Tree Stress and Mortality from Emerald Ash Borer Does Not Systematically Alter Short-Term Soil Carbon Flux in a Mixed Northeastern U.S. Forest
Abstract
:1. Introduction
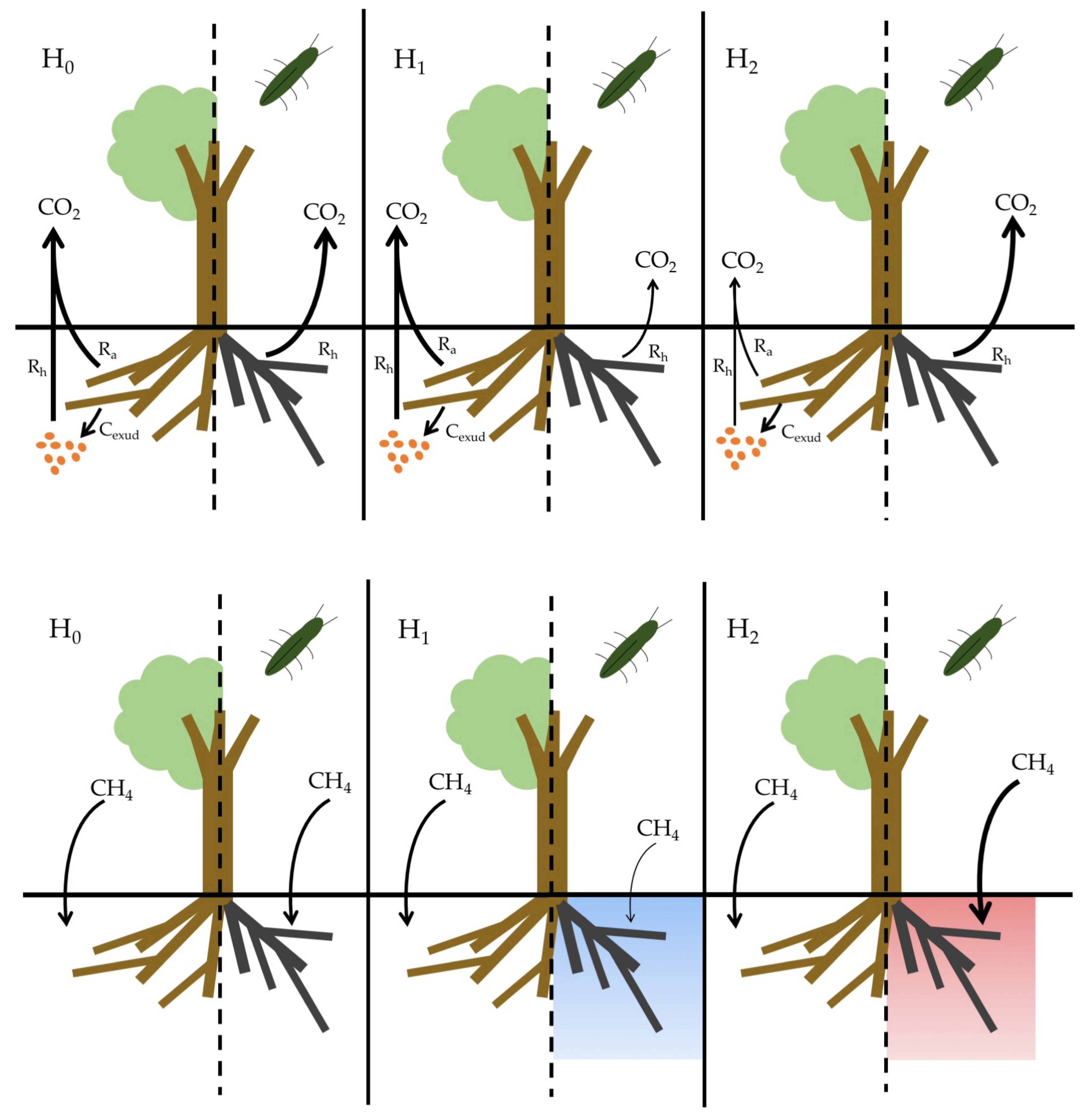
- CO2 flux, H1: Soils beneath EAB-impacted Fraxinus americana L. (F. americana) will have lower rates of CO2 flux due to lower active root metabolism and exudation of labile carbon substrates compared to soil beneath visibly healthy trees.
- CO2 flux, H2: Soils beneath EAB-impacted F. americana will have higher rates of CO2 flux due to an increase in dead root biomass available for decomposition, as well as an increase in soil temperature and moisture, as infestation results in large canopy gaps and reduced evapotranspiration.
- CH4 uptake, H1: Soils beneath EAB-impacted F. americana will have lower rates of CH4 uptake due to an increase in soil moisture from decreased evapotranspiration.
- CH4 uptake, H2: Soils beneath EAB-impacted F. americana will have higher rates of CH4 uptake due to an increase in soil temperature, as canopy gaps allow more sunlight to reach the forest floor.
2. Materials and Methods
2.1. Study Site
2.2. Study Design
2.3. Chamber Measurements and Flux Calculations
2.4. Soil Characteristics
2.5. Statistical Methods
3. Results
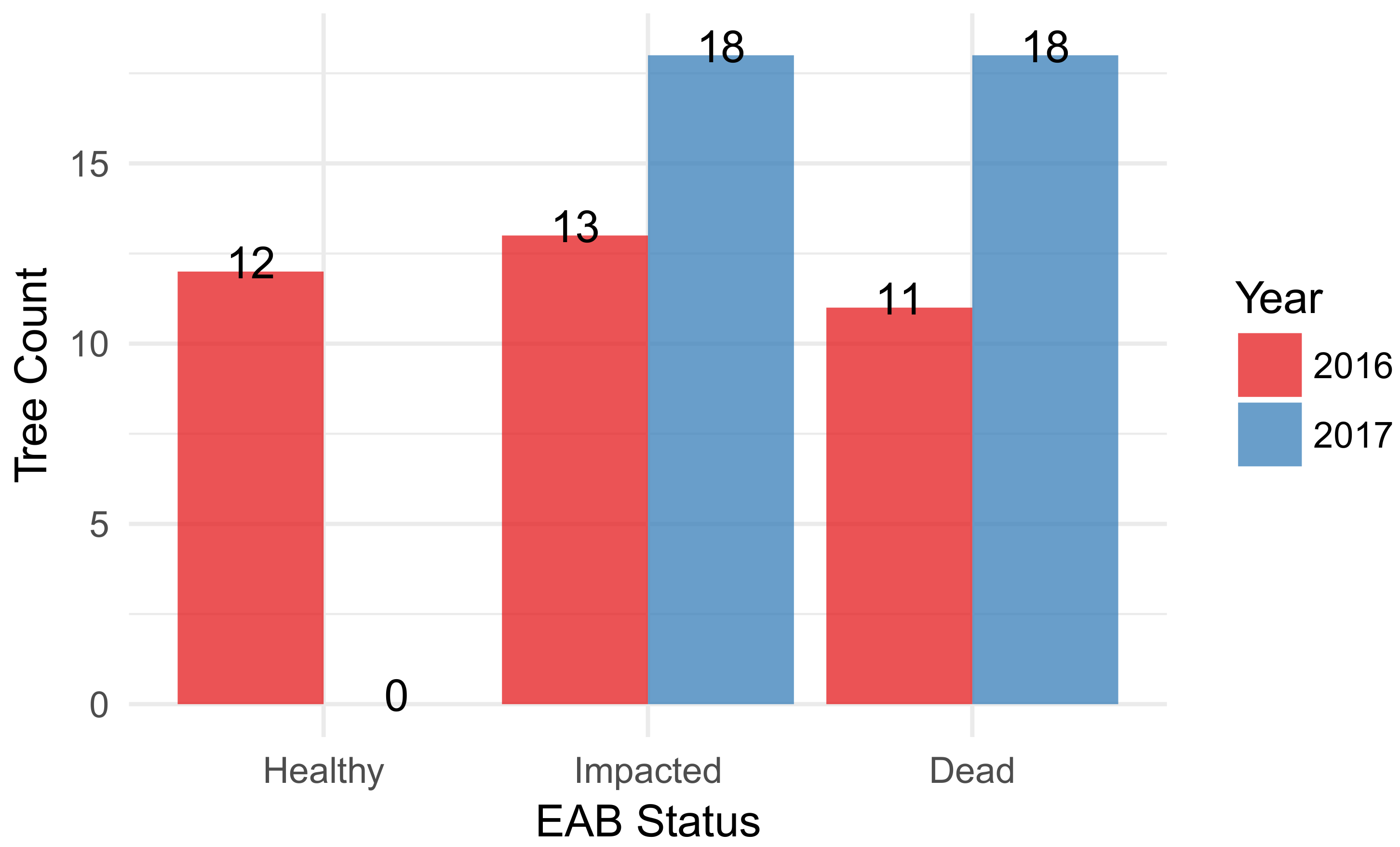
3.1. F. americana Decline over Time
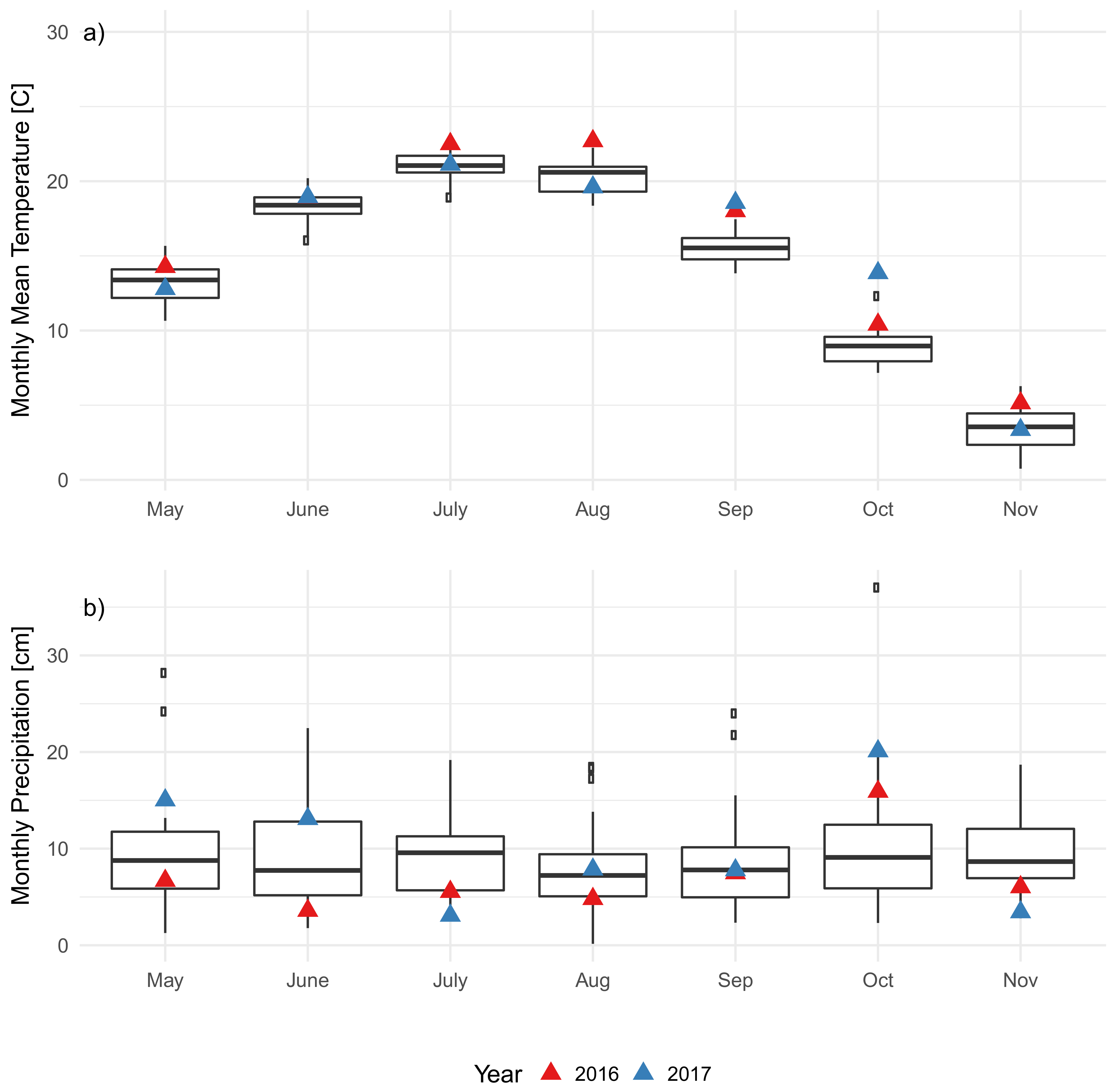
3.2. Soil Characteristics and Soil Microclimate Trends
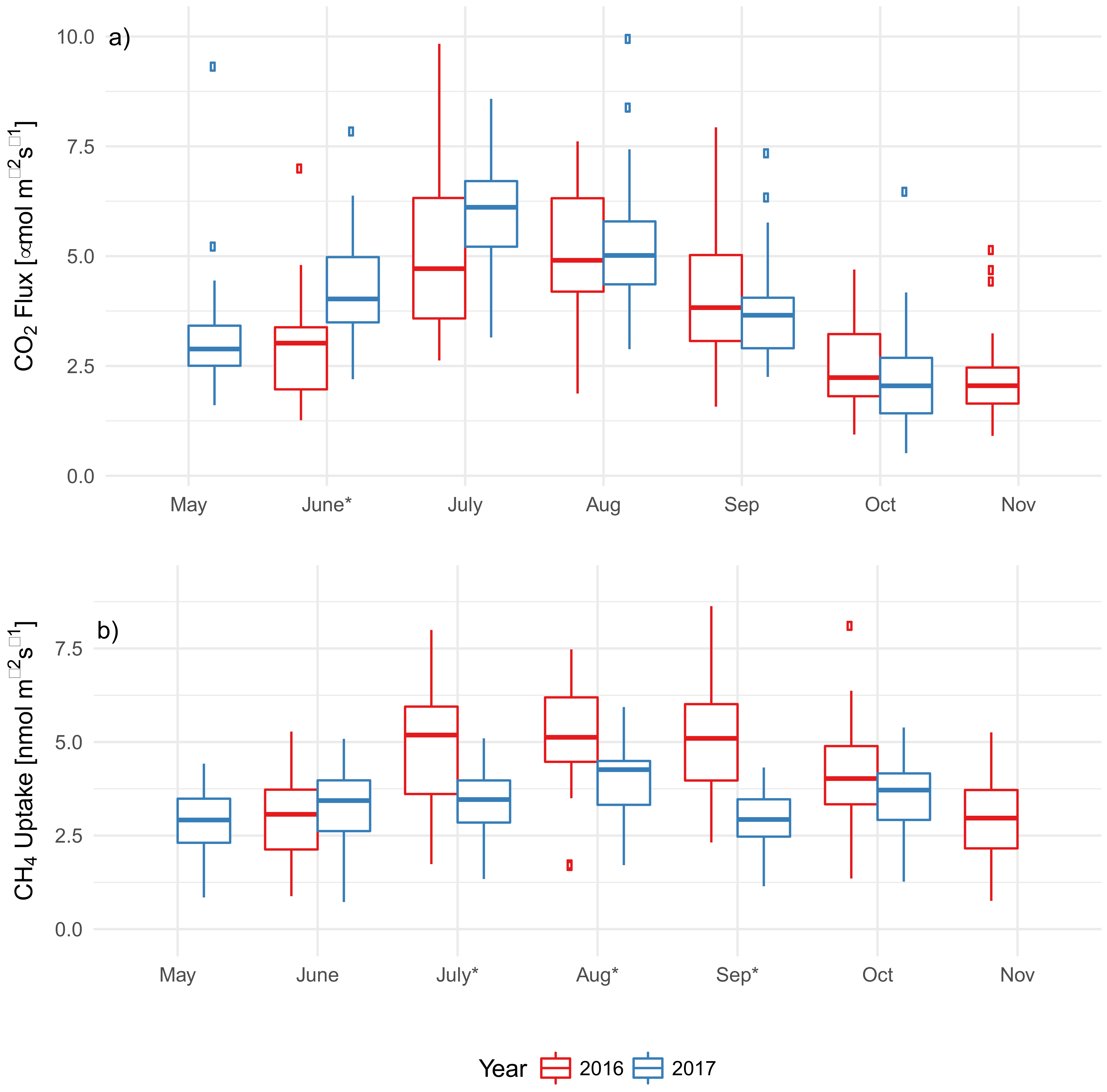
3.3. EAB Impact on Soil Microclimate and Gas Flux
3.4. Modeled Predictors of CO2 Flux and CH4 Uptake
4. Discussion
4.1. Impact of EAB on Microclimate
4.2. Impact of EAB on CO2 Flux
4.3. Impact of EAB on CH4 Uptake
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Skowronski, N.; Hom, J. Invasive insects impact forest carbon dynamics. Glob. Chang. Biol. 2010, 16, 88–101. [Google Scholar] [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Hogg, E.H.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Peltzer, D.A.; Allen, R.B.; Lovett, G.M.; Whitehead, D.; Wardle, D.A. Effects of biological invasions on forest carbon sequestration. Glob. Chang. Biol. 2010, 16, 732–746. [Google Scholar] [CrossRef]
- Schultz, J.C.; Appel, H.M.; Ferrieri, A.P.; Arnold, T.M. Flexible resource allocation during plant defense responses. Front. Plant Sci. 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T. Tree physiology and forest pests. J. For. 1969, 67, 118–123. [Google Scholar]
- Herms, D.A.; Mc Cullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.; Robert, P.; Rebbeck, J. How fast will trees die? A transition matrix model of ash decline in forest stands infested by emerald ash borer. In Proceedings of the Emerald Ash Borer Research and Development Meeting, Pittsburgh, PA, USA, 23–24 October 2008; pp. 28–29. [Google Scholar]
- McCullough, D.G.; Katovich, S.A. Pest Alert: Emerald Ash Borer; Canadian Food Inspection Agency: Ottawa, ON, USA, 2004; pp. 4–5. [Google Scholar]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Vannatta, A.R.; Hauer, R.H.; Schuettpelz, N.M. Economic Analysis of Emerald Ash Borer (Coleoptera: Buprestidae) Management Options. J. Econ. Entomol. 2012, 105, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Frank, D.A.; Hinchey, P.M.; Murray, T.R. Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol. Biochem. 2008, 40, 2865–2873. [Google Scholar] [CrossRef]
- Guitian, R.; Bardgett, R.D. Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant Soil 2000, 220, 271. [Google Scholar] [CrossRef]
- Craine, J.M.; Wedin, D.A.; Stuart Chapin, F. Predominance of ecophysiological controls on soil CO2 flux in a Minnesota grassland. Plant Soil 1999, 207, 77–86. [Google Scholar] [CrossRef]
- Uhlířová, E.; Šimek, M.; Šantrůčková, H. Microbial transformation of organic matter in soils of montane grasslands under different management. Appl. Soil Ecol. 2005, 28, 225–235. [Google Scholar] [CrossRef]
- Gavrichkova, O.; Moscatelli, M.C.; Kuzyakov, Y.; Grego, S.; Valentini, R. Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agric. Ecosyst. Environ. 2010, 136, 87–96. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Ruess, R.W.; Hendrick, R.L.; Bryant, J.P. Regulation of fine root dynamics by mammalian browsers in early successional Alaskan taiga forests. Ecology 1998, 79, 2706–2720. [Google Scholar] [CrossRef]
- Moore, D.J.P.; Trahan, N.A.; Wilkes, P.; Quaife, T.; Stephens, B.B.; Elder, K.; Desai, A.R.; Negron, J.; Monson, R.K. Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol. Lett. 2013, 16, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Nuckolls, A.E.; Wurzburger, N.; Ford, C.R.; Hendrick, R.L.; Vose, J.M.; Kloeppel, B.D. Hemlock Declines Rapidly with Hemlock Woolly Adelgid Infestation: Impacts on the Carbon Cycle of Southern Appalachian Forests. Ecosystems 2009, 12, 179–190. [Google Scholar] [CrossRef]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, M. Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests. For. Ecol. Manag. 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Frank, D.A. Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 2001, 82, 2397–2402. [Google Scholar] [CrossRef]
- Classen, A.T.; Hart, S.C.; Whitman, T.G.; Cobb, N.S.; Koch, G.W. Insect Infestations Linked to Shifts in Microclimate: Important Climate Change Implications. Soil Sci. Soc. Am. J. 2006, 69, 2049–2057. [Google Scholar] [CrossRef]
- Edburg, S.L.; Hicke, J.A.; Lawrence, D.M.; Thornton, P.E. Simulating coupled carbon and nitrogen dynamics following mountain pine beetle outbreaks in the western United States. J. Geophys. Res. 2011, 116, G04033. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Curry, C.L. Modeling the soil consumption of atmospheric methane at the global scale. Glob. Biogeochem. Cycles 2007, 21, GB4012. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Sarria-Guzmán, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Mancinelli, R.L. The regulation of methane oxidation in soil. Annu. Rev. Microbiol. 1995, 49, 581–605. [Google Scholar] [CrossRef] [PubMed]
- Adamsen, A.P.S.; King, G.M. Methane consumption in temperate and subarctic forest soils: Rates, vertical zonation, and responses to water and nitrogen. Appl. Environ. Microbiol. 1993, 59, 485–490. [Google Scholar] [PubMed]
- Striegl, R.G. Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere 1993, 26, 715–720. [Google Scholar] [CrossRef]
- Castro, M.S.; Steudler, P.; Melillo, J.M. Factors controlling atmospheric methane consumption by temperate forest soils. Glob. Biogeochem. Cycles 1995, 9, 1–10. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Sundquist, E.T.; Steudler, P. Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil. Soil Biol. Biochem. 2006, 38, 1388–1395. [Google Scholar] [CrossRef]
- Billings, S.A.; Richter, D.D.; Yarie, J. Sensitivity of soil methane fluxes to reduced precipitation in boreal forest soils. Soil Biol. Biochem. 2000, 32, 1431–1441. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. National Centers for Environmental Information Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 14 December 2017).
- Davidson, E.A.; Savage, K.; Verchot, L.V.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Cueva, A.; Bullock, S.H.; López-Reyes, E.; Vargas, R. Potential bias of daily soil CO2 efflux estimates due to sampling time. Sci. Rep. 2017, 7, 11925. [Google Scholar] [CrossRef] [PubMed]
- Pirk, N.; Mastepanov, M.; Parmentier, F.-J.W.; Lund, M.; Crill, P.; Christensen, T.R. Calculations of automatic chamber flux measurements of methane and carbon dioxide using short time series of concentrations. Biogeosciences 2016, 13, 903–912. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Soil Mechanics Level 1, Module 3: USDA Textural Soil Classification; United States Department of Agriculture: Washington, DC, USA, 1987.
- Stan Development Team. RStan: The R Interface to Stan; Stan Development Team: New York, NY, USA, 2017. [Google Scholar]
- Brooks, S.P.; Gelman, A. Interface Foundation of America General Methods for Monitoring Convergence of Iterative Simulations General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- EAB_soilflux GitHub Code Respository. Available online: https://github.com/jhmatthes/EAB_soilflux (accessed on 12 January 2018).
- Wickham, H. Tidyverse: Easily Install and Load “Tidyverse” Packages, version 1.2.1; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Wickham, H. Forcats: Tools for Working with Categorical Variables (Factors), version 0.2.0; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Wickham, H. Stringr: Simple, Consistent Wrappers for Common String Operations, version 1.2.0; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Wilke, C.O. ggridges: Ridgeline Plots in “ggplot2”, version 0.4.1; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics, version 2.3; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Reed, D.E.; Ewers, B.E.; Pendall, E. Impact of mountain pine beetle induced mortality on forest carbon and water fluxes. Environ. Res. Lett. 2014, 9. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Brown, J.R.; Dijkstra, P.; Allwright, M.C.; Hungate, B.A. Response of Terrestrial CH4 Uptake to Interactive Changes in Precipitation and Temperature Along a Climatic Gradient. Ecosystems 2010, 13, 1157–1170. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.F.; Lovett, G.M.; Arthur, M.A.; Weathers, K.C. Long-term effects of pest-induced tree species change on carbon and nitrogen cycling in northeastern U.S. forests: A modeling analysis. For. Ecol. Manag. 2016, 372, 269–290. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Reardon, R.C. Biology and Control of Emerald Ash Borer; USDA: Washington, DC, USA, 2015; p. 171. [Google Scholar]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]


| Parameter | Variable Range | CO2 Param Median | CO2 95% Interval | CH4 Param Median | CH4 95% Interval |
|---|---|---|---|---|---|
| Intercept | n/a | −7.65 | −15.64–0.53 | −4.25 | −21.0–12.2 |
| Soil Temperature | 7–20 °C | 0.06 | 0.03–0.08 | 0.03 | −0.03–0.08 |
| Soil Moisture | 0.009–0.530 m3 m−3 | 0.21 | −0.35–0.76 | −6.00 | −7.99–−3.91 |
| Monthly Rainfall | 5.64–19.9 cm | −0.02 | −0.03–0.00 | −0.01 | −0.06–0.03 |
| Fraction Sand | 0.92–0.99 | 7.74 | 0.60–14.6 | 12.63 | −1.45–27.11 |
| pH | 4.05–5.43 | 0.09 | −0.34–0.55 | −0.83 | −1.73–0.08 |
| DBH | 24.7–66.5 cm | 0.01 | −0.01–0.01 | n/a | n/a |
| Root Biomass | 0.67–7.55 g | −0.06 | −0.14–0.03 | n/a | n/a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthes, J.H.; Lang, A.K.; Jevon, F.V.; Russell, S.J. Tree Stress and Mortality from Emerald Ash Borer Does Not Systematically Alter Short-Term Soil Carbon Flux in a Mixed Northeastern U.S. Forest. Forests 2018, 9, 37. https://doi.org/10.3390/f9010037
Matthes JH, Lang AK, Jevon FV, Russell SJ. Tree Stress and Mortality from Emerald Ash Borer Does Not Systematically Alter Short-Term Soil Carbon Flux in a Mixed Northeastern U.S. Forest. Forests. 2018; 9(1):37. https://doi.org/10.3390/f9010037
Chicago/Turabian StyleMatthes, Jaclyn Hatala, Ashley K. Lang, Fiona V. Jevon, and Sarah J. Russell. 2018. "Tree Stress and Mortality from Emerald Ash Borer Does Not Systematically Alter Short-Term Soil Carbon Flux in a Mixed Northeastern U.S. Forest" Forests 9, no. 1: 37. https://doi.org/10.3390/f9010037





