Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites
Abstract
:1. Introduction
2. Materials and Methods
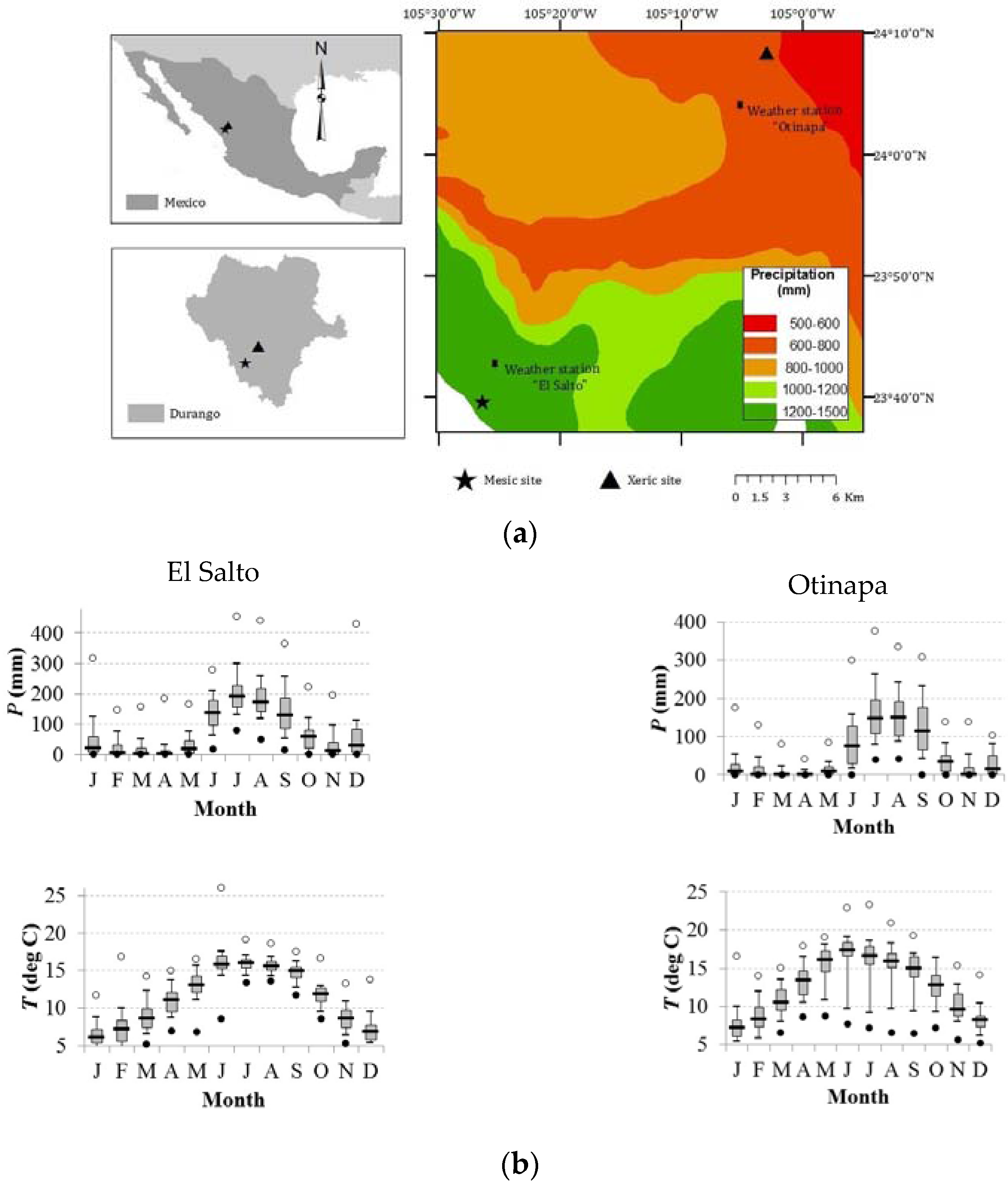
2.1. Study Area and Sampled Tree Species
2.2. Collection of Samples and Development of Growth Chronologies
2.3. Relationships between Climate, Drought, and Growth
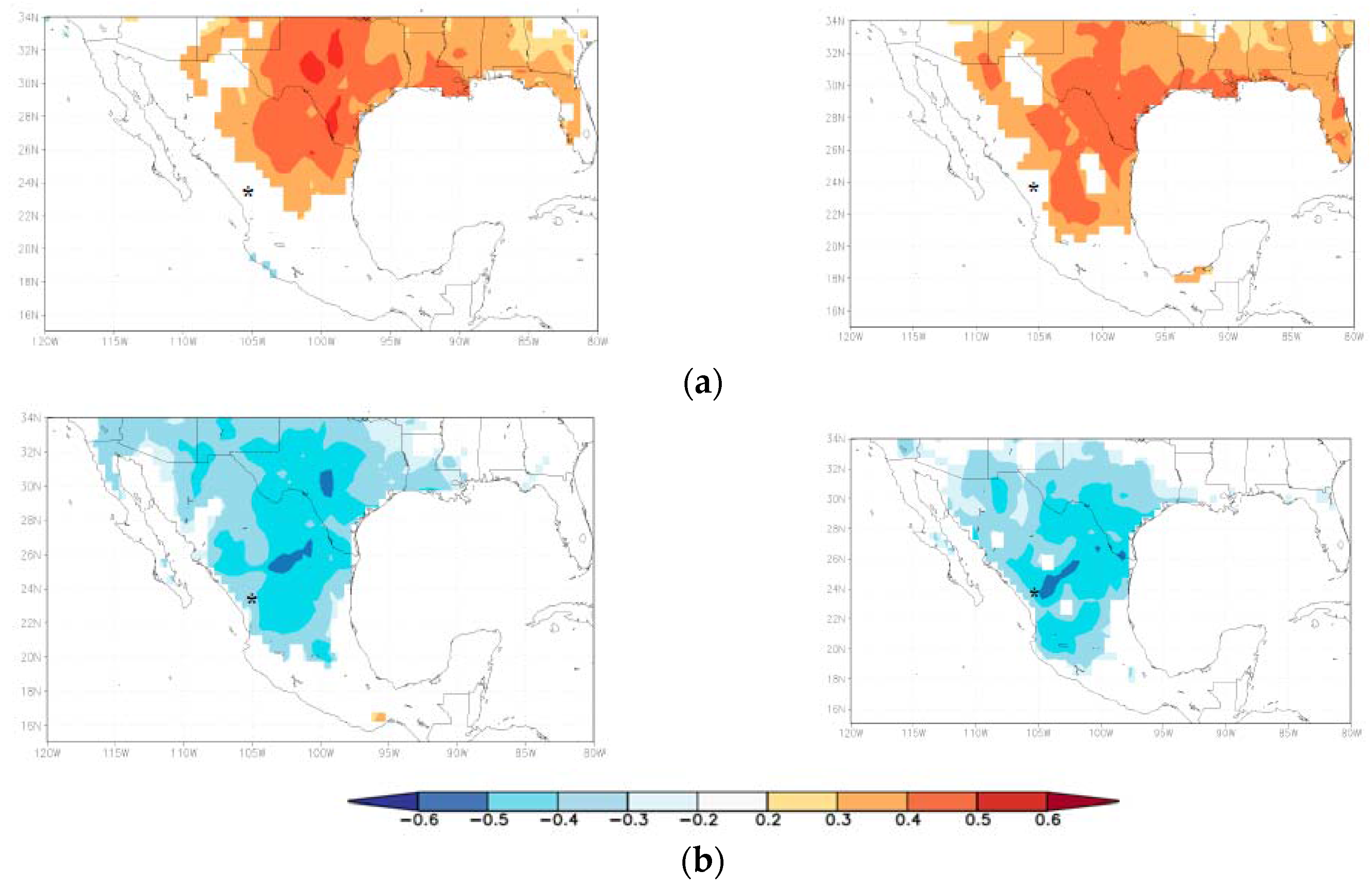
3. Results
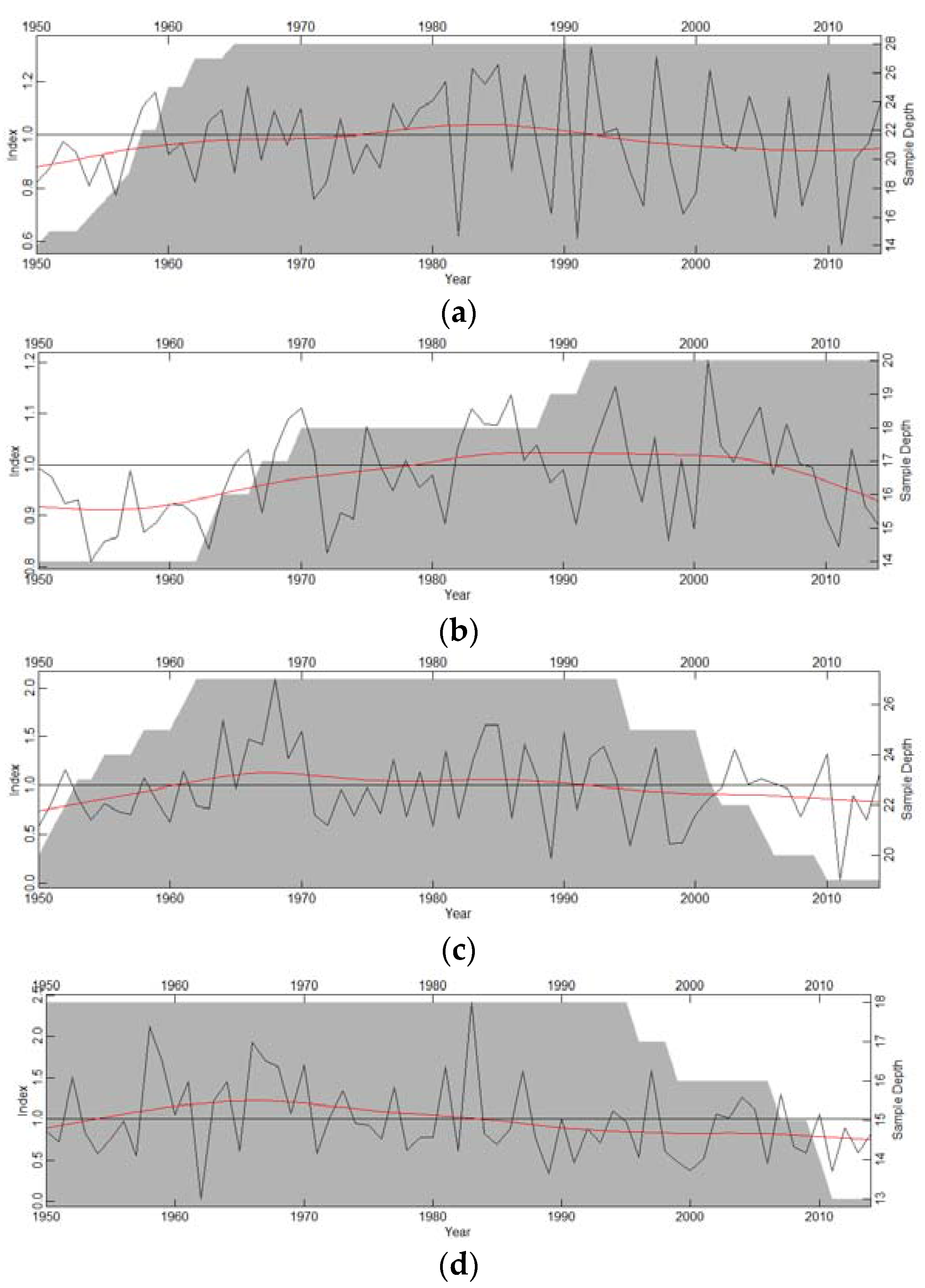
3.1. Growth Patterns
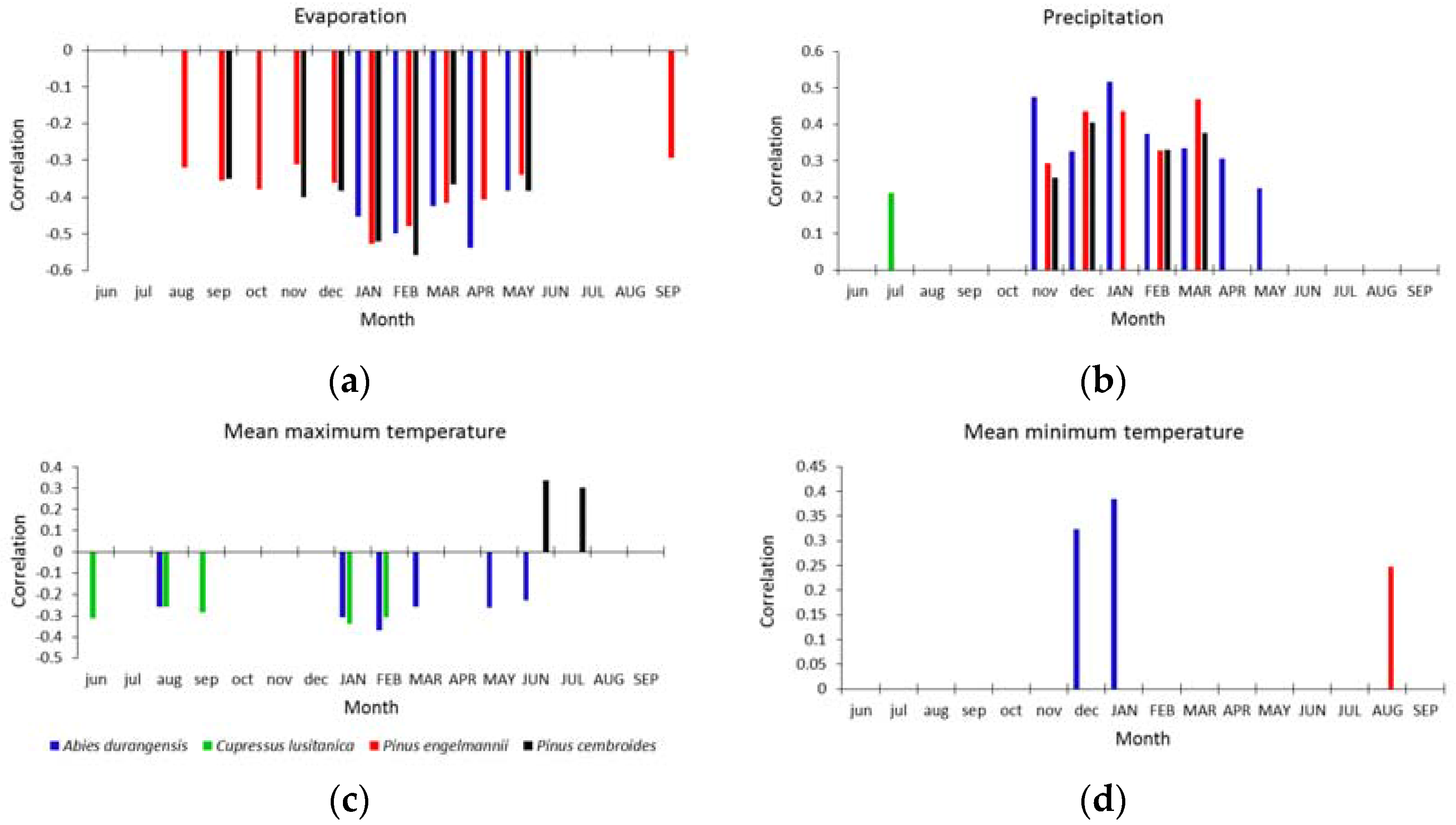
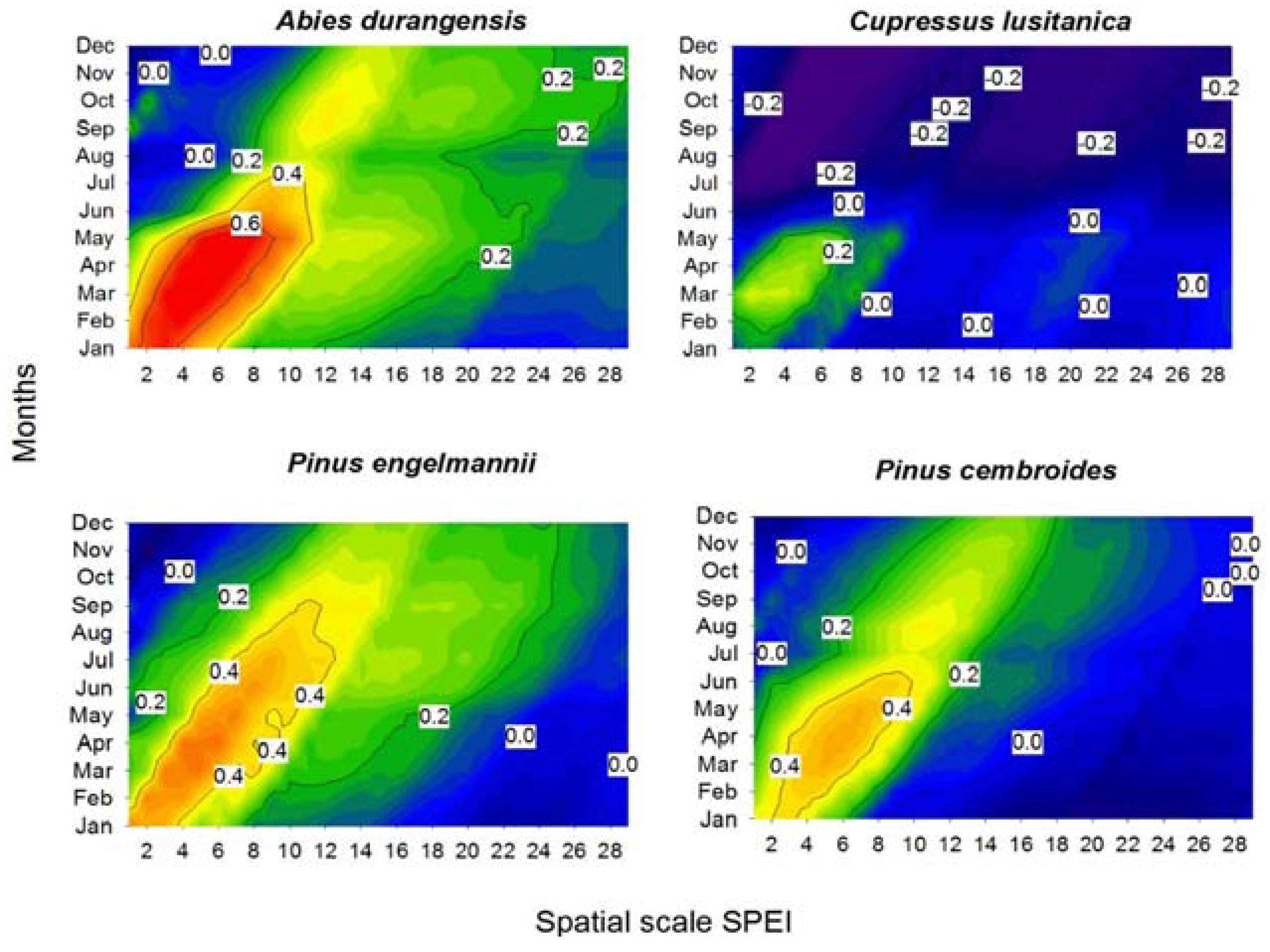
3.2. Climate-Growth Correlations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Summary for Policymakers. In Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Bigler, C.; Gavin, D.G.; Gunning, C.; Veblen, T.T. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos 2007, 116, 1983–1994. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro, R.M.; Camarero, J.J.; Fernández-Cancio, Á. Drought-induced growth decline of Aleppo and maritime pine forests in south-eastern Spain. For. Syst. 2010, 19, 458–470. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C.; Camarero, J.J.; Bowker, M.A.; Ochoa, V.; Carreira, J.A. Stand-structural effects on Heterobasidion abietinum-related mortality following drought events in Abies pinsapo. Oecologia 2010, 164, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Chhin, S. Influence of climate on the growth of hybrid poplar in Michigan. Forests 2010, 1, 209–229. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Dragoni, D.; Schmid, H.P.; Rahman, A.F.; Sims, D.; Wayson, C.A.; Johnson, D.J.; Phillips, R.P. Chronic water stress reduces tree growth and the carbon sink of deciduous hardwood forests. Glob. Chang. Biol. 2014, 20, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Correa-Díaz, A.; Gómez-Guerrero, A.; Villanueva-Díaz, J.; Castruita-Esparza, L.U.; Martínez-Trinidad, T.; Cervantes-Martínez, R. Análisis dendroclimático de Ahuehuete (Taxodium mucronatum Ten.) en el centro de México. Agrociencia 2014, 48, 537–551. [Google Scholar]
- Vicente-Serrano, S.M.; Camarero, J.J.; Zabalza, J.; Sangüesa-Barreda, G.; López-Moreno, J.I.; Tague, C.L. Evapotranspiration deficit controls net primary production and growth of silver fir: Implications for circum-Mediterranean forests under forecasted warmer and drier conditions. Agric. For. Meteorol. 2015, 206, 45–54. [Google Scholar] [CrossRef]
- Rodríguez-Catón, M.; Villalba, R.; Morales, M.; Srur, A. Influence of droughts on Nothofagus pumilio forest decline across northern Patagonia, Argentina. Ecosphere 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Gutiérrez, E.; González-Rouco, F.; Gazol, A.; Sangüesa-Barreda, G.; Andreu-Hayles, L.; Linares, J.C.; Seftigen, K. Assessing forest vulnerability to climate warming using a process-based model of tree growth: Bad prospects for rear-edges. Glob. Chang. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Kolb, T.E. Tree growth response to drought and temperature along an elevation gradient on a mountain landscape. J. Biogeogr. 2005, 32, 1629–1640. [Google Scholar] [CrossRef]
- Linares, J.C.; Tíscar, P.A. Climate change impacts and vulnerability of the southern populations of Pinus nigra subsp. salzmannii. Tree Physiol. 2010, 30, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.; Kitzberger, T. Differential effects of climate variability on forest dynamics along a precipitation gradient in northern Patagonia. J. Ecol. 2010, 98, 1023–1034. [Google Scholar] [CrossRef]
- Pompa-García, M.; Cerano-Paredes, J.; Fulé, P.Z. Variation in radial growth of Pinus cooperi response to climatic signals across an elevational gradient. Dendrochronologia 2013, 31, 198–204. [Google Scholar] [CrossRef]
- Sanders, T.G.M.; Heinrich, I.; Günther, B.; Beck, W. Increasing water use efficiency comes at a cost for Norway spruce. Forests 2016, 7, 296. [Google Scholar] [CrossRef]
- Bunn, A.G.; Hughes, M.K.; Salzer, M.W. Topographically modified tree-ring chronologies as a potential means to improve paleoclimate inference. Clim. Chang. 2011, 105, 627–634. [Google Scholar] [CrossRef]
- Rodríguez-Catón, M.; Villalba, R.; Srur, A.M.; Luckman, B. Long-term trends in radial growth associated with Nothofagus pumilio forest decline in Patagonia: Integrating local- into regional-scale patterns. For. Ecol. Manag. 2015, 339, 44–56. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; Linares, J.C.; Hernández, R.; Oliva, J.; Gazol, A.; González, E.; Montes, F.; García-Martín, A.; De la Riva, J. Papel de los factores bióticos y las sequías en el decaimiento del bosque: Aportaciones desde la dendroecología. Ecosistemas 2015, 24, 15–23. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Wang, X.Q.; Fei, B.H.; Ren, H.Q.; Liu, X.E. Effect of stand and tree attributes on growth and wood quality characteristics from a spacing trial with Populus xiaohei. Ann. For. Sci. 2007, 64, 807–814. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Pompa-García, M.; Camarero, J.J. Reconstructing evaporation from pine tree rings in northern Mexico. Tree Ring Res. 2015, 71, 95–105. [Google Scholar] [CrossRef]
- Bickford, I.N.; Fulé, P.Z.; Kolb, T.E. Growth sensitivity to drought of co-occurring Pinus spp. along an elevation gradient in northern Mexico. West. N. Am. Nat. 2011, 71, 338–348. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la Sierra Madre Occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–403. [Google Scholar] [CrossRef]
- Pompa-García, M.; Domínguez-Calleros, P.A. Respuesta de madera temprana y tardía a la sequía en una conífera mexicana bajo dos condiciones ecológicas. Rev. Ecosistemas 2015, 24, 37–42. [Google Scholar] [CrossRef]
- Cerano, J.; Rivera, M.; Estrada, J.; Trucios, R.; Rios, J.C. Análisis dendrocronológico de Pinus cooperi en Durango, México. Agrofaz 2012, 12, 81–88. [Google Scholar]
- Villanueva, J.; Cerano, J.; Fulé, P.Z.; Cortés, C.; Vázquez, L.; Yocom, L.L.; Ruiz-Corral, J.A. Cuatro siglos de variabilidad hidroclimática en el noroeste de Chihuahua, México, reconstruida con anillos de árboles. Investig. Geogr. 2015, 87, 141–153. [Google Scholar] [CrossRef]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in climate–growth relationship indicate diverse drought tolerances among five pine species coexisting in Northwestern Mexico. Trees 2016, 31, 531–544. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; De Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Aguirre-Díaz, G.J.; Labarthe-Hernández, G. Fissure ignimbrites: Fissure-source origin for voluminous ignimbrites of the Sierra Madre Occidental and its relationship with Basin and Range faulting. Geology 2003, 31, 773–776. [Google Scholar] [CrossRef]
- González-Elizondo, M.; Jurado, E.; Návar, J.; González-Elizondo, M.S.; Villanueva, J.; Aguirre, O.; Jiménez, J. Tree-rings and climate relationships for Douglas-fir chronologies from the Sierra Madre Occidental, Mexico: A 1681–2001 rain reconstruction. For. Ecol. Manag. 2005, 213, 39–53. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in treering dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Science; Kluwer: Alphen aan den Rijn, The Netherlands, 1990; p. 394. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Comisión Nacional del Agua (CNA). Datos Climáticos de Estaciones Meteorológicas de Durango: El Salto, Otinapa. México, 2016. Available online: http://smn1.conagua.gob.mx/index.php?option =com_content& view=article&id=180:durango&catid=14:normales-por-estacion (accessed on 10 February 2017).
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index, R Package Version 1.6. 2013. Available online: http://CRAN.R-project.org/package=SPEI (accessed on 10 February 2017).
- Stahle, D.W.; Cook, E.R.; Burnette, D.J.; Villanueva, J.; Cerano, J.; Burns, J.N.; Griffin, D.; Cook, B.I.; Acuña, R.; Torbenson, M.C.A.; et al. The Mexican Drought Atlas: Tree-ring reconstructions of the soil moisture balance during the late pre-Hispanic, colonial, and modern eras. Quat. Sci. Rev. 2016, 149, 34–60. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Van Oldenborgh, G.J.; Burgers, G. Searching for decadal variations in ENSO precipitation teleconnections. Geophys. Res. Lett. 2005, 32, L15701. [Google Scholar] [CrossRef]
- García-Arévalo, A. Vegetación y flora de un bosque relictual de Picea chihuahuana Martínez del norte de México. Polibotánica 2008, 25, 45–68. [Google Scholar]
- Constante-García, V.; Villanueva-Díaz, J.; Cerano-Paredes, J.; Cornejo-Oviedo, E.H.; Valencia-Manzo, S. Dendrocronología de Pinus cembroides Zucc. y reconstrucción de precipitación estacional para el Sureste de Coahuila. Cienc. For. Méx. 2009, 34, 17–39. [Google Scholar]
- Pompa-García, M.; Yerena-Yamalliel, J.I. Concentración de carbono en Pinus cembroides Zucc: Fuente potencial de mitigación del calentamiento global. Rev. Chapingo Ser. Cie. 2014, 20, 169–175. [Google Scholar]
- Pasho, E.; Camarero, J.J.; Vicente-Serrano, S.M. Climatic impacts and drought control of radial growth and seasonal wood formation in Pinus halepensis. Trees 2012, 26, 1875–1886. [Google Scholar] [CrossRef]
- Castruita-Esparza, L.U. Variabilidad Climática, Eficiencia de uso de Agua Intrínseca y Crecimiento del Área Basal en Bosques del Norte de México. Ph.D. Thesis, Colegio de Postgraduados, Texcoco, México, 2014. [Google Scholar]
- Gutiérrez-García, J.V.; Rodríguez-Trejo, D.A.; Villanueva-Morales, A.; García-Díaz, S.; Romo-Lozano, J.L. Calidad del agua en la producción de Pinus cembroides Zucc. en vivero. Agrociencia 2015, 49, 205–219. [Google Scholar]
- Gómez-Guerrero, A.; Silva, L.C.; Barrera-Reyes, M.; Kishchuk, B.; Velázquez-Martínez, A.; Martínez-Trinidad, T.; Plascencia-Escalante, F.O.; Horwath, W.R. Growth decline and divergent tree ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Glob. Change Biol. 2013, 19, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Farjon, A. A Monograph of Cupressaceae and Sciadopitys; Royal Botanic Gardens: Kew, UK, 2005. [Google Scholar]
- Pederson, N.; Dyer, J.M.; McEwan, R.W.; Hessl, A.E.; Mock, C.J.; Orwig, D.A.; Rieder, H.E.; Cook, B.I. The legacy of episodic climatic events in shaping temperate, broadleaf forests. Ecol. Monogr. 2014, 84, 599–620. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Flint, A.; Huang, C.Y.; Flint, L.; Berry, J.A.; Davis, F.W.; Sperry, J.S.; Field, C.B. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef]
- Chakrabortya, T.; Sahab, S.; Matzarakisd, A.; Reifa, A. Influence of multiple biotic and abiotic factors on the crown die-back of European beech trees at their drought limit. Flora 2017, 229, 58–70. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
| Habitat Type | Species | Diameter (cm) | No. Trees/No. Radii | Best-Replicated Period # | Mean Tree-Ring Width (mm) | AC | Rbar |
|---|---|---|---|---|---|---|---|
| Mesic habitat | A. durangensis | 36.3 ± 2.1 | 15/28 | 1946–2015 | 2.13 ± 0.12 c | 0.72 ± 0.03 b | 0.48 |
| C. lusitanica | 31.5 ± 1.9 | 15/20 | 1919–2015 | 1.77 ± 0.15 b | 0.70 ± 0.03 b | 0.37 | |
| Xeric habitat | P. engelmannii | 40.4 ± 0.9 | 15/27 | 1941–2015 | 2.17 ± 0.08 c | 0.60 ± 0.02 a | 0.65 |
| P. cembroides | 34.2 ± 1.6 | 15/18 | 1880–2015 | 1.04 ± 0.05 a | 0.61 ± 0.02 a | 0.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompa-García, M.; González-Cásares, M.; Acosta-Hernández, A.C.; Camarero, J.J.; Rodríguez-Catón, M. Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites. Forests 2017, 8, 175. https://doi.org/10.3390/f8050175
Pompa-García M, González-Cásares M, Acosta-Hernández AC, Camarero JJ, Rodríguez-Catón M. Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites. Forests. 2017; 8(5):175. https://doi.org/10.3390/f8050175
Chicago/Turabian StylePompa-García, Marín, Marcos González-Cásares, Andrea C. Acosta-Hernández, Jesús Julio Camarero, and Milagros Rodríguez-Catón. 2017. "Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites" Forests 8, no. 5: 175. https://doi.org/10.3390/f8050175
APA StylePompa-García, M., González-Cásares, M., Acosta-Hernández, A. C., Camarero, J. J., & Rodríguez-Catón, M. (2017). Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites. Forests, 8(5), 175. https://doi.org/10.3390/f8050175







