CrossRef]
- Vance, E.D. Agricultural site productivity: Principles derived from long-term experiments and their implications for intensively managed forests. For. Ecol. Manag. 2000, 138, 369–396. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Jurgensen, M.; Terry, T. Maintaining soil productivity during forest or biomass-to-energy thinning harvests in the western United States. West. J. Appl. For. 2010, 25, 5–11. [Google Scholar]
- Liu, S.M.; Tian, D.L.; Xiang, W.H.; Yan, W.D.; Liu, Y.G.; Hu, X.J. The impacts of thinning intensity on overland flow in a Chinese fir plantation. Acta Ecol. Sin. 2015, 35, 5769–5775, (In Chinese with English Abstract). [Google Scholar]
- Costa, A.D.; Albuquerque, J.A.; Costa, A.D.; Warmling, M.T.; Magro, B.A. Pine harvest impact on soil structure of a dystric cambisol (Humic). Rev. Bras. Ciênc. Solo 2016, 40, e0140643. [Google Scholar] [CrossRef]
- Korb, J.E.; Johnson, N.C.; Covington, W.W. Arbuscular mycorrhizal propagule densities respond rapidly to ponderosa pine restoration treatments. J. Appl. Ecol. 2003, 40, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, D.C.; Bakker, J.D.; Daniels, M.L.; Moore, M.M.; Casey, C.A.; Springer, J.D. Restoring plant species diversity and community composition in a ponderosa pine-bunch-grass ecosystem. Plant Ecol. 2008, 197, 139–151. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, D.M.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Grady, K.C.; Hart, S.C. Influences of thinning, prescribed burning, and wildfire on soil processes and properties in southwestern ponderosa pine forests: A retrospective study. For. Ecol. Manag. 2006, 234, 123–135. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zouhar, K.L. Effects of selection harvest and prescribed fire on the soil nitrogen status of ponderosa pine forests. For. Ecol. Manag. 2000, 138, 263–271. [Google Scholar] [CrossRef]
- Holden, S.R.; Treseder, K.K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 2013, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 33, 1599–1611. [Google Scholar] [CrossRef]
- Metzger, L.; Levanon, D.; Mingelgrin, U. The effect of sewage-sludge on soil structural stability-microbiological aspects. Soil Sci. Soc. Am. J. 1987, 51, 346–351. [Google Scholar] [CrossRef]
- Chenu, C. Influence of a fungal polysaccharide, scleroglucan, on clay microstructures. Soil Biol. Biochem. 1989, 21, 299–305. [Google Scholar] [CrossRef]
- Tisdall, J.M. Fungal hyphae and structural stability of soil. Aust. J. Soil Res. 1991, 29, 729–743. [Google Scholar] [CrossRef]
- Lynch, J.M.; Bragg, E. Microorganisms and soil aggregate stability. Adv. Soil Sci. 1985, 2, 134–170. [Google Scholar]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef] [PubMed]
- Amellal, N.; Burtin, G.; Bartoli, F.; Heulin, T. Colonization of wheat roots by an exopolysaccharide-producing Pantoea agglomerans strain and its effect on rhizosphere soil aggregation. Appl. Environ. Microbiol. 1998, 64, 3740–3747. [Google Scholar] [PubMed]
- Kihara, J.; Martius, C.; Bationo, A.; Thuita, M.; Lesueur, D.; Herrmann, L.; Amelung, W.L.; Vlek, P.L. Soil aggregation and total diversity of bacteria and fungi in various tillage systems of sub-humid and semi-arid Kenya. Appl. Soil Ecol. 2012, 58, 12–20. [Google Scholar] [CrossRef]
- Yu, X.T. A summary of the studies on Chinese fir in 2000–2005. I. The research development on physiological ecology of Chinese fir. J. Fujian Coll. For. 2006, 26, 177–185, (In Chinese with English Abstract). [Google Scholar]
- Zhou, H.; Meng, S.; Liu, Q. Diameter growth, biological rotation age and biomass of Chinese fir in burning and clearing site preparations in subtropical China. Forests 2016, 7, 177. [Google Scholar] [CrossRef]
- Xu, J.L.; Mao, Y.M.; Cheng, X.R.; Yu, M.K. Effect of thinning on growth and timber outturn in Cunninghamia lanceolata plantation. For. Res. 2014, 27, 99–107, (In Chinese with English Abstract). [Google Scholar]
- Zhang, X.; Duan, A.; Zhang, J. Tree biomass estimation of Chinese fir (Cunninghamia lanceolata) based on Bayesian method. PLoS ONE 2013, 8, e79868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H. Influence of thinning on soil fertility in artificial forests. Chin. J. Appl. Ecol. 2001, 12, 672–676, (In Chinese with English abstract). [Google Scholar]
- Bao, S.D. Analytical Methods of Soil Agro-Chemistry; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Tan, Z.; Lal, R.; Owens, L.; Izaurralde, R.C. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007, 92, 53–59. [Google Scholar] [CrossRef]
- Le Guillou, C.; Angers, D.A.; Maron, P.A.; Leterme, P.; Menasseri-Aubry, S. Linking microbial community to soil water-stable aggregation during crop residue decomposition. Soil Biol. Biochem. 2012, 50, 126–133. [Google Scholar] [CrossRef]
- Pereira, L.B.; Vicentini, R.; Ottoboni, L.M.M. Changes in the Bacterial Community of Soil from a Neutral Mine Drainage Channel. PLoS ONE 2014, 9, e96605. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Baumgartl, T.; Horn, R. Effect of aggregate stability on soil compaction. Soil Tillage Res. 1991, 19, 203–213. [Google Scholar] [CrossRef]
- Oades, J.M. Soil organic-matter and structural stability e mechanisms and implications for management. Plant Soil 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Duchicela, J.; Sullivan, T.S.; Bontti, E.; Bever, J.D.; Wan, S. Soil aggregate stability increase is strongly related to fungal community succession along an abandoned agricultural field chronosequence in the Bolivian Altiplano. Appl. Soil Ecol. 2013, 50, 1266–1273. [Google Scholar] [CrossRef]
- Marchi, E.; Picchio, R.; Spinelli, R.; Verani, S.; Venanzi, R.; Certini, G. Environmental impact assessment of different logging methods in pine forests thinning. Ecol. Eng. 2014, 70, 429–436. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Yan, H.; Jiang, Y.; Zhao, T.; An, S. Dynamics of soil nitrogen fractions and their relationship with soil microbial communities in two forest species of northern China. PLoS ONE 2018, 13, e0196567. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Neill, A.R.; Puettmann, K.J. Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. For. Ecol. Manag. 2010, 260, 1104–1113. [Google Scholar] [CrossRef]
- Cai, W. Effect of thinning on biodiversity and soil nutrient in the low-benefit Chinese fir forest. J. Anhui Agric. Univ. 2017, 44, 649–653, (In Chinese with English abstract). [Google Scholar]
- Lin, W.R.; Wang, P.H.; Chen, W.C.; Lai, C.M.; Winder, R.S. Responses of soil fungal populations and communities to the thinning of Cryptomeria japonica forests. Microbes Environ. 2016, 31, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2016, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2015, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Howes, C.G.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Nilsson, R.H.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overby, S.T.; Hart, S.C. Short-term belowground responses to thinning and burning treatments in Southwestern ponderosa pine forests of the USA. Forests 2016, 7, 45. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Aleklett, K.; Hart, M. The root microbiota—A fingerprint in the soil? Plant Soil 2013, 370, 671–686. [Google Scholar]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest management type influences diversity and community composition of soil fungi across temperate forest ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.R.; Xu, J.L.; Liu, J.; Yu, M.K. Effect of thinning on understory vegetation diversity and its nutrient stocks in Cunninghamia lanceolata plantation. Ecol. Environ. Sci. 2014, 23, 30–34, (In Chinese with English abstract). [Google Scholar]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; van der Putten, W.H.; Roggero, P.P.; Seddaiu, G. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Baena, C.W.; Andrés-Abellán, M.; Lucas-Borja, M.E.; Martínez-García, E.; García-Morote, F.A.; Rubio, E.; López-Serrano, F.R. Thinning and recovery effects on soil properties in two sites of a Mediterranean forest, in Cuenca Mountain (South-eastern of Spain). For. Ecol. Manag. 2013, 308, 223–230. [Google Scholar] [CrossRef]


| Treatments | Control | MT | HT |
|---|---|---|---|
| Stem density (tree ha−1) | 1960 ± 107 a | 1530 ± 81 b | 1334 ± 88 c |
| Canopy coverage (%) | 98.5 ± 1.3 | 98.1 ± 1.7 | 97.8 ± 1.9 |
| Height (m) | 17.5 ± 0.6 | 18.0 ± 0.7 | 18.6 ± 0.5 |
| Diameter at breast height (cm) | 20.3 ± 0.5 b | 22.5 ± 0.8 a | 23.5 ± 0.8 a |
| Volume (m3 ha−1) | 562.4 ± 31.7 | 547.2 ± 28.6 | 531.8 ± 33.9 |
| Forest floor biomass (t ha−1) | 7.35 ± 1.28 | 7.83 ± 1.62 | 8.06 ± 0.84 |
| Understory vegetation biomass (t ha−1) | 1.41 ± 0.47 | 1.55 ± 0.72 | 1.72 ± 0.40 |
| Treatment | >0.250 mm | 0.053–0.250 mm | <0.053 mm | MWD |
|---|---|---|---|---|
| Control | 55.89 ± 5.81 | 17.63 ± 2.13 | 26.48 ± 5.05 | 0.52 ± 0.05 |
| MT | 62.15 ± 3.85 | 15.79 ± 1.75 | 21.06 ± 2.68 | 0.58 ± 0.04 |
| HT | 63.53 ± 5.46 | 17.27 ± 1.82 | 19.20 ± 4.84 | 0.58 ± 0.05 |
| Microbial community | Treatment | OTUs | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| Bacteria | Control | 825 ± 58 | 916 ± 77 | 5.40 ± 0.23 | 0.010 ± 0.004 |
| MT | 818 ± 52 | 949 ± 71 | 5.29 ± 0.31 | 0.014 ± 0.007 | |
| HT | 884 ± 61 | 985 ± 52 | 5.45 ± 0.27 | 0.011 ± 0.003 | |
| Fungi | Control | 217 ± 74 | 218 ± 75 | 3.24 ± 0.47 | 0.104 ± 0.036 |
| MT | 253 ± 67 | 259 ± 64 | 3.20 ± 0.42 | 0.098 ± 0.026 | |
| HT | 230 ± 30 | 255 ± 37 | 2.80 ± 0.19 | 0.109 ± 0.017 |
| Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|
| Phylum | Control | MT | HT | Phylum | Control | MT | HT |
| Acidobacteria (%) | 17.29 ± 3.60 | 20.24 ± 0.50 | 19.96 ± 1.40 | Ascomycota (%) | 61.05 ± 11.15 | 50.03 ± 7.79 | 72.95 ± 10.10 |
| Actinobacteria (%) | 5.70 ± 0.79 b | 5.66 ± 1.13 b | 7.63 ± 2.15 a | Basidiomycota (%) | 17.04 ± 4.67 b | 29.31 ± 7.69 a | 13.33 ± 4.39 b |
| Bacteroidetes (%) | 3.14 ± 1.17 | 2.63 ± 0.61 | 3.35 ± 0.32 | Chytridiomycota (%) | 0.04 ± 0.03 | 0.06 ± 0.03 | 0.02 ± 0.01 |
| Chloroflexi (%) | 11.85 ± 5.56 | 9.70 ± 3.01 | 9.75 ± 7.17 | Entomophthoromycota (%) | 0.20 ± 0.18 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| Firmicutes (%) | 12.86 ± 8.81 | 17.90 ± 11.56 | 8.96 ± 4.36 | Fungi_norank (%) | 0.49 ± 0.26 b | 2.14 ± 1.24 a | 0.53 ± 0.45 b |
| Fusobacteria (%) | 0.66 ± 0.43 | 0.50 ± 0.32 | 1.01 ± 0.62 | Glomeromycota (%) | 3.36 ± 1.56 | 3.63 ± 2.07 | 2.59 ± 3.65 |
| Gemmatimonadetes (%) | 2.14 ± 0.61 | 1.80 ± 0.60 | 1.95 ± 0.32 | Unclassified (%) | 17.81 ± 8.74 | 14.83 ± 6.09 | 10.55 ± 6.45 |
| Planctomycetes (%) | 2.35 ± 1.16 a | 2.48 ± 0.69 a | 1.32 ± 0.84 b | ||||
| Proteobacteria (%) | 40.33 ± 7.94 | 35.24 ± 10.38 | 42.68 ± 10.59 | ||||
| Verrucomicrobia (%) | 1.88 ± 0.72 | 2.01 ± 0.65 | 1.56 ± 0.34 | ||||
| Others (%) | 1.80 ± 0.45 | 1.84 ± 0.59 | 1.83 ± 0.26 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Xing, W.; Yuan, H.; Yu, M. Long-Term Thinning Does not Significantly Affect Soil Water-Stable Aggregates and Diversity of Bacteria and Fungi in Chinese Fir (Cunninghamia lanceolata) Plantations in Eastern China. Forests 2018, 9, 687. https://doi.org/10.3390/f9110687
Cheng X, Xing W, Yuan H, Yu M. Long-Term Thinning Does not Significantly Affect Soil Water-Stable Aggregates and Diversity of Bacteria and Fungi in Chinese Fir (Cunninghamia lanceolata) Plantations in Eastern China. Forests. 2018; 9(11):687. https://doi.org/10.3390/f9110687
Chicago/Turabian StyleCheng, Xiangrong, Wenli Xing, Haijing Yuan, and Mukui Yu. 2018. "Long-Term Thinning Does not Significantly Affect Soil Water-Stable Aggregates and Diversity of Bacteria and Fungi in Chinese Fir (Cunninghamia lanceolata) Plantations in Eastern China" Forests 9, no. 11: 687. https://doi.org/10.3390/f9110687





