Functional Diversity of Small and Large Trees along Secondary Succession in a Tropical Dry Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Species Selection
2.3. Functional Traits
2.4. Statistical Analyses
2.4.1. Functional Indices
2.4.2. Null Models
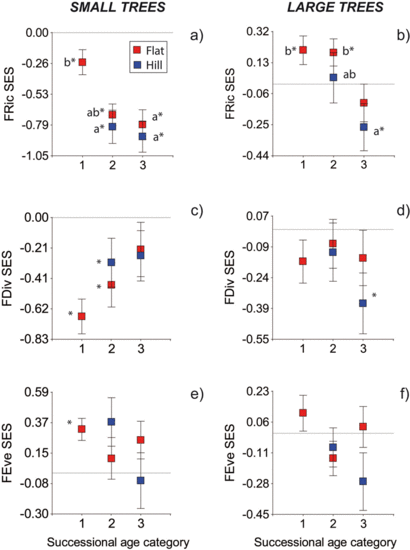
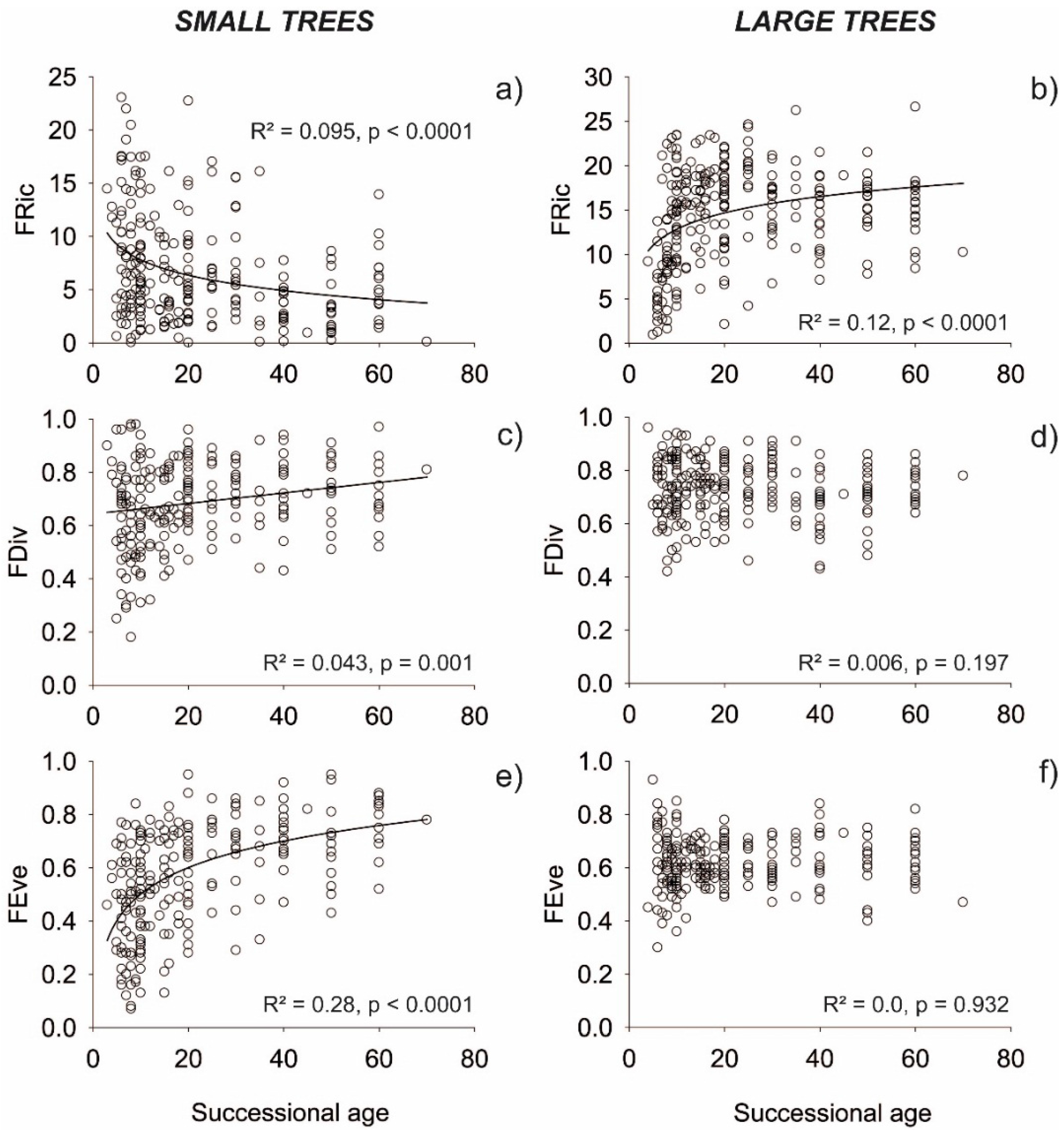
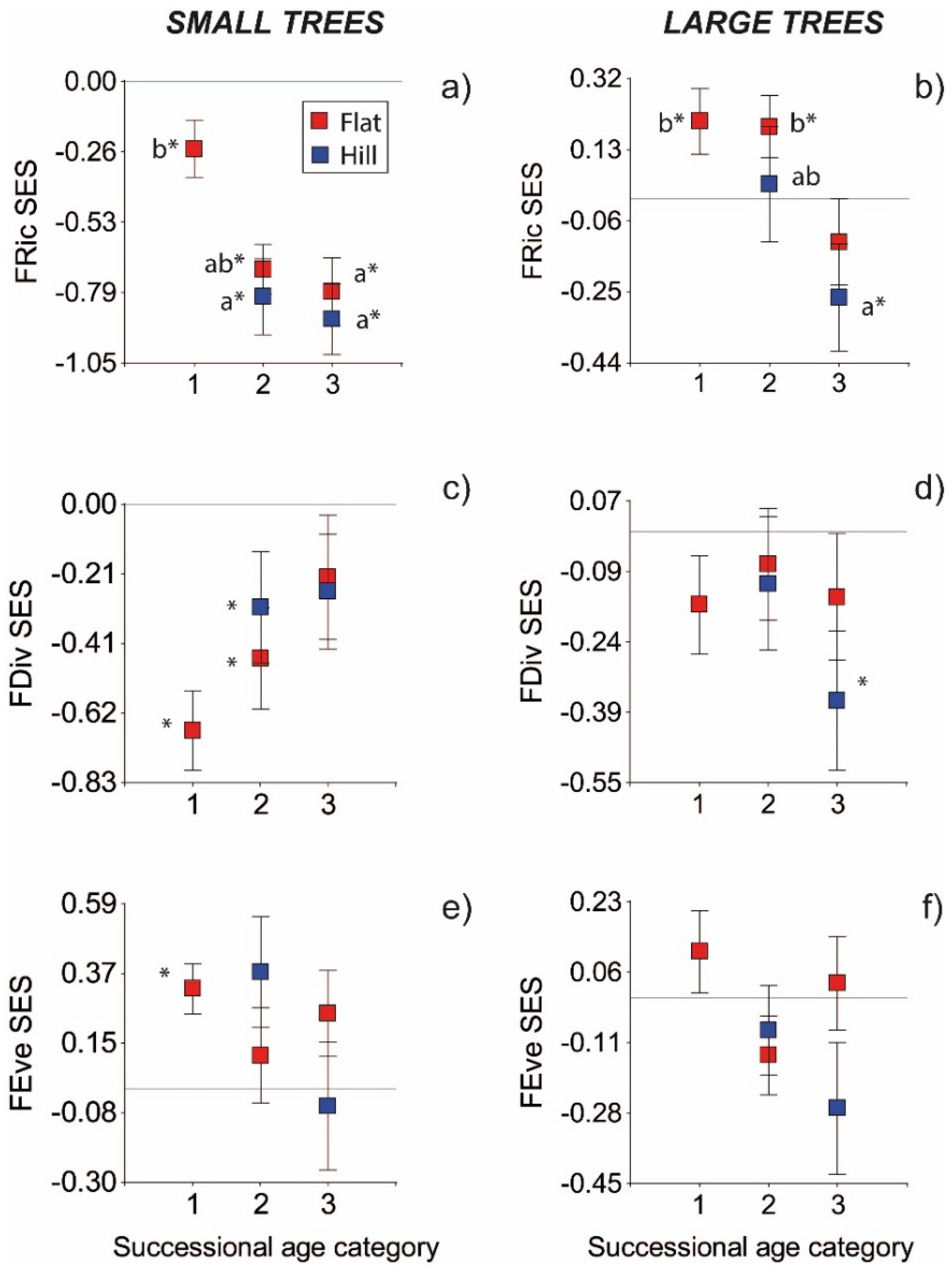
3. Results
3.1. Functional Indices
3.2. Null Models
4. Discussion
- (1)
- Relatively higher FRic values early in succession may reflect the coexistence of acquisitive and conservative species that show different constellations of functional traits in young stands. Pioneers (which represent the conservative strategy in dry forests) may establish from seeds of nearby forests (since slash-and-burn agriculture may destroy the seed bank) [57], whereas more acquisitive shade-tolerant species may avoid unfavorable conditions for seed establishment by getting established mainly from resprouts [58]. The subsequent reduction in FRic over succession may result from environmental filtering associated with lower light and air evaporative demand, which would decrease the success of the conservative species [14].
- (2)
- Alternatively, modern coexistence theory states that functional convergence results not only form environmental filtering, but also from competitive exclusion based on hierarchical competitive abilities among species [59,60]. The Functional Richness index may thus reflect the combined effect of both environmental filtering and competitive processes [60]. In line with this, early in succession negative FRic values of small trees may reflect the effect of environmental filters (high irradiance load, air temperature and evaporative demand), whereas at latter successional ages the lower FRic values may reflect the elimination of poor competitors [60]. Competitive hierarchy of species can be determined by multiple factors, but in a dry forests it may be defined mostly by water availability, which is the most limiting resource [2]. Previous studies of microenvironment in tropical dry forests have shown that soil water content increases with successional age, as radiation load and air evaporative demand decrease with canopy development [11,12,13]. However, other studies have found a steady and sometimes marked increase in fine root biomass along succession [61,62], but see [63], and a decrease in soil water potential [12]. Theoretically, the increasing importance of soil drying by transpiration as canopy closes and forests mature [64] could be a sign of stronger competitive processes, if plants pre-empt water supply from coming in contact with other species (which may be considered as a filter), or if they reduce soil water potential to low levels [65]. The effect of below-ground competition on soil water availability has been poorly quantified [65]. The relative importance of soil drying by evaporation vs. transpiration during dry forests succession, and its effects on tree community assembly need further investigation.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DOAJ | Directory of open access journals |
| PCoA | Principal Coordinate Analysis |
| FRic | Functional Richness |
| FDiv | Functional Divergence |
| FEve | Functional Evenness |
| SES | Standard Effect Size |
References
- Gerhardt, K.; Hytteborn, H. Natural dynamics and regeneration methods in tropical dry forests—An introduction. J. Veg. Sci. 1992, 3, 361–364. [Google Scholar]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar]
- Sanchez-Azofeifa, G.A.; Quesada, M.; Rodriguez, J.P.; Nassar, J.M.; Stoner, K.E.; Castillo, A.; Garvin, T.; Zent, E.L.; Calvo-Alvarado, J.C.; Kalacska, M.E.R.; et al. Research Priorities for Neotropical Dry Forests. Biotropica 2005, 37, 477–485. [Google Scholar]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar]
- Quesada, M.; Sanchez-Azofeifa, G.A.; Alvarez-Añorve, M.; Stoner, K.E.; Avila-Cabadilla, L.; Calvo-Alvarado, J.; Castillo, A.; Espírito-Santo, M.M.; Fagundes, M.; Fernandes, G.W.; et al. Succession and management of tropical dry forests in the Americas: Review and new perspectives. For. Ecol. Manag. 2009, 258, 1014–1024. [Google Scholar]
- Kennard, D.K. Secondary forest succession in a tropical dry forest: Patterns of development across a 50-year chronosequence in lowland Bolivia. J. Trop. Ecol. 2002, 18, 53–66. [Google Scholar]
- Madeira, B.G.; Espírito-Santo, M.M.; Neto, S.D.; Nunes, Y.R.F.; Arturo Sánchez Azofeifa, G.; Wilson Fernandes, G.; Quesada, M. Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol. 2009, 201, 291–304. [Google Scholar]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, mechanisms and predictability of vegetation change during tropical dry forest succession. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar]
- Alvarez-Añorve, M.Y.; Quesada, M.; Sanchez-Azofeifa, G.A.; Avila-Cabadilla, L.D.; Gamon, J.A. Functional regeneration and spectral reflectance of trees during succession in a highly diverse tropical dry forest ecosystem. Am. J. Bot. 2012, 99, 816–826. [Google Scholar]
- Williams-Linera, G.; Alvarez-Aquino, C.; Hernández-Ascención, E.; Toledo, M. Early successional sites and the recovery of vegetation structure and tree species of the tropical dry forest in Veracruz, Mexico. New For. 2011, 42, 131–148. [Google Scholar]
- Buzzard, V.; Hulshof, C.M.; Birt, T.; Violle, C.; Enquist, B.J. Re-growing a tropical dry forest: Functional plant trait composition and community assembly during succession. Funct. Ecol. 2015. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar]
- Pineda-García, F.; Paz, H.; Meinzer, F.C. Drought resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Paz, H.; Pérez-García, E.A.; Romero-Pérez, I.E.; Tauro, A.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar]
- Lohbeck, M.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional trait strategies of trees in dry and wet tropical forests are similar but differ in their consequences for succession. PLoS ONE 2015, 10, e0123741. [Google Scholar] [CrossRef]
- Purschke, O.; Schmid, B.C.; Sykes, M.T.; Poschlod, P.; Michalski, S.G.; Durka, W.; Kühn, I.; Winter, M.; Prentice, H.C. Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: Insights into assembly processes. J. Ecol. 2013, 101, 857–866. [Google Scholar]
- Becker, P.; Rabenold, P.E.; Idol, J.R.; Smith, A.P. Water potential gradients for gaps and slopes in a Panamanian tropical moist forest’s dry season. J. Trop. Ecol. 1988, 4, 173–184. [Google Scholar]
- Gallardo-Cruz, J.A.; Pérez-García, E.A.; Meave, J.A. β-Diversity and vegetation structure as influenced by slope aspect and altitude in a seasonally dry tropical landscape. Landsc. Ecol. 2009, 24, 473–482. [Google Scholar]
- Markesteijn, L.; Iraipi, J.; Bongers, F.; Poorter, L. Seasonal variation in soil and plant water potentials in a Bolivian tropical moist and dry forest. J. Trop. Ecol. 2010, 26, 497–508. [Google Scholar]
- Schwinning, S.; Weiner, J. Mechanisms Determining the Degree of Size Asymmetry in Competition among Plants. Oecologia 1998, 113, 447–455. [Google Scholar]
- Cavender-Bares, J.; Kitajima, K.; Bazzaz, F.A. Multiple trait associations in relation to habitat differentiation among 17 Floridian oak species. Ecol. Monogr. 2004, 74, 635–662. [Google Scholar]
- Mason, N.W.H.; Lanoiselée, C.; Mouillot, D.; Wilson, J.B.; Argillier, C. Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. J. Anim. Ecol. 2008, 77, 661–669. [Google Scholar]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar]
- Weiher, E.; Keddy, P.A. Assembly Rules, Null Models, and Trait Dispersion: New Questions from Old Patterns. Oikos 1995, 74, 159–164. [Google Scholar]
- Tilman, D. The importance of the mechanisms of interspecific competition. Am. Nat. 1987, 129, 769–774. [Google Scholar]
- Mason, N.W.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar]
- Ricotta, C.; Bacaro, G.; Moretti, M. A New Measure of Functional Evenness and Some of Its Properties. PLoS ONE 2014, 9, e104060. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community Assembly Rules, Morphological Dispersion, and the Coexistence of Plant Species. Oikos 1998, 81, 309–322. [Google Scholar]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science 2008, 322, 580–582. [Google Scholar]
- Macarthur, R.; Levins, R. The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 1967, 101, 377–385. [Google Scholar]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (para Adaptarlo a las Condiciones de la República Mexicana); Instituto de Geografía Universidad Nacional Autónoma de México: Mexico DF, Mexico, 1981. [Google Scholar]
- Orellana, R.; Islebe, G.A.; González-Iturbe, J.A. Presente, pasado y futuro de los climas de la Península de Yucatán. In Naturaleza y Sociedad en el Área Maya; Colunga-García Marín, P., Larqué Saavedra, A., Eds.; Academia Mexicana de Ciencias, Centro de Investigación Científica de Yucatán: Mérida, Yucatán, Mexico, 2003; pp. 37–52. [Google Scholar]
- Flores, J.S.; Espejel, I. Tipos de Vegetación de la Península de Yucatán; Flores, J.S., Ed.; Etnoflora Yucatanense, Universidad Autónoma de Yucatán: Mérida, Yucatán, Mexico, 1994. [Google Scholar]
- Bautista-Zúñiga, F.; Batllori-Sampedro, E.; Ortiz-Pérez, M.A.; Palacio-Aponte, G.; Castillo-González, M. Geoformas, agua y suelo en la Península de Yucatán. In Naturaleza y sociedad en el área maya: Pasado, Presente y Futuro; Colunga, P., Larque, A., Eds.; Academia Mexicana de Ciencias y Centro de Investigación Científica de Yucatán: Mérida, Yucatán, Mexico, 2003; pp. 21–35. [Google Scholar]
- Dupuy, J.M.; Hernández-Stefanoni, J.L.; Hernández-Juárez, R.A.; Tetetla-Rangel, E.; López-Martínez, J.O.; Leyequién-Abarca, E.; Tun-Dzul, F.J.; May-Pat, F. Patterns and Correlates of Tropical Dry Forest Structure and Composition in a Highly Replicated Chronosequence in Yucatan, Mexico. Biotropica 2012, 44, 151–162. [Google Scholar]
- Rico-Gray, V.; García-Franco, J.G. The Maya and the vegetation of the Yucatan Peninsula. J. Ethnobiol. 1991, 11, 135–142. [Google Scholar]
- Hernández-Stefanoni, J.L.; Dupuy, J.M. Effects of landscape patterns on species density and abundance of trees in a tropical subdeciduous forest of the Yucatan Peninsula. For. Ecol. Manag. 2008, 255, 3797–3805. [Google Scholar]
- Hernández-Stefanoni, J.L.; Pineda, J.B.; Valdes-Valadez, G. Comparing the Use of Indigenous Knowledge with Classification and Ordination Techniques for Assessing the Species Composition and Structure of Vegetation in a Tropical Forest. Environ. Manag. 2006, 37, 686–702. [Google Scholar]
- Hernández-Stefanoni, J.L.; Dupuy, J.M.; Tun-Dzul, F.; May-Pat, F. Influence of landscape structure and stand age on species density and biomass of a tropical dry forest across spatial scales. Landsc. Ecol. 2011, 26, 355–370. [Google Scholar]
- Turner, B.L., II; Villar, S.C.; Foster, D.; Geoghegan, J.; Keys, E.; Klepeis, P.; Lawrence, D.; Mendoza, P.M.; Manson, S.; Ogneva-Himmelberger, Y. Others Deforestation in the southern Yucatán peninsular region: An integrative approach. For. Ecol. Manag. 2001, 154, 353–370. [Google Scholar]
- Pakeman, R.J.; Quested, H.M. Sampling plant functional traits: What proportion of the species need to be measured? Appl. Veg. Sci. 2007, 10, 91–96. [Google Scholar]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 2014. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity...Correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar]
- Muller-Landau, H.C. Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 2004, 36, 20–32. [Google Scholar]
- Grime, J.P. Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar]
- Swenson, N.G. Functional and Phylogenetic Ecology in R; Springer: New York, NY, USA, 2014. [Google Scholar]
- Cailliez, F. The analytical solution of the additive constant problem. Psychometrika 1983, 48, 305–308. [Google Scholar]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring functional diversity from multiple traits, and other tools for functional ecology. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 27 July 2016).
- R Development Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2015. [Google Scholar]
- Dalling, J.W.; Denslow, J.S. Soil seed bank composition along a forest chronosequence in seasonally moist tropical forest, Panama. J. Veg. Sci. 1998, 9, 669–678. [Google Scholar]
- Kammesheidt, L. Forest recovery by root suckers and above-ground sprouts after slash-and-burn agriculture, fire and logging in Paraguay and Venezuela. J. Trop. Ecol. 1999, 15, 143–157. [Google Scholar]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities: Phylogeny and coexistence. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar]
- Vargas, R.; Allen, M.F.; Allen, E.B. Biomass and carbon accumulation in a fire chronosequence of a seasonally dry tropical forest. Glob. Chang. Biol. 2008, 14, 109–124. [Google Scholar]
- Deans, J.D.; Moran, J.; Grace, J. Biomass relationships for tree species in regenerating semi-deciduous tropical moist forest in Cameroon. For. Ecol. Manag. 1996, 88, 215–225. [Google Scholar]
- Powers, J.S.; Peréz-Aviles, D. Edaphic factors are a more important control on surface fine roots than stand age in secondary tropical dry forests. Biotropica 2013, 45, 1–9. [Google Scholar]
- Marthews, T.R.; Burslem, D.F.R.P.; Paton, S.R.; Yangüez, F.; Mullins, C.E. Soil drying in a tropical forest: Three distinct environments controlled by gap size. Ecol. Model. 2008, 216, 369–384. [Google Scholar]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar]
- Warner, R.R.; Chesson, P.L. Coexistence mediated by recruitment fluctuations: A field guide to the storage effect. Am. Nat. 1985, 125, 769–787. [Google Scholar]
| Plant Trait | Functional Role |
|---|---|
| Minimal photosyntethic unit | Leaf cooling |
| Leaf petiole | Light capture efficiency |
| Leaf dry matter content | Leaf cost, leaf life span, herbivory defense |
| Specific leaf area | Growth rate, photosynthetic rate |
| Leaf compoundness | Leaf cooling |
| Leaf deciduousness | Drought avoidance |
| Leaf pulvination | Minimization of photo-damage and water loss by transpiration |
| Leaf pubescence | Herbivory defense |
| Wood specific gravity | Growth rate, hydraulic conductivity and safety, mechanical resistance |
| Plant exudates | Herbivory defense |
| Plant spininess | Herbivory defense |
| Dispersal syndrome | Biotic interactions, dispersal |
| Functional Component | Formula | Ecological Interpretation | |
|---|---|---|---|
| Positive Values 1 | Negative Values 1 | ||
| Richness (FRic) [28,32] | Quickhull algorithm. Volume of the minimal convex hull, which includes all the species considered in the community. | Favorable conditions that allow multiple combinations of traits and a more thorough use of the resources available [28,32]. | Environmental filtering favoring a narrow range of functional traits [28,32]. |
| Divergence (FDiv) (abundance-weighted) [28] | where is the sum of abundance-weighted deviances from the center of gravity; is the absolute abundance-weighted deviances from the center of gravity, and is the mean distance of the S species to the center of gravity. | The most abundant species have extreme functional trait values, reflecting either niche partitioning by competitive exclusion, or local environmental heterogeneity favoring different ecological strategies [27]. | Environmental filtering favoring a narrow range of functional traits, resulting in little functional differentiation among dominant species [28]. |
| Evenness (FEve)(Abundance-weighted) [29] | The average community uniqueness U is the expected dissimilarity between one individual of species i chosen at random from a given community and all other j species in the community: where dij is the functional dissimilarity between species i and j; p is the relative abundance of species i or j, and U+ is the weighted dissimilarity between species i and all other species in the community. Functional evenness is the regularity in the distribution of single species contributions to U. With the relative contribution of species i to U (), the regularity in the distributions of is calculated with the index of Bulla: | An even dispersion of the strategies of co-occurring species in relation to resource use, it can be interpreted as an indication of niche partitioning by competitive exclusion [33]. | Environmental filters favoring one or few strategies, so the species that survive are functionally more similar than expected by chance [24,51]. |
| Successional Age/Topographic Class | Functional Index | Z | p | Z | p |
|---|---|---|---|---|---|
| Large Trees | Small Trees | ||||
| 1 Flat | FRic | 1.9659 | 0.049 | −3.148 | 0.002 |
| 2 Flat | 2.4179 | 0.016 | −5.155 | 0.000 | |
| 2 Hill | 0.0514 | ns 1 | −4.054 | 0.000 | |
| 3 Flat | −0.7012 | ns | −4.577 | 0.000 | |
| 3 Hill | −1.9821 | 0.046 | −4.253 | 0.000 | |
| 1 Flat | FDiv | −0.7991 | ns | −4.800 | 0.000 |
| 2 Flat | −0.2076 | ns | −2.914 | 0.004 | |
| 2 Hill | 0.0103 | ns | −2.065 | 0.037 | |
| 3 Flat | −0.4007 | ns | −1.200 | ns | |
| 3 Hill | −2.0756 | 0.037 | −1.215 | ns | |
| 1 Flat | FEve | 0.5085 | ns | 2.776 | 0.006 |
| 2 Flat | −1.4597 | ns | 1.239 | ns | |
| 2 Hill | −1.1210 | ns | 1.784 | ns | |
| 3 Flat | −0.3916 | ns | 1.730 | ns | |
| 3 Hill | −1.4959 | ns | 0.137 | ns | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes-García, C.; Paz, H.; Jackson, P.C. Functional Diversity of Small and Large Trees along Secondary Succession in a Tropical Dry Forest. Forests 2016, 7, 163. https://doi.org/10.3390/f7080163
Sanaphre-Villanueva L, Dupuy JM, Andrade JL, Reyes-García C, Paz H, Jackson PC. Functional Diversity of Small and Large Trees along Secondary Succession in a Tropical Dry Forest. Forests. 2016; 7(8):163. https://doi.org/10.3390/f7080163
Chicago/Turabian StyleSanaphre-Villanueva, Lucía, Juan Manuel Dupuy, José Luis Andrade, Casandra Reyes-García, Horacio Paz, and Paula C. Jackson. 2016. "Functional Diversity of Small and Large Trees along Secondary Succession in a Tropical Dry Forest" Forests 7, no. 8: 163. https://doi.org/10.3390/f7080163