How Time since Forest Fire Affects Stand Structure, Soil Physical-Chemical Properties and Soil CO2 Efflux in Hemiboreal Scots Pine Forest Fire Chronosequence?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil C Content and CO2 Efflux
2.3. Statistical Analyses
3. Results
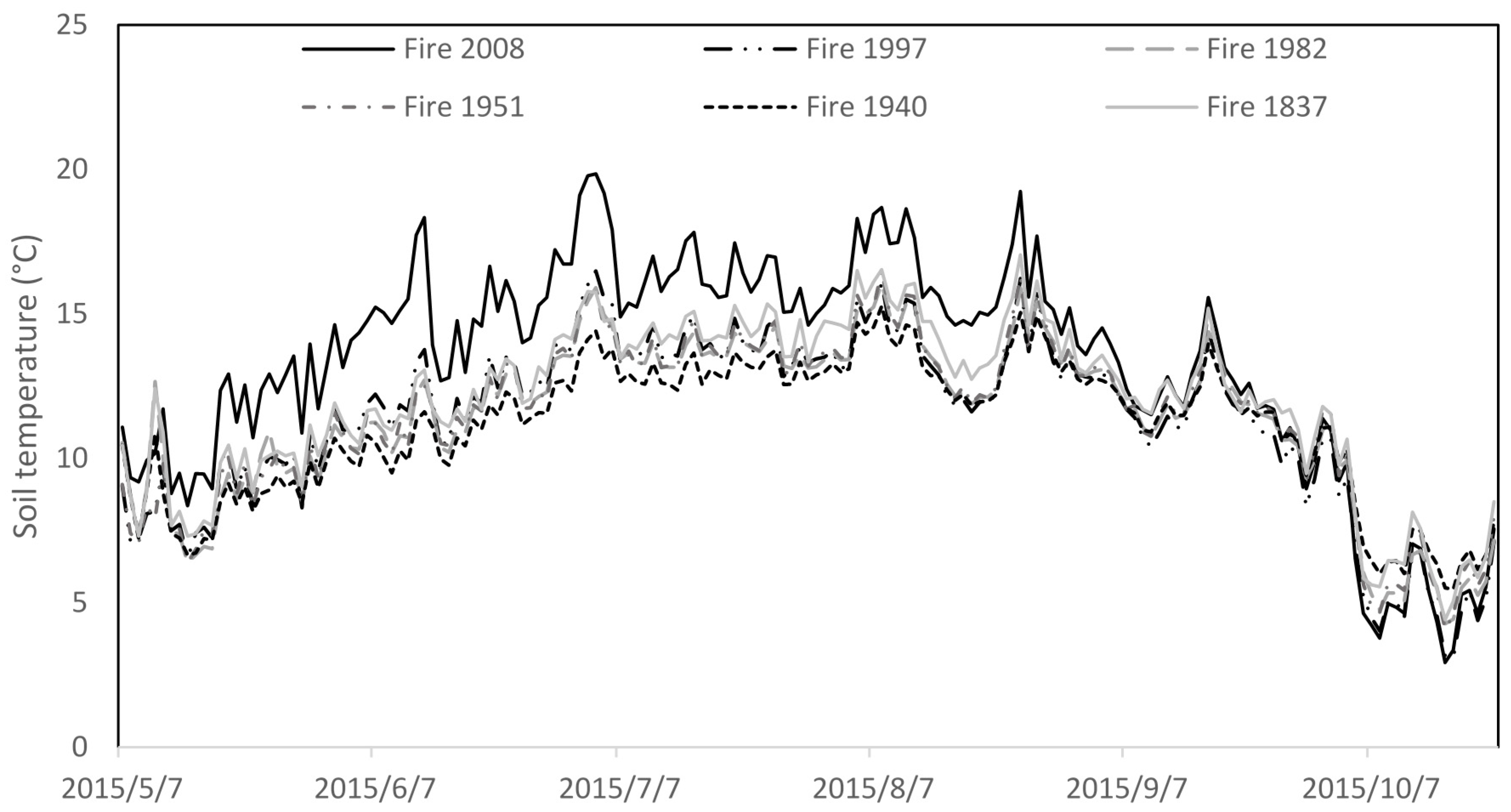
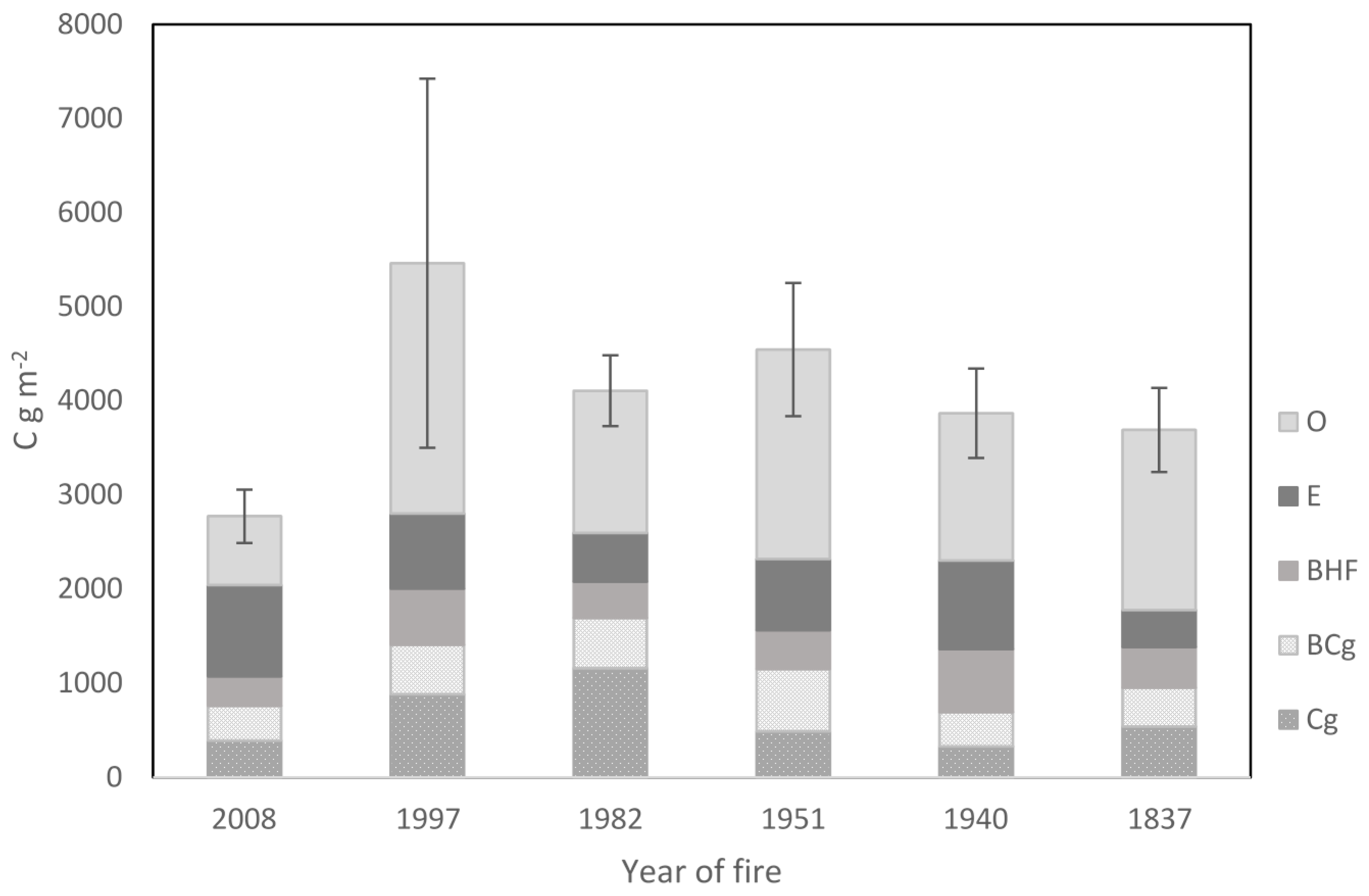
3.1. Soil Physical-Chemical Properties and Above- and Belowground Biomasses
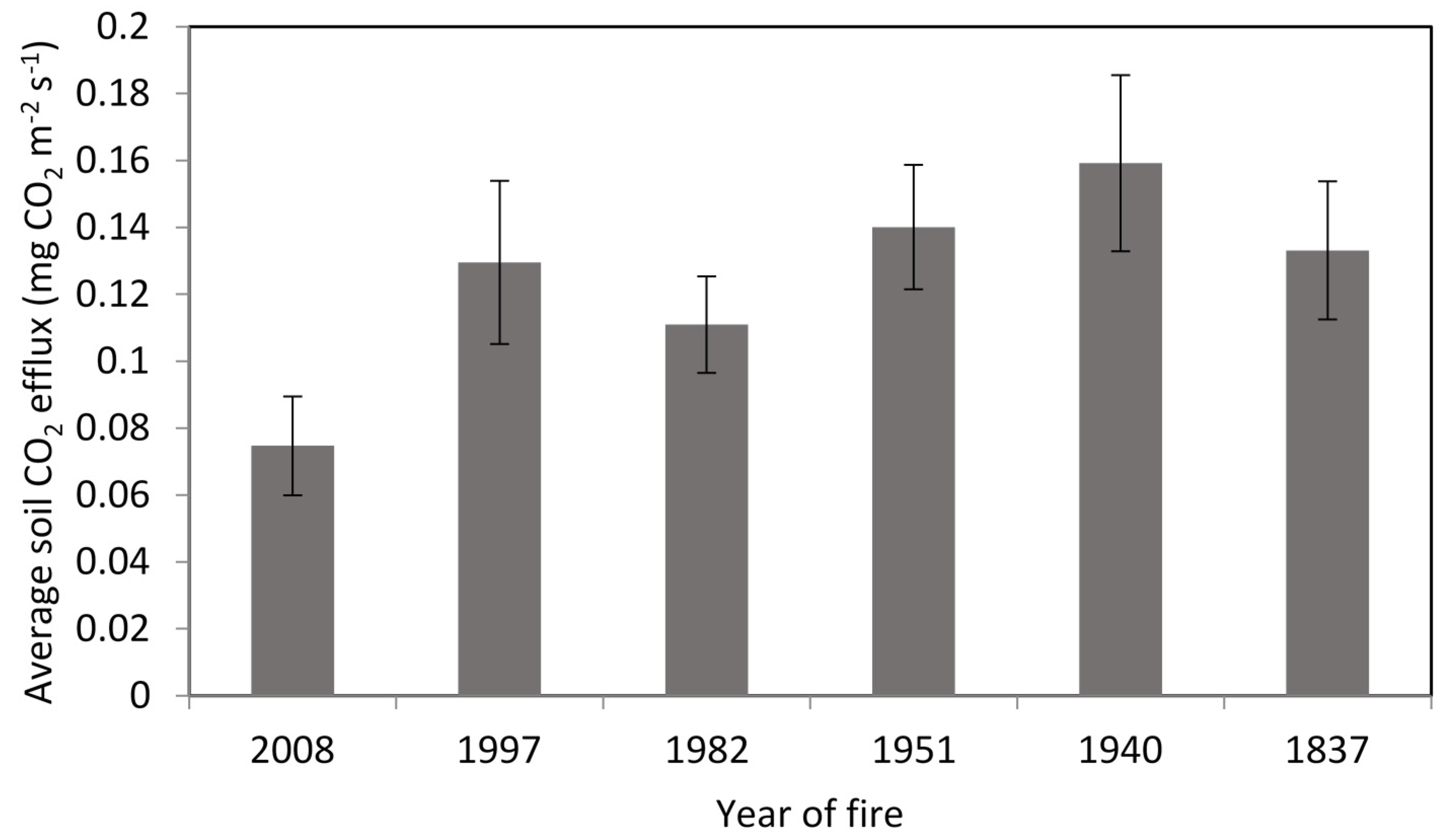
3.2. Soil CO2 Efflux
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franklin, J.F.; Spies, T.A.; Pelt, R.V.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Parro, K.; Metslaid, M.; Renel, G.; Sims, A.; Stanturf, J.A.; Jõgiste, K.; Köster, K. Impact of postfire management on forest regeneration in a managed hemiboreal forest, Estonia. Can. J. For. Res. 2015, 45, 1192–1197. [Google Scholar] [CrossRef]
- Kashian, D.M.; Romme, W.H.; Tinker, D.B.; Turner, M.G.; Ryan, M.G. Carbon storage on landscapes with stand-replacing fires. BioScience 2006, 56, 598–606. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Gower, S.T.; Wang, C.; Cyr, P.; Veldhuis, H. Nitrogen dynamics of a boreal black spruce wildfire chronosequence. Biogeochemistry 2006, 81, 1–16. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Yarie, J.; Billings, S. Carbon balance of the taiga forest within Alaska: Present and future. Can. J. For. Res. 2002, 32, 757–767. [Google Scholar] [CrossRef]
- Shorohova, E.; Kuuluvainen, T.; Kangur, A.; Jõgiste, K. Natural stand structures, disturbance regimes and successional dynamics in the Eurasian boreal forests: A review with special reference to Russian studies. Ann. For. Sci. 2009, 66, 201p1–201p20. [Google Scholar] [CrossRef]
- Conard, S.G.; Sukhinin, A.I.; Stocks, B.J.; Cahoon, D.R.; Davidenko, E.P.; Ivanova, G.A. Determining Effects of Area Burned and Fire Severity on Carbon Cycling and Emissions in Siberia. Clim. Chang. 2002, 55, 197–211. [Google Scholar] [CrossRef]
- Goetz, S.J.; Mack, M.C.; Gurney, K.R.; Randerson, J.T.; Houghton, R.A. Ecosystem responses to recent climate change and fire disturbance at northern high latitudes: Observations and model results contrasting northern Eurasia and North America. Environ. Res. Lett. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Högberg, P.; Read, D.J. Towards a more plant physiological perspective on soil ecology. Trends Ecol. Evol. 2006, 21, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Pumpanen, J.; Heinonsalo, J.; Rasilo, T.; Hurme, K.-R.; Ilvesniemi, H. Carbon balance and allocation of assimilated CO2 in Scots pine, Norway spruce, and Silver birch seedlings determined with gas exchange measurements and 14C pulse labelling. Trees 2009, 23, 611–621. [Google Scholar] [CrossRef]
- Köster, K.; Berninger, F.; Lindén, A.; Köster, E.; Pumpanen, J. Recovery in fungal biomass is related to decrease in soil organic matter turnover time in a boreal fire chronosequence. Geoderma 2014, 235–236, 74–82. [Google Scholar] [CrossRef]
- Wan, S.; Hui, D.; Luo, Y. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Goulden, M.L.; McMillan, A.M.S.; Winston, G.C.; Rocha, A.V.; Manies, K.L.; Harden, J.W.; Bond-Lamberty, B.P. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Chang. Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef]
- Parro, K.; Köster, K.; Jõgiste, K.; Vodde, F. Vegetation dynamics in a fire damaged forest area: The response of major ground vegetation species. Balt. For. 2009, 15, 206–215. [Google Scholar]
- Repola, J. Biomass equations for birch in Finland. Silva Fenn. 2008, 42, 605–624. [Google Scholar] [CrossRef]
- Repola, J. Biomass equations for Scots pine and Norway spruce in Finland. Silva Fenn. 2009, 43, 625–647. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014. [Google Scholar]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Gromtsev, A. Natural disturbance dynamics in the boreal forests of European Russia: A review. Silva Fenn. 2002, 36, 41–55. [Google Scholar] [CrossRef]
- Schimmel, J.; Granström, A. Fire severity and vegetation response in the boreal Swedish forest. Ecology 1996, 77, 1436–1450. [Google Scholar] [CrossRef]
- Ruokolainen, L.; Salo, K. The effect of fire intensity on vegetation succession on a sub-xeric heath during ten years after wildfire. Ann. Bot. Fenn. 2009, 46, 30–42. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Bormann, B.T.; Homann, P.S.; Darbyshire, R.L.; Morrissette, B.A. Intense forest wildfire sharply reduces mineral soil C and N: The first direct evidence. Can. J. For. Res. 2008, 38, 2771–2783. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Kurz, W.A.; Neilson, E.T.; Stinson, G. Implications of future disturbance regimes on the carbon balance of Canada’s managed forest (2010–2100). Tellus B 2010, 62, 719–728. [Google Scholar] [CrossRef]
- Liski, J.; Ilvesniemi, H.; Mäkelä, A.; Starr, M. Model analysis of the effects of soil age, fires and harvesting on the carbon storage of boreal forest soils. Eur. J. Soil Sci. 1998, 49, 407–416. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Adams, D.E.; Wild, A. Model estimates of CO2 emissions from soil in response to global warming. Nature 1991, 351, 304–306. [Google Scholar] [CrossRef]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; Calvo de Anta, R.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Berryman, E.M.; Marshall, J.D.; Kavanagh, K. Decoupling litter respiration from whole-soil respiration along an elevation gradient in a Rocky Mountain mixed-conifer forest. Can. J. For. Res. 2014, 44, 432–440. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Certini, G.; Nocentini, C.; Knicker, H.; Arfaioli, P.; Rumpel, C. Wildfire effects on soil organic matter quantity and quality in two fire-prone Mediterranean pine forests. Geoderma 2011, 167–168, 148–155. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier: New York, NY, USA, 2006; p. 320. [Google Scholar]
- Czimczik, C.I.; Trumbore, S.E.; Carbone, M.S.; Winston, G.C. Changing sources of soil respiration with time since fire in a boreal forest. Glob. Chang. Biol. 2006, 12, 957–971. [Google Scholar] [CrossRef]
- Köster, K.; Berninger, F.; Heinonsalo, J.; Lindén, A.; Köster, E.; Ilvesniemi, H.; Pumpanen, J. The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests. Int. J. Wildland Fire 2016, 25, 213–223. [Google Scholar] [CrossRef]
- Kulmala, L.; Aaltonen, H.; Berninger, F.; Kieloaho, A.-J.; Levula, J.; Bäck, J.; Hari, P.; Kolari, P.; Korhonen, J.F.J.; Kulmala, M.; et al. Changes in biogeochemistry and carbon fluxes in a boreal forest after the clear-cutting and partial burning of slash. Agric. For. Meteorol. 2014, 188, 33–44. [Google Scholar] [CrossRef]
- Köster, E.; Köster, K.; Berninger, F.; Pumpanen, J. Carbon dioxide, methane and nitrous oxide fluxes from podzols of a fire chronosequence in the boreal forests in Värriö, Finnish Lapland. Geoderma Reg. 2015, 5, 181–187. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and soil respiration: Correlation and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Widén, B.; Majdi, H. Soil CO2 efflux and root respiration at three sites in a mixed pine and spruce forest: Seasonal and diurnal variation. Can. J. For. Res. 2001, 31, 786–796. [Google Scholar] [CrossRef]
- Raich, J.W.; Schelesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bergeron, Y.; Chen, H.Y.H. The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol. Biochem. 2012, 53, 18–27. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Köster, K.; Püttsepp, Ü.; Pumpanen, J. Comparsion of soil CO2 flux between uncleared and cleared windthrow areas in Estonia and Latvia. For. Ecol. Manag. 2011, 262, 65–70. [Google Scholar] [CrossRef]
- Köster, K.; Voolma, K.; Jõgiste, K.; Metslaid, M.; Laarmann, D. Assessment of tree mortality after windthrow using photo-derived data. Ann. Bot. Fenn. 2009, 46, 291–298. [Google Scholar] [CrossRef]
- Moser, B.; Temperli, C.; Schneiter, G.; Wohlgemuth, T. Potential shift in tree species composition after interaction of fire and drought in the Central Alps. Eur. J. For. Res. 2010, 129, 625–633. [Google Scholar] [CrossRef]


| Study Area (Geographical Coordinate) | Site Type | Tree Species Composition (%) | Living Trees/ha | D1,3 (cm) | H (m) | Soil Texture | Average Thickness of: O-/E-/BHF-/BCg-/Cg-Horizon in Soil (cm) | Soil pH (O-/E-/Mineral Horizons) | Average Soil Temperature (Growing Season) (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Fire 2008 (59°11′ N 23°46′ E) | Calluna/Cladina | 56 Pi, 44 Bi | 1422 | 1.9 | 1.1 | Loamy sand | 3.3/10.3/12.9/11.2/11.5 | 4.0/4.1/4.7 | 13.4 |
| Fire 1997 (59°12′ N 23°49′ E) | Calluna/Vaccinium uliginosum | 91 Pi, 9 Bi | 2683 | 3.9 | 2.9 | Loamy sand | 8.8/7.8/12.1/11.3/15.3 | 4.0/4.2/4.5 | 11.4 |
| Fire 1982 (59°12′ N 23°48′ E) | Vaccinium uliginosum | 100 Pi | 3167 | 7.2 | 5.5 | Loamy sand | 8.7/5.5/7.9/9.1/20.8 | 4.0/3.9/4.4 | 11.5 |
| Fire 1951 (59°14′ N 23°49′ E) | Calluna | 93 Pi, 7 Bi | 1583 | 12.5 | 11.1 | Loamy sand | 13.2/7.2/11.1/9.4/10.3 | 3.8/3.9/4.3 | 11.5 |
| Fire 1940 (59°10′ N 23°42′ E) | Calluna | 100 Pi | 3117 | 10.4 | 9.4 | Loamy sand | 9.4/10.4/11.7/10.1/10.2 | 3.6/3.7/4.6 | 11.1 |
| Fire 1837 (59°13′ N 23°36′ E) | Calluna/Cladina | 100 Pi | 558 | 21.8 | 13.4 | Loamy sand | 8.9/9.9/12.6/10.7/17.5 | 3.7/4.0/4.5 | 11.4 |
| Factor | Complex Model | p-Value | ||
|---|---|---|---|---|
| NDF | DDF | F | ||
| Time since fire (year of fire) | 5 | 924 | 116.99 | <0.001 |
| Time of measurement (month) | 6 | 924 | 31.60 | <0.001 |
| Soil water content | 1 | 924 | 0.87 | 0.3508 |
| Soil temperature | 1 | 924 | 141.89 | <0.001 |
| Soil C content | 1 | 924 | 0.14 | 0.8929 |
| Soil N content | 1 | 924 | 3.36 | 0.0672 |
| Ground vegetation biomass | 1 | 924 | 3.39 | 0.0701 |
| Root biomass (tree and ground vegetation roots) | 1 | 924 | 19.23 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köster, K.; Köster, E.; Orumaa, A.; Parro, K.; Jõgiste, K.; Berninger, F.; Pumpanen, J.; Metslaid, M. How Time since Forest Fire Affects Stand Structure, Soil Physical-Chemical Properties and Soil CO2 Efflux in Hemiboreal Scots Pine Forest Fire Chronosequence? Forests 2016, 7, 201. https://doi.org/10.3390/f7090201
Köster K, Köster E, Orumaa A, Parro K, Jõgiste K, Berninger F, Pumpanen J, Metslaid M. How Time since Forest Fire Affects Stand Structure, Soil Physical-Chemical Properties and Soil CO2 Efflux in Hemiboreal Scots Pine Forest Fire Chronosequence? Forests. 2016; 7(9):201. https://doi.org/10.3390/f7090201
Chicago/Turabian StyleKöster, Kajar, Egle Köster, Argo Orumaa, Kristi Parro, Kalev Jõgiste, Frank Berninger, Jukka Pumpanen, and Marek Metslaid. 2016. "How Time since Forest Fire Affects Stand Structure, Soil Physical-Chemical Properties and Soil CO2 Efflux in Hemiboreal Scots Pine Forest Fire Chronosequence?" Forests 7, no. 9: 201. https://doi.org/10.3390/f7090201







