Aftermath of Mountain Pine Beetle Outbreak in British Columbia: Stand Dynamics, Management Response and Ecosystem Resilience
Abstract
:1. Introduction
2. Stand Structure and Composition
3. Growth Response of Residual Live Trees
4. Mid-Term Timber Supply
5. Forest Fire
6. Carbon Dynamics
7. Species Range Expansion
8. Biodiversity
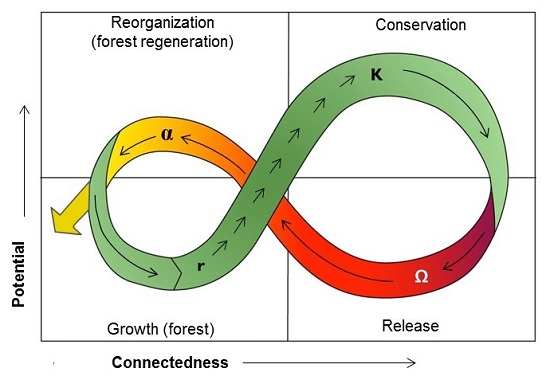
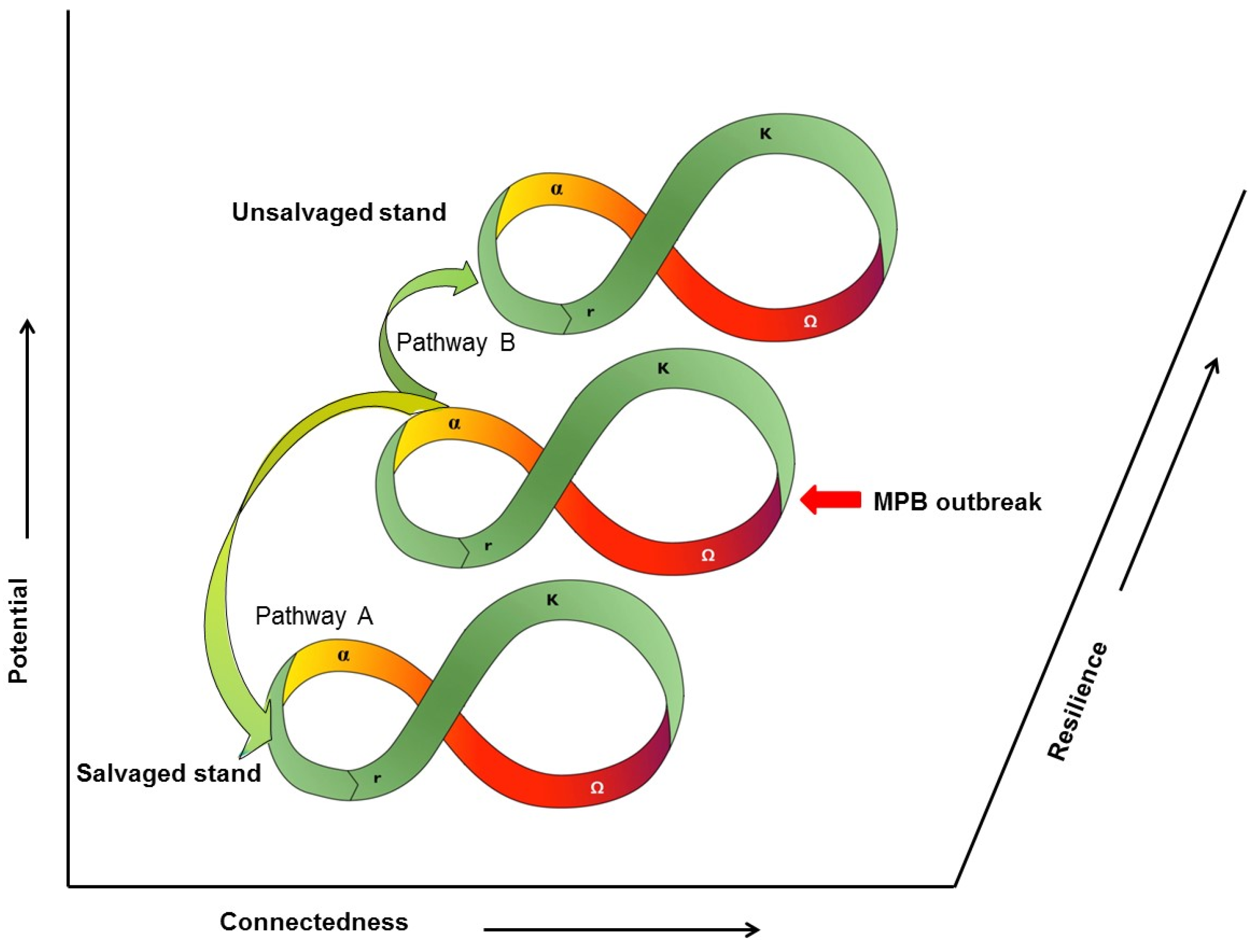
9. Ecosystem Resilience
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Axelson, J.; Alfaro, R.; Hawkes, B. Influence of fire and mountain pine beetle on the dynamics of lodgepole pine stands in British Columbia, Canada. For. Ecol. Manag. 2009, 257, 1874–1882. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Campbell, E.; Hawkes, B.C. Historical Frequency, Intensity and Extent of Mountain Pine Beetle Disturbance in British Columbia; MPB Working Paper 2009-30; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2010; p. 52.
- Safranyik, L.; Carroll, A.L. The Biology and Epidemiology of the Mountain Pine Beetle in Lodgepole Pine Forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Natural Resources Canada: Victoria, BC, Canada, 2006; pp. 3–66. [Google Scholar]
- Lee, S.; Kim, J.J.; Breuil, C. Leptographium longiclavatum sp. nov., a new species associated with the mountain pine beetle, Dendroctonus ponderosae. Mycol. Res. 2005, 109, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Kim, J.-J.; Lu, M.; Breuil, C. Determining fungal diversity on Dendroctonus ponderosae and Ips pini affecting lodgepole pine using cultural and molecular methods. Fungal Divers. 2005, 19, 79–94. [Google Scholar]
- Rice, A.V.; Thormann, M.N.; Langor, D.L. Mountain pine beetle-associated blue-stain fungi are differentially adapted to boreal temperatures. For. Path. 2008, 38, 113–123. [Google Scholar] [CrossRef]
- Whitney, H.; Spanier, O. An improved method for rearing axenic mountain pine beetles, Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. Entomol. 1982, 114, 1095–1100. [Google Scholar] [CrossRef]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of tree defences by Ophiostomatoid fungi can explain attack success of bark beetles on conifers. Ann. For. Sci. 2009, 66, 801p1–801p22. [Google Scholar] [CrossRef]
- Barras, S. Reduction of progeny and development in southern pine beetle following removal of symbiotic fungi. Can. Entomol. 1973, 105, 1295–1299. [Google Scholar] [CrossRef]
- Ayres, M.; Wilkens, R.; Ruel, J.; Lombardero, M.; Vallery, E. Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi. Ecology 2000, 81, 2198–2210. [Google Scholar] [CrossRef]
- Klepzig, K.; Moser, J.; Lombardero, M.; Ayres, M. Symbiosis and competition: Complex interactions among beetles, fungi and mites. Symbiosis 2001, 30, 93–96. [Google Scholar]
- Bleiker, K.; Six, D. Dietary benefits of fungal associates to an eruptive herbivore: Potential implications of multiple associates on host population dynamics. Environ. Entomol. 2007, 36, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Amman, G.D.; McGregor, M.D.; Cahill, D.B.; Klein, W.H. Guidelines for Reducing Losses of Lodgepole Pine to the Mountain Pine Beetle in Unmanaged Stands in The Rocky Mountains; General Technical Report INT-36; USDA Forest Service: Ogden, Utah, UT, USA, 1977; p. 19.
- Shore, L.T.; Safranyik, L.; Hawkes, C.B.; Taylor, W.S. Effects of the mountain pine beetle on lodgepole pine stand structure and dynamics. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Natural Resources Canada: Victoria, BC, Canada, 2006; pp. 95–116. [Google Scholar]
- Price, T.S.; Doggett, C.; Pye, J.M.; Smith, B. A History of Southern Pine Beetle Outbreaks in the Southeastern United States; Georgia Forestry Commission: Atlanta, GA, USA, 1992; p. 65. [Google Scholar]
- Safranyik, L.; Linton, D.A. Unseasonably low fall and winter temperatures affecting mountain pine beetle and pine engraver beetle populations and damage in the British Columbia Chilcotin Region. J. Entomol. Soc. B.C. 1991, 88, 17–21. [Google Scholar]
- Safranyik, L. Mountain pine beetle epidemiology in lodgepole pine. In Proceedings of the Stone Mountain Pine Beetle Symposium: Challenges and Solutions, Victoria, BC, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Eds.; Information Report BC-X-399. Natural Resources Canada, Pacific Forestry Centre: Victoria, BC, Canada, 2004; p. 298. [Google Scholar]
- Alfaro, R.I.; Campbell, R.; Vera, P.; Hawkes, B.; Shore, T. Dendroecological reconstruction of mountain pine beetle outbreaks in the Chilcotin Plateau of British Columbia. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, BC, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Information Report BC-X-399. Resources Canada, Canadian, Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004; pp. 245–256. [Google Scholar]
- Taylor, S.W.; Carroll, A.L.; Alfaro, R.I.; Safranyik, L. Forest, climate and mountain pine beetle outbreak dynamics in western Canada. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, W.R., Eds.; Canadian Forest Service: Victoria, BC, Canada, 2006; pp. 67–94. [Google Scholar]
- Cole, W.E.; Amman, G.D. Mountain Pine Beetle Dynamics in Lodgepole Pine Forests, Part 1: Course of An Infestation; General Technical Report INT-89; U.S. Department of Agriculture Forest Service, Intermountain Research Station: Ogden, UT, USA, 1980; p. 64.
- McIntosh, A.C.S.; Macdonald, S.E. Potential for lodgepole pine regeneration after mountain pine beetle attack in newly invaded Alberta stands. For. Ecol. Manag. 2013, 295, 11–19. [Google Scholar] [CrossRef]
- Walton, A. Provincial-Level Projection of the Current Mountain Pine Beetle Outbreak: Update of the Infestation Projection Based on the Provincial Aerial Overview Surveys of Forest Health Conducted from 1999 through 2012 and the BCMPB Model (Year 10); BC Ministry of Forests, Lands and Natural Resource Operations: Victoria, BC, Canada, 2013. Available online: http://www.for.gov.bc.ca/ftp/hre/external/!publish/web/bcmpb/year10/BCMPB.v10.BeetleProjection.Update.pdf (accessed on 3 December 2015).
- Dhar, A.; Balliet, N.A.; Runzer, K.D; Hawkins, C.D.B. Impact of a mountain pine beetle outbreak on young lodgepole pine stands in central British Columbia. Forests 2015, 6, 3483–3500. [Google Scholar] [CrossRef]
- Association of BC Forest Professional (ABCFP). Mid-Term Timber Supply Advocacy Report; Association of BC Forest Professionals: Victoia, BC, Canada, 2011; Available online: http://www.abcfp.ca/publications_forms/publications/documents/Mid-term_TimberSupply_ABCFP_Summary_Report.pdf (accessed on 22 October 2015).
- Dhar, A.; Parrott, L.; Heckbert, S. Mapping the impact of mountain pine beetle outbreaks on forest ecosystem services in British Columbia, Canada. In Proceedings of the IUFRO Landscape Ecology Conference Sustaining Ecosystem Services in Forest Landscapes Concepts, Research, and Applications, Tartu, Estonia, 23–30 August 2015; Available online: http://iufrole2015.to.ee/download/m55d4ea9be5748#iufrole_2015_abstracts_pdf (accessed on 22 February 2016).
- Coops, N.C.; Timko, J.A.; Wulder, M.A.; White, J.C.; Ortlepp, S.M. Investigating the effectiveness of Mountain Pine Beetle mitigation strategies. Int. J. Pest Manag. 2008, 54, 151–165. [Google Scholar] [CrossRef]
- Fettig, C.J.; Gibson, K.E.; Munson, A.S.; Negrón, J.F. Cultural practices for prevention and mitigation of mountain pine beetle infestations. For. Sci. 2014, 60, 450–463. [Google Scholar]
- Gillette, N.E.; Wood, D.L.; Hines, S.J.; Runyon, J.B.; Negrón, J.F. Consequences of mountain pine beetle treatment decisions. For. Sci. 2014, 60, 527–538. [Google Scholar]
- Fettig, C.J.; Munson, A.S.; Grosman, D.M.; Bush, P.B. Evaluations of emamectin benzoate and propiconazole for protecting individual Pinus contorta from mortality attributed to colonization by Dendroctonus ponderosae and associated fungi. Pest. Manag. Sci. 2013, 70, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Progar, R.A.; Gillette, N.E.; Fettig, C.J.; Hrinkevich, K.H. Applied chemical ecology of the mountain pine beetle. For. Sci. 2014, 60, 414–433. [Google Scholar]
- Eng, M.A. Forest Stewardship in the Context of Large-Scale Salvage Operations: An Interpretation Paper; Technical Report 019; British Columbia Ministry of Forests, Forest Science Program: Victoria, BC, Canada, 2004. Available online: https://www.for.gov.bc.ca/hfd/pubs/docs/tr/tr019.pdf (accessed on 22 January 2016).
- Lindenmayer, D.B.; Burton, P.J.; Franklin, F.J. Salvage Logging and its Ecological Consequences; Island Press: Washington, DC, USA, 2008; p. 246. [Google Scholar]
- Six, L.D.; Biber, E.; Long, E. Management for Mountain Pine Beetle Outbreak Suppression: Does Relevant Science Support Current Policy? Forests 2014, 5, 103–133. [Google Scholar] [CrossRef]
- Schoennagel, T.; Veblen, T.T.; Negron, J.F.; Smith, J.M. Effects of Mountain Pine Beetle on Fuels and Expected Fire Behavior in Lodgepole Pine Forests, Colorado, USA. PLoS ONE 2012, 7, e30002. [Google Scholar] [CrossRef] [PubMed]
- Harveya, B.J.; Donatob, D.C.; Turnera, M.G. Recent mountain pine beetle outbreaks, wildfire severity, and postfire tree regeneration in the US Northern Rockies. Proc. Natl. Acad. Sci. 2014, 111, 15120–15125. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.J.; Schoennagela, T.; Veblena, T.T.; Chapmana, T.B. Area burned in the western United States is unaffected by recent mountain pine beetle outbreaks. Proc. Natl. Acad. Sci. 2015, 111, 4375–4380. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.L.; Amman, G.D. The Mountain Pine Beetle in Lodgepole Pine Forests; Research Paper INT-71; US Department of Agriculture Forest Service, Intermountain Research Station: Ogden, UT, USA, 1970; p. 28.
- Stuart, J.D.; Agee, J.K.; Gara, R.I. Lodgepole pine regeneration in an old, self-perpetuating forest in south central Oregon. Can. J. For. Res. 1989, 19, 1096–1104. [Google Scholar] [CrossRef]
- Heath, R.; Alfaro, R. Growth response in a Douglas-fir/lodgepole pine stand after thinning of lodgepole pine by the mountain pine beetle: A case study. J. Entomol. Soc. B.C. 1990, 87, 16–21. [Google Scholar]
- Hawkes, B.; Taylor, S.W.; Stockdale, C.; Shore, T.L.; Alfaro, R.I.; Campbell, R.; Vera, P. Impact of mountain pine beetle on stand dynamics in British Columbia. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, BC, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Information Report BC-X-399. Natural Resources Canada, Canadian, Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004; pp. 177–199. [Google Scholar]
- Berg, E.E.; Henry, J.D.; Fastie, C.L.; Volder, A.D.; Matsuoka, S.M. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to summer temperatures and regional differences in disturbance regimes. For. Ecol. Manag. 2006, 227, 219–232. [Google Scholar] [CrossRef]
- Hawkins, C.D.B.; Dhar, A.; Balliet, N. Radial growth of residual overstory trees and understory saplings after mountain pine beetle attack in central British Columbia. For. Ecol. Manag. 2013, 310, 348–356. [Google Scholar] [CrossRef]
- Dhar, A.; Hawkins, C.D.B. Regeneration and growth following mountain pine beetle attack: A synthesis of knowledge. J. Ecosyst. Manag. 2011, 12, 1–16. [Google Scholar]
- Dhar, A.; Parrott, L.; Heckbert, S. Consequences of mountain pine beetle outbreak on forest ecosystem services in western Canada. Can. J. For. Res. 2016, 46, 987–999. [Google Scholar] [CrossRef]
- British Columbia Ministry of Forests and Range. Forests for Tomorrow: Planning, Reforestation and Brushing Focused in Catastrophic Event-Impacted Management Units; Forest Practices Branch: Victoria, BC, Canada, 2005; p. 27.
- Stone, W.E.; Wolfe, M.L. Response of understory vegetation to variable tree mortality following a mountain pine beetle epidemic in lodgepole pine stands in northern Utah. Vegetatio 1996, 122, 1–12. [Google Scholar] [CrossRef]
- Purdon, M.; Brais, S.; Bergeron, Y. Initial response of understorey vegetation to fire severity and salvage-logging in the southern boreal forest of Québec. Appl. Veg. Sci. 2004, 7, 49–60. [Google Scholar] [CrossRef]
- Marzano, R.; Garbarino, M.; Marcolin, E.; Pividori, M.; Lingua, E. Deadwood anisotropic facilitation on seedling establishment after a stand-replacing wildfire in Aosta Valley (NW Italy). Ecol. Eng. 2013, 51, 117–122. [Google Scholar] [CrossRef]
- Kurulok, S.E.; Macdonald, S.E. Impacts of post fire salvage logging on understory plant communities of the boreal mixedwood forest 2 and 34 years after disturbance. Can. J. For. Res. 2007, 37, 2637–2651. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Fraver, S.; Palik, B.J.; Bradford, J.B.; Patty, L. Singular and interactive effects of blowdown, salvage logging, and wildfire in sub-boreal pine systems. For. Ecol. Manag. 2011, 262, 2070–2078. [Google Scholar] [CrossRef]
- Coates, K.D.; DeLong, C.; Burton, P.J.; Sachs, D.L. Abundance of Secondary Structure in Lodgeople Pine Stands Affected by Mountain Pine Beetle; Report of Chief Forester; Bulkley Valley Centre for Natural Resources Research and Management: Smithers, BC, Canada, 2006; p. 17.
- Coates, K.D.; Glover, T.; Henderson, B. Abundance of Secondary Structure in Lodgepole Pine Stands Affected by the Mountain Pine Beetle in the Cariboo-Chilcotin; Mountain Pine Beetle Working Paper; Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2009; Available online: http://cfs.nrcan.gc.ca/pubwarehouse/pdfs/31195.pdf (accessed on 7 April 2016).
- Vyse, A.; Ferguson, C.; Huggard, D.J.; Roach, J.; Zimonick, B. Regeneration beneath lodgepole pine dominated stands attacked or threatened by the mountain pine beetle in the south central Interior, British Columbia. For. Ecol. Manag. 2006, 258, S36–S43. [Google Scholar] [CrossRef]
- Hawkins, C.D.B.; Dhar, A.; Balliet, N.A.; Runzer, K.D. Residual mature trees and secondary stand structure after mountain pine beetle attack in central British Columbia. For. Ecol. Manag. 2012, 277, 107–115. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Coates, K.D.; Astrup, R. Stand recovery and self-organization following large-scale mountain pine beetle induced canopy mortality in northern forests. For. Ecol. Manag. 2013, 310, 300–311. [Google Scholar] [CrossRef]
- DeLong, C.; Rogers, B.; Kaytor, B. Response of understory trees to MPB attack in permanent sample plots three years after establishment in the Sub-Boreal Spruce zone. In Proceedings of the Growth and Yield Modelling Workshop “Regeneration and Growth Following MPB Attack: A synthesis of Knowledge”, University of Northern British Columbia, Prince George, BC, Canada, 23–24 September 2008; Forsythe, P., Hawkins, C., Hassegawa, M., Eds.; University of Northern British Columbia: Prince George, BC, Canada, 2009; pp. 6–7. [Google Scholar]
- Statland, C.B. Advanced regeneration in pine thinning trials killed by mountain pine beetle. In Proceedings of the Growth and Yield Modelling Workshop “Regeneration and Growth Following MPB Attack: A synthesis of Knowledge”, University of Northern British Columbia, Prince George, BC, Canada, 23–24 September 2008; Forsythe, P., Hawkins, C., Hassegawa, M., Eds.; University of Northern British Columbia: Prince George, BC, Canada, 2009; pp. 4–5. [Google Scholar]
- Zumrawi, A.; Sattler, D.; LeMay, V.; Marshall, P.; Lee, T. Predicting natural regeneration within the Prognosis BC framework following MPB attack: Imputation and hybrid modeling. In Proceedings of the Growth and Yield Modelling Workshop “Regeneration and Growth Following MPB Attack: A synthesis of Knowledge”, University of Northern British Columbia, Prince George, BC, Canada, 23–24 September 2008; Forsythe, P., Hawkins, C., Hassegawa, M., Eds.; University of Northern British Columbia: Prince George, BC, Canada, 2009; pp. 8–10. [Google Scholar]
- Astrup, R.; Coates, D.K.; Hall, E. Recruitment limitation in forests: Lessons from an unprecedented mountain pine beetle epidemic. For. Ecol. Manag. 2008, 256, 1743–1750. [Google Scholar] [CrossRef]
- Perovich, P.; Sibold, J.S. Forest composition change after a mountain pine beetle outbreak, Rocky Mountain National Park, CO, USA. For. Ecol. Manag. 2016, 366, 184–192. [Google Scholar] [CrossRef]
- Nigh, G.D.; Antos, J.A.; Parish, R. Density and distribution of advance regeneration in mountain pine beetle killed lodgepole pine stands of the Montane Spruce zone of southern British Columbia. Can. J. For. Res. 2008, 38, 2826–2836. [Google Scholar] [CrossRef]
- Griesbauer, H.; Green, S. Examining the utility of advance regeneration for reforestation and timber production in unsalvaged stands killed by the mountain pine beetle: Controlling factors and management implications. J. Ecosyst. Manag. 2006, 7, 81–92. [Google Scholar]
- Dale, V.H.; Lugo, A.E.; MacMahon, J.A.; Pickett, S.T.A. Ecosystem management in the context of large infrequent disturbances. Ecosystems 1998, 1, 546–557. [Google Scholar] [CrossRef]
- Waring, R.H.; Pitman, G.B. Modifying lodgepole pine stands to change susceptibility to mountain pine beetle attack. Ecology 1985, 66, 889–897. [Google Scholar] [CrossRef]
- Murphy, T.E.L.; Adams, D.L.; Ferguson, D.E. Response of advance lodgepole pine regeneration to overstory removal in eastern Idaho. For. Ecol. Manag. 1999, 120, 234–244. [Google Scholar]
- Romme, W.H.; Knight, D.H.; Fedders, J. Mountain pine beetle outbreaks in the Rocky Mountains: Effects on fuels and fire in lodgepole pine forest (abstract). In Program of the Annual Meeting of the Ecological Society of America; Syracuse University: Syracuse, NY, USA, 1986; p. 290. [Google Scholar]
- Dhar, A.; Coates, K.D.; Rogers, B.; Hardy, K. Impact of Mountain pine beetle on mid-term timber supply in sub boreal spruce zone of British Columbia. In 16th International Boreal Forest Research Association (IBFRA) Conference; Comeau, P., Ed.; IBRF: Edmonton, AB, Canada, 2013; p. 28. [Google Scholar]
- Kimmins, J.P. Forest Ecology; Macmillan Publishing Company: New York, NY, USA, 1987; p. 531. [Google Scholar]
- Smith, F.W.; Resh, S.C. Age-related changes in production and below-ground carbon allocation in Pinus contorta forests. For. Sci. 1999, 45, 333–341. [Google Scholar]
- Kashian, D.M.; Romme, W.H.; Tinker, D.B.; Turner, M.G.; Ryan, M.G. Post-fire changes in forest carbon storage over a 300-year chronosequence of Pinus contorta-dominated forests. Ecol. Monogr. 2013, 83, 49–66. [Google Scholar] [CrossRef]
- Bogdanski, B.; Sun, L.; Peter, B.; Stennes, B. Markets for Forest Products Following A Large Disturbance: Opportunities and Challenges from the Mountain Pine Beetle Outbreak in Western Canada; Report BC-X-429; Canada Forest Services: Victoria, BC, Canada, 2011; Available online: http://cfs.nrcan.gc.ca/pubwarehouse/pdfs/32226.pdf (accessed on 16 February 2015).
- BC Ministry of Forests and Range. Timber Supply and the Mountain Pine Beetle Infestation in British Columbia: 2007 Update; Forest Analysis and Inventory Branch, BC Ministry of Forests and Range: Victoria, BC, Canada, 2007; p. 32.
- Pousette, J.; Hawkins, C. An assessment of critical assumptions supporting the timber supply modelling for mountain-pine-beetle-induced allowable annual cut uplift in the Prince George Timber Supply Area. J. Ecosyst. Manag. 2006, 7, 93–104. [Google Scholar]
- Patriquin, M.N.; Wellstead, A.M.; White, W.A. Beetles, trees, and people: Regional economic impact sensitivity and policy considerations related to the mountain pine beetle infestation in British Columbia, Canada. For. Policy Econ. 2007, 9, 938–946. [Google Scholar] [CrossRef]
- Coates, K.D. Evaluation of Stand Dynamics after A 25–30 Year Old Mpb Attack in the Flathead Region of South Eastern British Columbia; FIA-FSP project # M085196; Bulkley Valley Research Centre: Smithers, BC, Canada, 2008. Available online: http://www.for.gov.bc.ca/hfd/library/FIA/2008/FSP_M085196.pdf (accessed on 12 October 2015).
- Coates, K.D.; Hall, E.C. Implications of Alternative Silvicultural Strategies in Mountain Pine Beetle Damaged Stands; Technical Report for Forest Science Project Y051161; Bulkley Valley Centre for Natural Resources Research and Management: Smithers, BC, Canada, 2005; p. 23.
- Runzer, K.; Hassegawa, M.; Balliet, N.; Bittencourt, E.; Hawkins, C.D.B. Temporal Composition and Structure of Post-Beetle Lodgepole Pine Stands: Regeneration, Growth, Economics, and Harvest Implications; Mountain Pine Beetle Initiative Working Paper 2008-23; Canadian Forest Service: Victoria, BC, Canada, 2008; p. 76.
- Pousette, J. Secondary Stand Structure and Its Timber Supply Implication for Mountain Pine Beetle Attacked Forests on the Nechako Plateau of British Columbia. Master’s Thesis, University of Northern British Columbia, Prince George, BC, Canada, 2010; p. 182. [Google Scholar]
- Lynch, H.J.; Renkin, R.A.; Crabtree, R.L.; Moorcroft, P.R. The influence of previous mountain pine beetle (Dendroctonus ponderosae) activity on the 1988 Yellowstone fires. Ecosystems 2006, 9, 1318–1327. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Hebertson, E.; Page, W.; Jorgenson, C.A. Bark beetles, fuels, fires and implications for forest management in the intermountain west. For. Ecol. Manag. 2008, 254, 16–34. [Google Scholar] [CrossRef]
- Hicke, J.A.; Johnson, M.C.; Hayes, J.L.; Preisler, H.K. Effects of bark beetle-caused tree mortality on wildfire. For. Ecol. Manag. 2012, 271, 81–90. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Runyon, J.B.; Fettig, C.J.; Page, W.G.; Bentz, B.J. Interactions among the mountain pine beetle, fires, and fuels. For. Sci. 2014, 60, 489–501. [Google Scholar] [CrossRef]
- Jolly, W.M.; Parsonsa, R.A.; Hadlowa, A.M.; Cohna, G.M.; McAllister, S.S.; Popp, J.B.; Hubbard, R.M.; Negron, J.F. Relationships between moisture, chemistry, and ignition of Pinus contorta needles during the early stages of mountain pine beetle attack. For. Ecol. Manag. 2012, 269, 52–59. [Google Scholar] [CrossRef]
- Page, W.G.; Jenkins, M.J.; Runyon, J.B. Mountain pine beetle attack alters the chemistry and flammability of lodgepole pine foliage. Can. J. Res. 2012, 42, 1631–1647. [Google Scholar] [CrossRef]
- Collins, B.J.; Rhoades, C.C.; Battaglia, M.A.; Hubbard, R.M. The effects of bark beetle outbreaks on forest development, fuel loads and potential fire behavior in salvage logged and untreated lodgepole pine forests. For. Ecol. Manag. 2012, 284, 260–268. [Google Scholar] [CrossRef]
- Simard, M.; Romme, W.; Griffin, J. Do mountain pine beetle outbreaks change the probability of active crown fire in lodgepole pine forests? Ecol. Monogr. 2011, 81, 3–24. [Google Scholar] [CrossRef]
- Klutsch, G.J.; Battaglia, M.A.; West, D.R.; Costello, S.L.; Negrón, J.F. Evaluating potential fire behavior in lodgepole pine-dominated forests after a mountain pine beetle epidemic in north-central Colorado. West J. Appl. For. 2011, 26, 201–109. [Google Scholar]
- Bourbonnais, M.L.; Nelson, T.A.; Wulder, M.A. Geographic analysis of the impacts of mountain pine beetle infestation on forest fire ignition. Can. Geogr. 2014, 58, 188–202. [Google Scholar] [CrossRef]
- Meigs, G.W.; Campbell, J.L.; Zald, H.S.; Bailey, J.D.; Shaw, D.C.; Kennedy, R.E. Does wildfire likelihood increase following insect outbreaks in conifer forests? Ecosphere 2015, 6. [Google Scholar] [CrossRef]
- Kulakowski, D.; Jarvis, D. The influence of mountain pine beetle outbreaks and drought on severe wildfires in northwestern Colorado and southern Wyoming: A look at the past century. For. Ecol. Manag. 2011, 262, 1686–1696. [Google Scholar] [CrossRef]
- Mattson, W.J.; Addy, N.D. Photophagous insect as regulators of forest primary production. Science 1975, 190, 515–522. [Google Scholar] [CrossRef]
- Loreau, M. Consumers as maximizers of matter and energy flow in ecosystems. Am. Nat. 1995, 145, 22–42. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.K. Impacts of Mountain Pine Beetle (Dendroctonus Ponderosae) and Fire Disturbances on Forest Ecosystem Carbon Dynamics and Species Composition. Master’s Thesis, University of Colorado, Denver, CO, USA, 2012; p. 95. [Google Scholar]
- Mathys, A.; Black, T.A.; Nesic, Z.; Nishio, G. Carbon balance of a partially-harvested mixed conifer forest following mountain pine beetle attack and its comparison to a clearcut. Biogeosciences 2013, 10, 5451–5463. [Google Scholar] [CrossRef]
- Reed, E.D.; Ewers, E.B.; Pendall, E. Impact of mountain pine beetle induced mortality on forest carbon and water fluxes. Environ. Res. Lett. 2014, 9, 105004. [Google Scholar] [CrossRef]
- Emmel, C.; Paul-Limgoes, E.; Bowler, R.; Black, T.A.; Christen, A. Vertical distribution of Carbon dioxide sources and sinksin a recovering mountain pine beetle-attack lodgepole pine stand. Agric. For. Meteorol. 2014, 195, 108–122. [Google Scholar] [CrossRef]
- Fettig, C.J.; Reid, M.L.; Bentz, B.J.; Sevanto, S.; Spittlehouse, D.L.; Wang, T. Changing climates, changing forests: A western North American perspective. J. For. 2013, 111, 214–228. [Google Scholar] [CrossRef]
- Hansen, E.M.; Amacher, C.M.; Miegroet, H.V.; Long, J.; Ryan, M.G. Carbon Dynamics in Central US Rockies Lodgepole Pine Type After Mountain Pine Beetle Outbreaks. For. Sci. 2015, 61, 665–679. [Google Scholar]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Brown, M.; Black, T.A.; Nesic, Z.; Foordb, V.N.; Spittlehousec, D.L.; Fredeen, A.L.; Grant, N.J.; Burton, P.J.; Trofymow, J.A. Impact of mountain pine beetle on the net ecosystem production of lodgepole pine stands in British Columbia. Agric. For. Meteorol. 2010, 150, 254–264. [Google Scholar] [CrossRef]
- Arora, V.K.; Peng, Y.; Kurz, W.A.; Fyfe, J.C.; Hawkins, B.; Werner, A.T. Potential near-future carbon uptake overcomes losses from a large insect outbreak in British Columbia, Canada. Geophys. Res. Lett. 2016, 43. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T.L. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Burton, P.J. Striving for sustainability and resilience in the face of unprecedented change: The case of the mountain pine beetle outbreak in British Columbia. Sustainability 2010, 2, 2403–2423. [Google Scholar] [CrossRef]
- Von Holle, B.; Delcourt, H.R.; Simberloff, D. The importance of biological inertia in plant community resistance to invasion. J. Veg. Sci. 2003, 14, 425–432. [Google Scholar] [CrossRef]
- Kovacic, D.A.; Dyer, M.I.; Cringan, A.T. Understory biomass in ponderosa pine following mountain pine beetle infestation. For. Ecol. Manag. 1985, 13, 53–67. [Google Scholar] [CrossRef]
- Pec, G.J.; Karst, J.; Sywenky, A.N.; Cigan, P.W.; Erbilgin, N.; Simard, S.W.; Cahill, J.F., Jr. Rapid Increases in Forest Understory Diversity and Productivity following a Mountain Pine Beetle (Dendroctonus ponderosae) Outbreak in Pine Forests. PLoS ONE 2015, 10, e0124691. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Krawchuk, M.M.; Burton, P.J. Short-interval disturbance in lodgepole pine forests, British Columbia, Canada: Understory and overstory response to mountain pine beetle and fire. For. Ecol. Manag. 2015, 335, 163–175. [Google Scholar] [CrossRef]
- Chan-McLeod, A.C.A. A review and synthesis of the effects of unsalvaged mountain-pine-beetle-attacked stands on wildlife and implications for forest management. J. Ecosyst. Manag. 2006, 7, 119–132. [Google Scholar]
- Martin, K.; Norris, A.; Drever, M. Effects of bark beetle outbreaks on avian biodiversity in the British Columbia interior: Implications for critical habitat management. J. Ecosyst. Manag. 2006, 7, 10–24. [Google Scholar]
- Saab, V.A.; Latif, Q.S.; Rowland, M.M.; Johnson, T.N.; Chalfoun, A.D.; Buskirk, S.W.; Heyward, J.E.; Dresser, M.A. Ecological Consequences of Mountain Pine Beetle Outbreaks for Wildlife in Western North American Forests. For. Sci. 2014, 60, 539–559. [Google Scholar]
- Stone, W.E. The Impact of A Mountain Pine Beetle Epidemic on Wildlife Habitat and Communities in Post Epidemic Stands of Lodgepole Pine Forests in Northern Utah. Ph.D. Thesis, Utah S University, Logan, UT, USA, 1995; p. 229. [Google Scholar]
- Drever, M.C.; Aitken, K.E.H.; Norris, A.R.; Martin, K. Woodpeckers as reliable indicators of bird richness, forest health and harvest. Biol. Conserv. 2008, 141, 624–634. [Google Scholar] [CrossRef]
- Walters, E.L.; Miller, E.H.; Lowther, P.E. Red-Breasted Sapsucker (Sphyrapicus Ruber) and Red-Naped Sapsucker (Sphyrapicus Nuchalis); Poole, A., Gill, F., Eds.; The Birds of North America: Philadelphia, PA, USA, 2002; Available online: http://www.ericlwalters.ca/rnsarbsaBNA.pdf (accessed on 5 July 2016).
- Bonnot, T.W.; Rumble, M.A.; Millspaugh, J.J. Nest success of black-backed woodpeckers in forests with mountain pine beetle outbreaks in the Black Hills, South Dakota. Condor 2008, 110, 450–457. [Google Scholar] [CrossRef]
- Bunnell, F.L.; Kremsater, L.L.; Houde, I. Mountain Pine Beetle: A Synthesis of the Ecological Consequences of Large-Scale Disturbances on Sustainable Forest Management, with Emphasis on Biodiversity; Canadian Forest Service: Victoria, BC, Canada, 2011; p. 99.
- Ferrari, M.R.; Miller, J.R.; Russell, G.L. Modeling changes in summer temperature of the Fraser River during the next century. J. Hydrol. 2007, 342, 336–346. [Google Scholar] [CrossRef]
- Johannes, M.R.S.; Kenney, A.; Pouliotte, J.; Steele, D. Mountain Pine Beetle Threats to Salmon and Fisheries Resources. In Proceedings of the Pacific Salmon Foundation and Fraser Basin Council Workshop, Prince George, BC, Canada, 30–31 January 2007; p. 71.
- Wong, C. Environmental Impacts of Mountain Pine Beetle in the Southern Interior; British Columbia Ministry of Environment: Prince George, BC, Canada, 2008. Available online: http://www.sibacs.com/wp-content/uploads/2009/02/environmental-impacts-report-final.pdf (accessed on 16 July 2016).
- Gunderson, L.H.; Holling, C.S. Panarchy: Understanding Transformations in Human and Natural Systems; Island Press: Washington, DC, USA, 2002; p. 507. [Google Scholar]
- Parrott, L.; Meyer, W.S.W. Future landscapes: Managing within complexity. Front. Ecol. Environ. 2012, 10, 382–389. [Google Scholar] [CrossRef]
- Messier, C.; Puettmann, K.; Chazdon, R.; Andersson, K.P.; Angers, V.A.; Brotons, L.; Filotas, E.; Tittler, R.; Parrott, L.; Levin, S.A. From Management to Stewardship: Viewing Forests as Complex Adaptive Systems in an Uncertain World. Conserv. Lett. 2015, 8, 368–377. [Google Scholar] [CrossRef]
- Hansen, E.M. Forest development and carbon dynamics after mountain pine beetle outbreaks. For. Sci. 2014, 60, 476–488. [Google Scholar]
- Bunnell, F.; Squires, K.A.; Houde, I. Evaluating Effects of Large-Scale Salvage Logging for Mountain Pine Beetle in Terrestrial and Aquatic Vertebrates; Natural Resources Canada: Victoria, BC, Canada, 2004; p. 57.
- Brown, S.; Schreier, H. Water quantity and quality related to rates of pine beetle infestation and salvage logging: A regional comparison; Water quality technical report, MPB Project #7.31; University of British Columbia: Vancouver, BC, Canada, 2009; p. 24. [Google Scholar]
- Mikkelson, K.; Bearup, L.A.; Maxwell, R.M.; Stednick, J.D.; McCray, J.E.; Sharp, J.O. Bark beetle infestation impacts on nutrient cycling, water quality and interdependent hydrological effects. Biogeochemistry 2013, 115, 1–21. [Google Scholar] [CrossRef]
- Foster, D.R.; Knight, D.H.; Franklin, J.F. Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems 1998, 1, 497–510. [Google Scholar] [CrossRef]
- Turner, M.G.; Dale, V.H. Comparing large, infrequent disturbances: What have we learned? Ecosystems 1998, 1, 493–496. [Google Scholar] [CrossRef]
- Schowalter, T.D. Forest pest management: A synopsis. Northwest Environ. J. 1988, 4, 313–318. [Google Scholar]
- Perry, D.A. The scientific basis of forestry. Annu. Rev. Ecol. Syst. 1998, 29, 435–466. [Google Scholar] [CrossRef]
- Coyle, D.R.; Nebeker, T.E.; Hart, E.R.; Mattson, W.J. Biology and management of insect pets in North American intensively managed hardwood forest systems. Annu. Rev. Entomol. 2005, 50, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.G.; Romme, W.H. Landscape dynamics in crown fire ecosystems. Landsc. Ecol. 1994, 9, 59–77. [Google Scholar] [CrossRef]
- Bergeron, Y.; Richard, P.J.H.; Carcaillet, C.; Gauthier, S.; Flannigan, M.; Prairie, Y.T. Variability in the Fire Frequency and Forest Composition in Canada’s Southeastern Boreal Forest: A Challenge For Sustainable Forest Management. Available online: http://www.consecol.org/vol2/iss2/art6/ (accessed on 12 November 2015).
- Agee, J.K. Fire Ecology of Pacific Northwest Forests; Island Press: Washington, DC, USA, 1993; p. 505. [Google Scholar]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention forestry to maintain multifunctional forests: A world perspective. BioScience 2012, 62, 633–645. [Google Scholar]
- Lindenmayer, D.B.; Franklin, J.F.; Lõhmus, A.; Bake, S.C.; Bauhus, J.; Beese, W.; Brodie, A.; Kiehl, B.; Kouki, J.; Pastur, G.M.; et al. A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conserv. Lett. 2012, 5, 421–431. [Google Scholar] [CrossRef]
- Fedrowitz, K.; Koricheva, J.; Bake, R S.C.; Lindenmayer, D.B.; Palik, B.; Rosenvald, R.; Beese, W.; Franklin, J.F.; Kouki, J.; Macdonald, E.; et al. Can retention forestry help conserve biodiversity? A meta-analysis. J. Appl. Ecol. 2014, 51, 1669–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drever, R.C.; Peterson, G.; Messier, C.; Bergeron, Y.; Flannigan, M. Can forest management based on natural disturbances maintain ecological resilience? Can. J. For. Res. 2006, 36, 2285–2299. [Google Scholar] [CrossRef] [Green Version]
- Park, A.; Puettmann, K.; Wilson, E.; Messier, C.; Kames, S.; Dhar, A. Can boreal and temperate forest management be adapted to the uncertainties of 21st century climate change? Crit. Rev. Plant Sci. 2014, 33, 251–285. [Google Scholar] [CrossRef]
- Bunnell, F. Forest-dwelling fauna and natural fire regimes in British Columbia: Patterns and implications for conservation. Conserv. Biol. 1995, 9, 636–644. [Google Scholar] [CrossRef]
- Covington, W.W. The evolutionary and history context. In Ecological Restoration of Southwestern Ponderosa Pine Forests; Friedrici, P., Ed.; Island press: Washington, DC, USA, 2003; pp. 26–47. [Google Scholar]
- Schneider, D.W. Experimental perturbation of whole lakes as tests of hypotheses concerning ecosystem structure and function. Oikos 1990, 57, 25–41. [Google Scholar]
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, A.; Lindelöw, Å.; Åsenblad, N. Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers. Conserv. 2005, 14, 3033–3053. [Google Scholar] [CrossRef]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a ‘‘benign neglect strategy’’ in a national park: Response of saproxylic beetles to dead wood accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Future directions for biodiversity conservation in managed forests: Indicator species, impact studies and monitoring programs. For. Ecol. Manag. 1999, 115, 277–287. [Google Scholar] [CrossRef]
- Arignan, V.; Villard, M.A. Selecting indicator species to monitor ecological integrity: A review. Environ. Monit. Assess. 2002, 78, 45–61. [Google Scholar] [CrossRef]
- Duncan, R.P. Flood disturbance and coexistence of species in a lowland podocarp forest, south Westland, New Zealand. J. Ecol. 1993, 81, 403–416. [Google Scholar] [CrossRef]
- Taylor, S.; Carroll, A. Disturbance, forest age, and mountain pine beetle outbreak dynamics in BC: A historical perspective. In Proceedings of the Mountain Pine Beetle Symposium: Challenges and Solutions, Kelowna, BC, Canada, 30–31 October 2003; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Information Report BC-X-399. Natural Resources Canada, Canadian, Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004; pp. 41–51. [Google Scholar]
- Dhar, A.; Parrott, L. Salvage logging after mountain pine beetle outbreaks reduces the social-ecological resilience of forest landscapes. In Proceedings of the Mountain Pine Beetle Information Exchange Forum, Edmonton, Alberta, AB, Canada, 22–23 April 2015; McClain, K., Ed.; Foothill Research Center: Hinton, AB, Canada, 2015; p. 17. [Google Scholar]
- Messier, C.; Puettmann, K.J.; Coates, K.D. Managing Forests as Complex Adaptive Systems: Building Resilience to the Challenge of Global Change; Routledge: New York, NY, USA, 2013; p. 368. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhar, A.; Parrott, L.; Hawkins, C.D.B. Aftermath of Mountain Pine Beetle Outbreak in British Columbia: Stand Dynamics, Management Response and Ecosystem Resilience. Forests 2016, 7, 171. https://doi.org/10.3390/f7080171
Dhar A, Parrott L, Hawkins CDB. Aftermath of Mountain Pine Beetle Outbreak in British Columbia: Stand Dynamics, Management Response and Ecosystem Resilience. Forests. 2016; 7(8):171. https://doi.org/10.3390/f7080171
Chicago/Turabian StyleDhar, Amalesh, Lael Parrott, and Christopher D.B. Hawkins. 2016. "Aftermath of Mountain Pine Beetle Outbreak in British Columbia: Stand Dynamics, Management Response and Ecosystem Resilience" Forests 7, no. 8: 171. https://doi.org/10.3390/f7080171
APA StyleDhar, A., Parrott, L., & Hawkins, C. D. B. (2016). Aftermath of Mountain Pine Beetle Outbreak in British Columbia: Stand Dynamics, Management Response and Ecosystem Resilience. Forests, 7(8), 171. https://doi.org/10.3390/f7080171