Land Use Affects the Soil C Sequestration in Alpine Environment, NE Italy
Abstract
:1. Introduction
2. Material and Methods
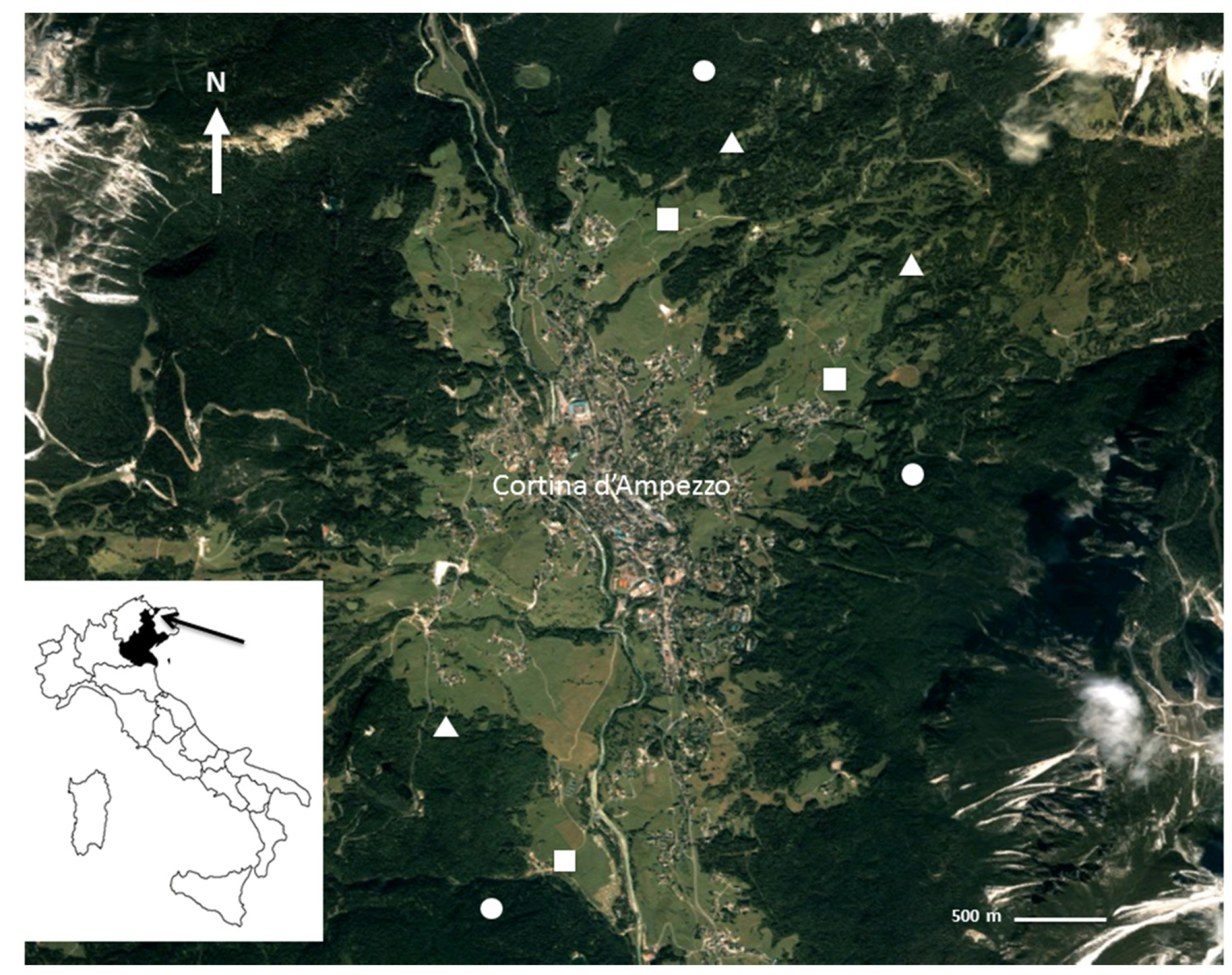
2.1. Study Site
2.2. Soil Sampling
2.3. Laboratory Analysis
2.4. Isotopic Determination
2.5. Statistical Analyses
3. Results
3.1. Morphology
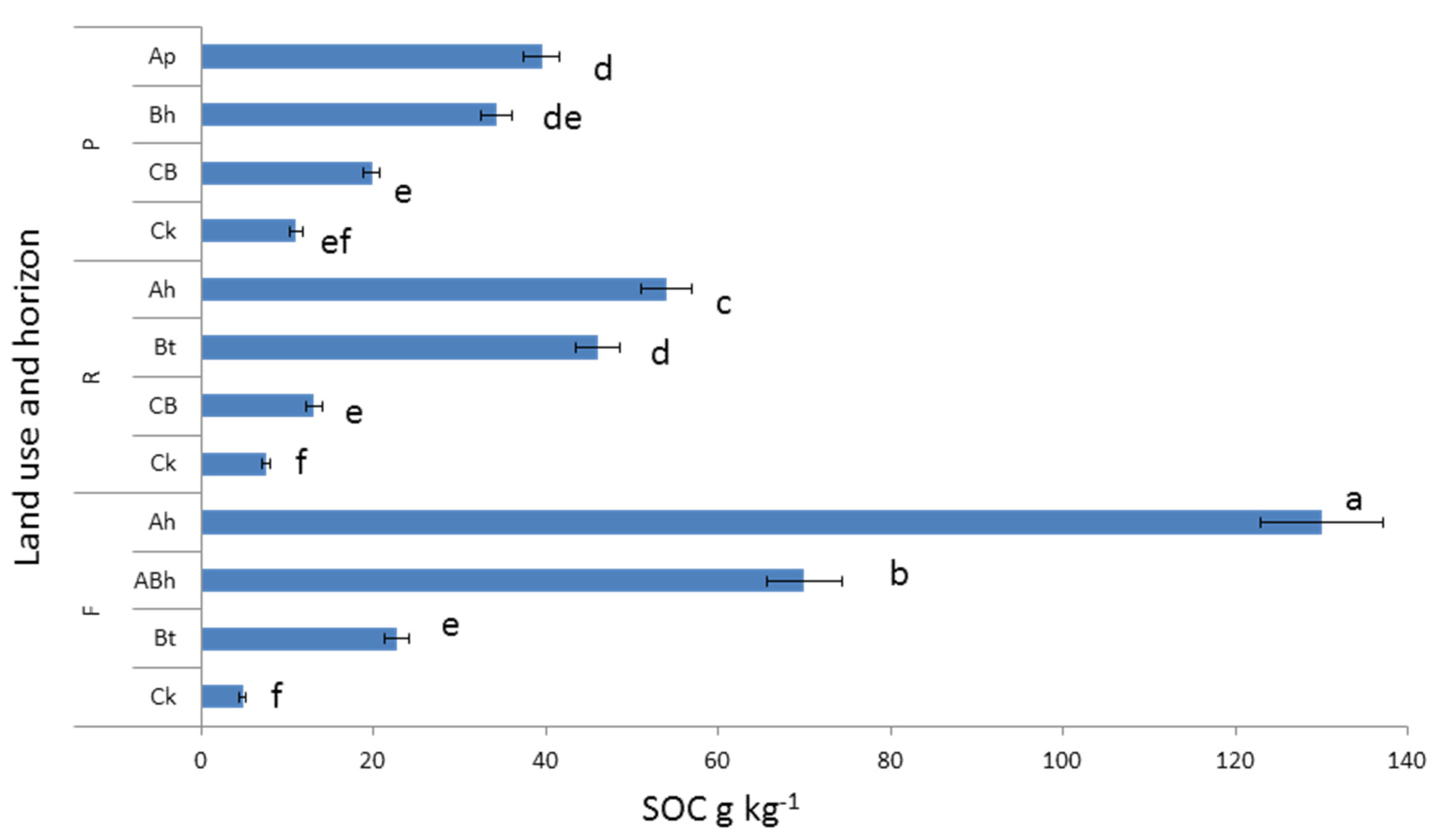
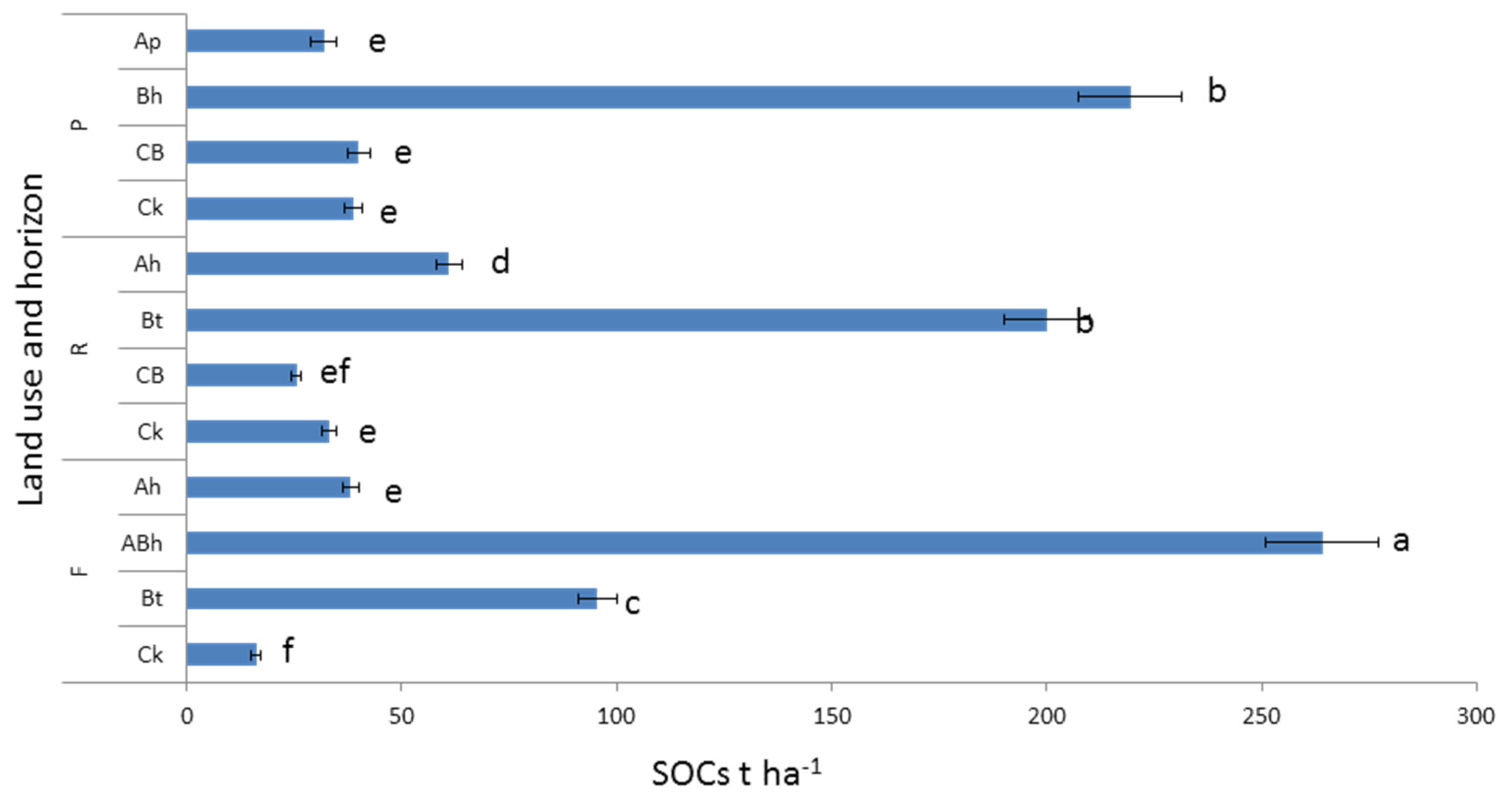
3.2. Soil Organic C and N Concentration and Stock under Different Land Uses and Depths
3.3. Humic Substance Features under Different Land Uses and Depths
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, X.; Zhang, C.; Wang, Q.; Chen, X.; Ding, J.; Karamage, F. Isolating and Quantifying the Effects of Climate and CO2 Changes (1980–2014) on the Net Primary Productivity in Arid and Semiarid China. Forests 2017, 8, 60. [Google Scholar] [CrossRef]
- Zanella, A.; Jabiol, B.; Ponge, J.F.; Sartore, G.; De Waal, R.; Van Delft, B.; Cools, U.; Katzensteiner, K.; Hager, H.; Englisch, M. A European morpho-functional classification of humus forms. Geoderma 2011, 164, 138–145. [Google Scholar] [CrossRef]
- Grüneberg, E.; Ziche, D.; Wellbrock, N. Organic carbon stocks and sequestration rates of forest soils in Germany. Glob. Chang. Biol. 2014, 20, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Mineral surfaces and soil organic matter. Eur. J. Soil Sci. 2003, 54, 219–236. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like activities of humic substances in different forest ecosystems. New Phytol. 2002, 155, 393–402. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Carletti, P.; Vendramin, E.; Pizzeghello, D.; Concheri, G.; Zanella, A.; Nardi, S.; Squartini, A. Soil humic compounds and microbial communities in six spruce forests as function of parent material, slope aspect and stand age. Plant Soil 2009, 315, 47–65. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- James, J.; Harrison, R. The Effect of Harvest on Forest Soil Carbon: A Meta-Analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. Tropical secondary forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Arnhold, S.; Otieno, D.; Onyango, J.; Koellner, T.; Huwe, B.; Tenhunen, J. Soil properties along a gradient from hillslopes to the savanna plains in the Lambwe Valley, Kenya. Soil Tillage Res. 2015, 154, 75–83. [Google Scholar] [CrossRef]
- Seyoum, B. Assessment of soil fertility status of Vertisols under selected three land uses in Girar Jarso District of North Shoa Zone, Oromia National Regional State, Ethiopia. Environ. Syst. Res. 2016, 5, 18. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Lei, Y.; Deng, Y.; Chen, H.; Sha, L.; Cao, M.; Deng, X. Estimating changes in soil organic carbon storage due to land use changes using a modified calculation method. iForest 2015, 8, 45–52. [Google Scholar] [CrossRef]
- Bravo, F.; Lucà, M.; Mercurio, R.; Sidari, M.; Muscolo, A. Soil and forest productivity: A case study from Stone pine (Pinus pinea L.) stands in Calabria (southern Italy). iForest 2011, 4, 25–30. [Google Scholar] [CrossRef]
- Montecchio, D.; Francioso, O.; Carletti, P.; Pizzeghello, D.; Chersich, S.; Previtali, F.; Nardi, S. Thermal analysis (TG-DTA) and DRIFT spectroscopy applied to investigate the evolution of humic acids in forest soil at different vegetation stages. J. Ther. Anal. Calorim. 2006, 83, 393–399. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Mallamaci, C.; Sidari, M. Biological indicators to assess short-term soil quality changes in forest ecosystems. Ecol. Ind. 2014, 45, 416–423. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Tatzber, M.; Gerzabek, M. Soil organic-matter stocks and characteristics along an Alpine elevation gradient. J. Plant Nutr. Soil Sci. 2010, 173, 30–38. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Marriott, C.A.; Hudson, G.; Hamilton, D.; Neilson, R.; Boag, B.; Handley, L.L.; Robinson, D. Spatial variability of soil total C and N and their stable isotopes in an upland Scottish grassland. Plant Soil 1997, 196, 151–162. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; p. 110. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd ed.; Agronomy Monograph; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; Volume 9, pp. 383–411. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kjeldahl, J. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley and Sons: New York, NY, USA, 1994. [Google Scholar]
- Candler, R.; Luping, C.; Michaelson, G.J.; Malcolm, R.L. Characterization of fractionated soil organic matter from two Alaskan arctic soils by 13C NMR and titration analysis. In The Role of Humic Substances in the Ecosystems and in Environmental Protection; IHSS Polish Society of Humic Substances: Wroclaw, Poland, 1997; pp. 301–307. [Google Scholar]
- Craig, H. Isotope standards for carbon and oxygen and correction factors for mass spectrometric analysis of carbon dioxide. Geochim. Cosmochim. Acta 1957, 12, 133–149. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 1st ed.; Freeman: San Francisco, CA, USA, 1969. [Google Scholar]
- Wang, T.; Kang, F.; Cheng, X.; Han, H.; Ji, W. Soil organic carbon and total nitrogen stocks under different land uses in a hilly ecological restoration area of North China. Soil Tillage Res. 2016, 163, 176–184. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil organic carbon and total nitrogen stocks under different land uses in a semi-arid watershed in Tigray, North Ethiopia. Agric. Ecosyst. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Lister, T.W.; Burger, J.A.; Patterson, S.C. Role of vegetation in mitigating soil quality impacted by forest harvesting. Soil Sci. Soc. Am. J. 2004, 68, 263–271. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Palm, C.A.; Parton, W.J.; Elliott, E.T.; Frey, S.D. Soil pH and organic carbon dynamics in tropical forest soils: Evidence from laboratory and simulation studies. Soil Biol. Biochem. 1995, 27, 1589–1599. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus-related properties in the profiles of three Italian soils after long-term mineral and manure applications. Agric. Ecosyst. Environ. 2014, 189, 216–228. [Google Scholar] [CrossRef]
- Beyer, L. The chemical composition of soil organic matter in classical humic compound fractions and in bulk samples—A review. J. Plant Nutr. Soil Sci. 1996, 159, 527–539. [Google Scholar] [CrossRef]
- Golchin, A.; Clarke, P.; Baldock, J.A.; Higashi, T.; Skjemstad, J.O.; Oades, J.M. The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by 13C NMR spectroscopy. I. Whole soil and humic acid fraction. Geoderma 1997, 76, 155–174. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Quideau, S.A.; Anderson, M.A.; Graham, R.C.; Chadwick, O.A.; Trumbore, S.E. Soil organic matter processes: Characterization by 13CNMR and 14C measurements. For. Ecol. Manag. 2000, 138, 19–27. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, G.; Chen, T.; Yang, J.; Yang, J.; Peters, M.; Wei, R.; Tian, L.; Han, X.; Hu, J. Spatial variation and environmental assessment of soil organic carbon isotopes for tracing sources in a typical contaminated site. J. Geochem. Explor. 2017, 175, 11–17. [Google Scholar] [CrossRef]
- Ågren, G.O.; Bosatta, E.; Balesdent, J. Isotope discrimination during decomposition of organic matter: A theoretical analysis. Soil Sci. Soc. Am. J. 1996, 60, 1121–1126. [Google Scholar] [CrossRef]
- Bol, R.; Harkness, D.D.; Huang, Y.; Howard, D.M. The influence of soil processes on carbon isotope distribution and turnover in the British uplands. Eur. J. Soil Sci. 1999, 50, 41–51. [Google Scholar] [CrossRef]
- Certini, G.; Agnelli, A.; Corti, G.; Capperucci, A. Composition and mean residence time of molecular weight fractions of organic matter extracted from two soils under different forest species. Biogeochemistry 2004, 71, 299–316. [Google Scholar] [CrossRef]

| Land Use | Soil Horizon | Depth cm | Colour a | Structure b | Roots c | Skeleton d % | Limit e | Observations |
|---|---|---|---|---|---|---|---|---|
| Soil under prairie (Aric Anthrosol, IUSS Working Group WRB, 2015) | ||||||||
| P | Ap | 0–20 | 10YR 2/2 (very dark brown) | 2,3 f, sbk | 3 vf, m | 5 | c, i | no effervescent |
| Bh | 20–80 | 10YR 3/3 (dark brown) | m, sbk | 1 vf, f | 5 | c, u | no effervescent | |
| CB | 80–103 | 10YR 4/2 (dark greyish brown) | sbk | 1 vf | 25 | c, u | weak effervescent | |
| Ck | 103–>150 | 10YR 4/1 (dark grey) | ms | - | 40 | uk | Effervescent | |
| Soil under reforestation (Haplic Luvisol, IUSS Working Group WRB, 2015) | ||||||||
| R | Ah | 0–32 | 10YR 2/2 (very dark brown) | 2,3 sbk | 3 vf, f | 5 | c, i | no effervescent |
| Bt | 32–71 | 10YR 3/3 (dark brown) | m, sbk | 1 f | 3 | c, u | no effervescent | |
| CB | 71–92 | 10YR 4/2 (dark greyish brown) | sbk | 1 vf | 15 | c, u | weak effervescent | |
| Ck | 92–>150 | 10YR 4/1 (dark grey) | ms | - | 40 | uk | effervescent | |
| Soil under forest (Haplic Luvisol, IUSS Working Group WRB, 2015) | ||||||||
| F | Ah | 0–16 | 10YR 2/2 (very dark brown) | 2 fr | 3 vf, m | 2 | c, i | no effervescent |
| ABh | 16–51 | 10YR 3/3 (dark brown) | 2 m, sbk | 2 vf, f | 2 | c, l | no effervescent | |
| Bt | 57–88 | 10YR 3/6 (dark greyish brown) | m, sbk | 1 f | 5 | c, u | weak effervescent | |
| Ck | 88–>150 | 10YR 4/1(dark grey) | ms | - | 50 | uk | effervescent | |
| Land Use | Chemical Shift of C (ppm) % | δ13C ‰ | |||||
|---|---|---|---|---|---|---|---|
| Hor | Carboxyl | Phenolic | Aromatic | O,N-Alkyl | Alkyl | ||
| P | Ah | 11.63 b * | 5.43 a | 13.73 d | 45.80 b | 25.80 c | −26.80 d |
| B | 11.13 b | 4.01 b | 20.10 b | 47.80 b | 19.90 e | −24.96 b | |
| 11.3 A | 4.72 A | 16.91 B | 46.80 A | 22.85 B | −25.88 B | ||
| R | Ah | 10.91 b | 4.00 b | 13.93 d | 43.73 bc | 27.50 b | −25.90 c |
| B | 5.90 d | 1.86 c | 18.53 bc | 55.13 a | 20.03 e | −23.93 a | |
| 8.40 B | 2.93 C | 16.23 B | 49.43 A | 23.76 B | −24.91 A | ||
| F | Ah | 16.16 a | 4.23 b | 15.53 c | 36.86 d | 28.93 a | −25.66 c |
| B | 7.36 c | 3.83 b | 24.86 a | 41.90 c | 23.00 d | −24.56 b | |
| 11.76 A | 4.03 B | 20.20 A | 39.38 B | 25.97 A | −25.11 A | ||
| Ah | 12.90 a | 4.55 a | 14.40 b | 42.13 b | 27.41 a | −26.12 b | |
| B | 8.13 b | 3.23 b | 21.16 a | 48.27 a | 20.98 b | −24.48 a | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzeghello, D.; Francioso, O.; Concheri, G.; Muscolo, A.; Nardi, S. Land Use Affects the Soil C Sequestration in Alpine Environment, NE Italy. Forests 2017, 8, 197. https://doi.org/10.3390/f8060197
Pizzeghello D, Francioso O, Concheri G, Muscolo A, Nardi S. Land Use Affects the Soil C Sequestration in Alpine Environment, NE Italy. Forests. 2017; 8(6):197. https://doi.org/10.3390/f8060197
Chicago/Turabian StylePizzeghello, Diego, Ornella Francioso, Giuseppe Concheri, Adele Muscolo, and Serenella Nardi. 2017. "Land Use Affects the Soil C Sequestration in Alpine Environment, NE Italy" Forests 8, no. 6: 197. https://doi.org/10.3390/f8060197
APA StylePizzeghello, D., Francioso, O., Concheri, G., Muscolo, A., & Nardi, S. (2017). Land Use Affects the Soil C Sequestration in Alpine Environment, NE Italy. Forests, 8(6), 197. https://doi.org/10.3390/f8060197









