Soil Respiration at Different Stand Ages (5, 10, and 20/30 Years) in Coniferous (Pinus tabulaeformis Carrière) and Deciduous (Populus davidiana Dode) Plantations in a Sandstorm Source Area
Abstract
:1. Introduction
2. Materials and Methods
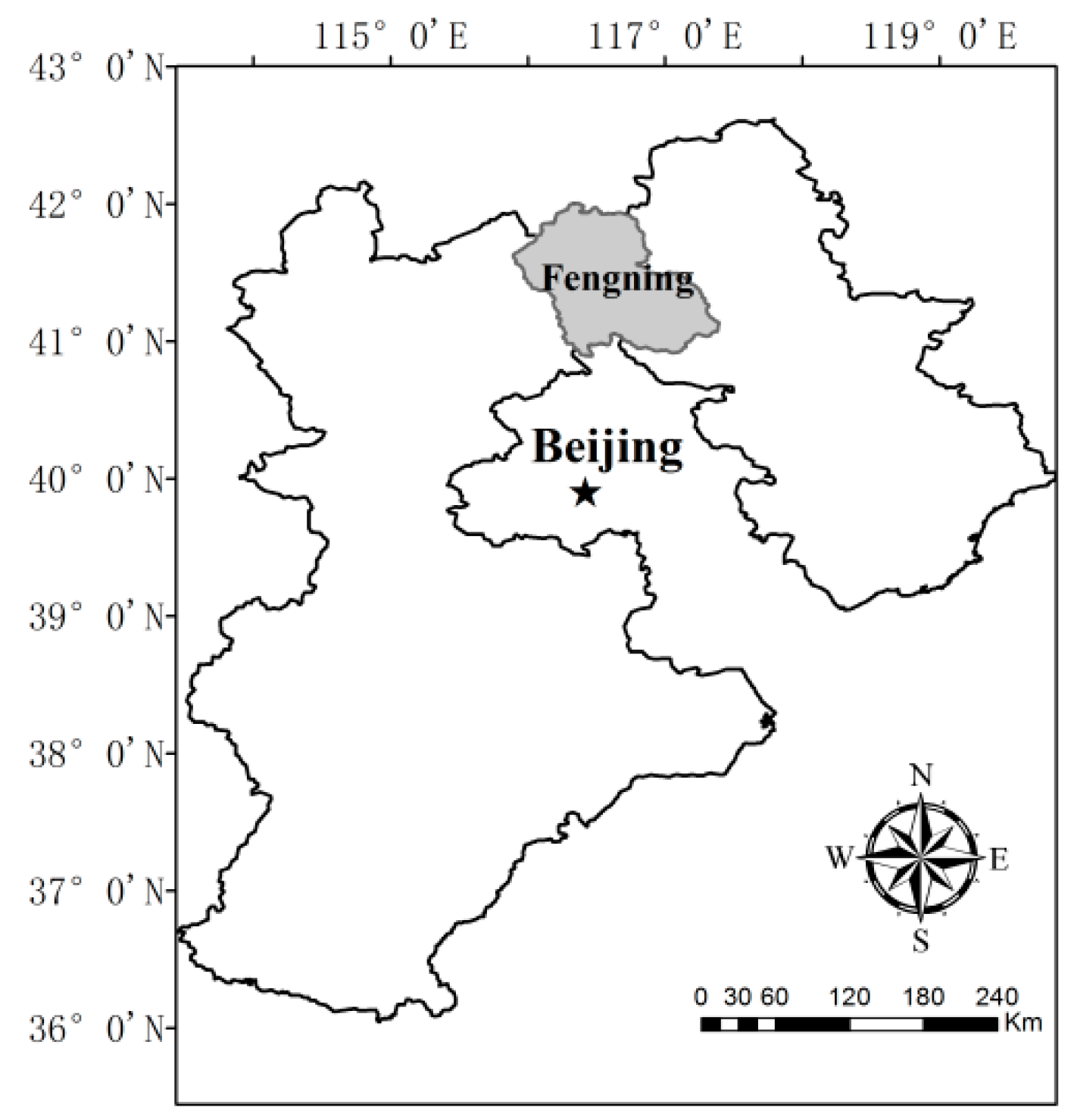
2.1. Site Description
2.2. Experimental Design
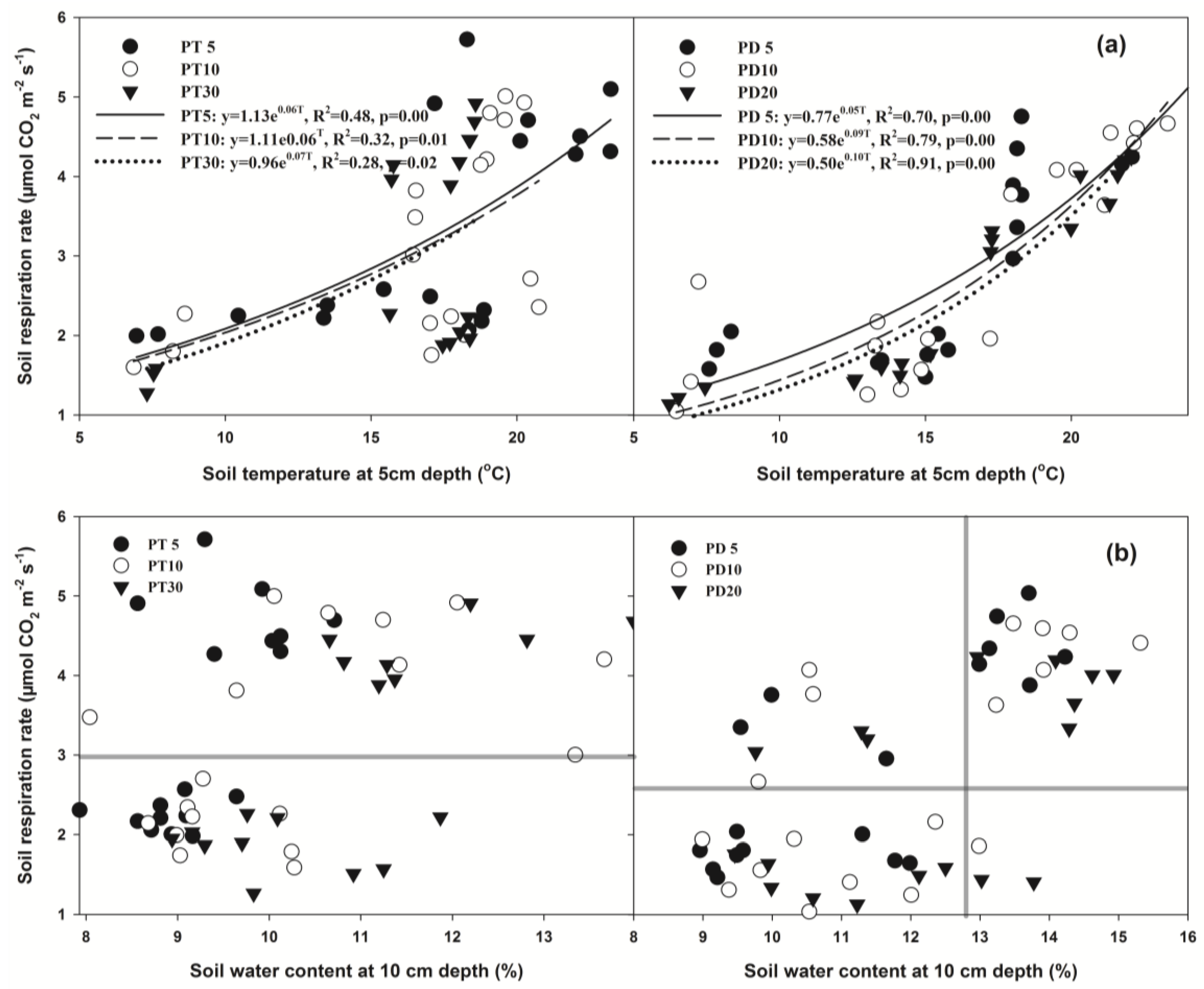
2.3. Measurements of Soil Respiration, Soil Temperature, and Soil Water Content
2.4. Soil Sampling and Laboratory Analysis
2.5. Statistical Analysis
3. Results
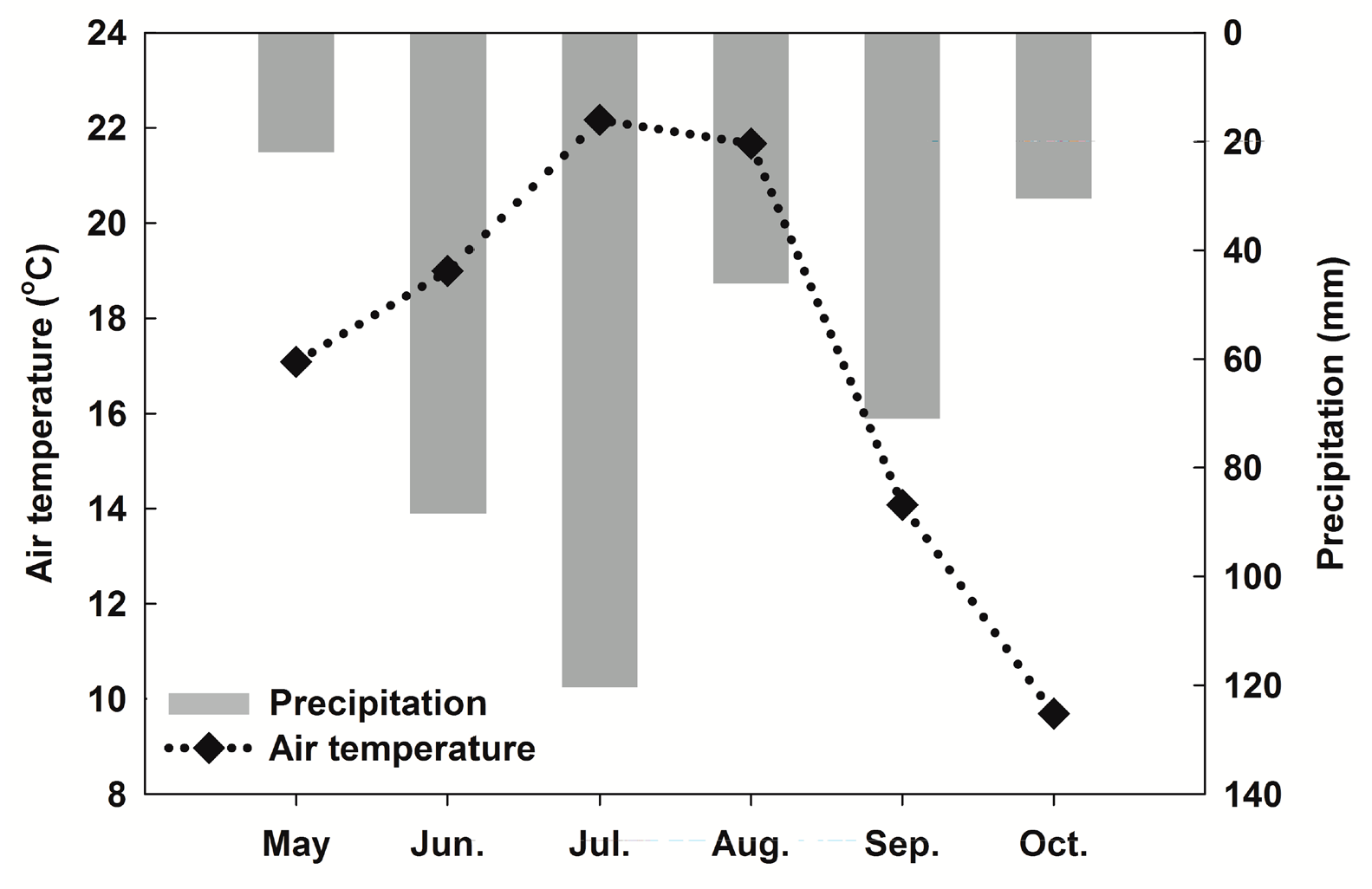
3.1. Meteorological Conditions
3.2. Soil Respiration
3.3. Soil Temperature and Soil Water Content
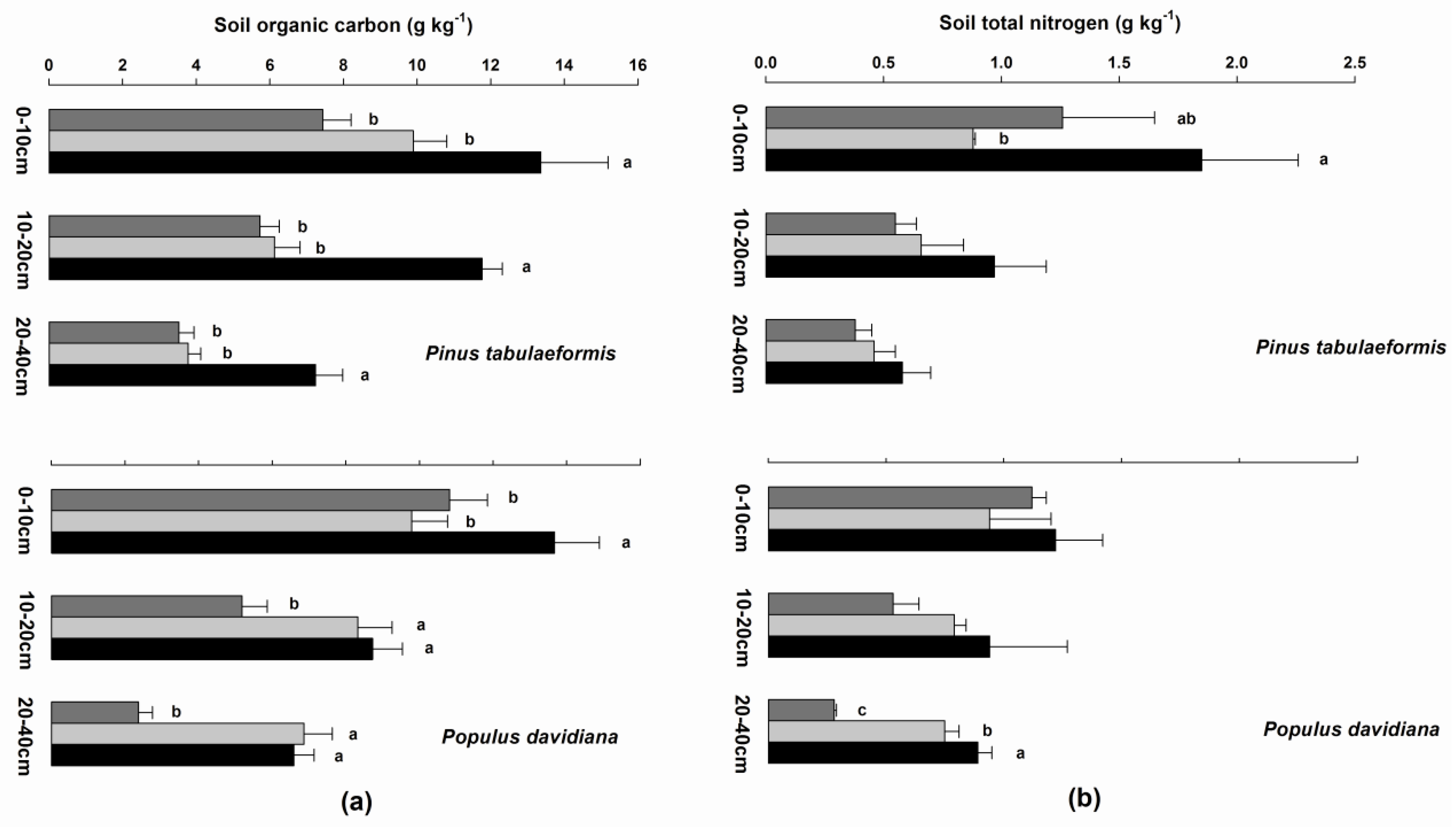
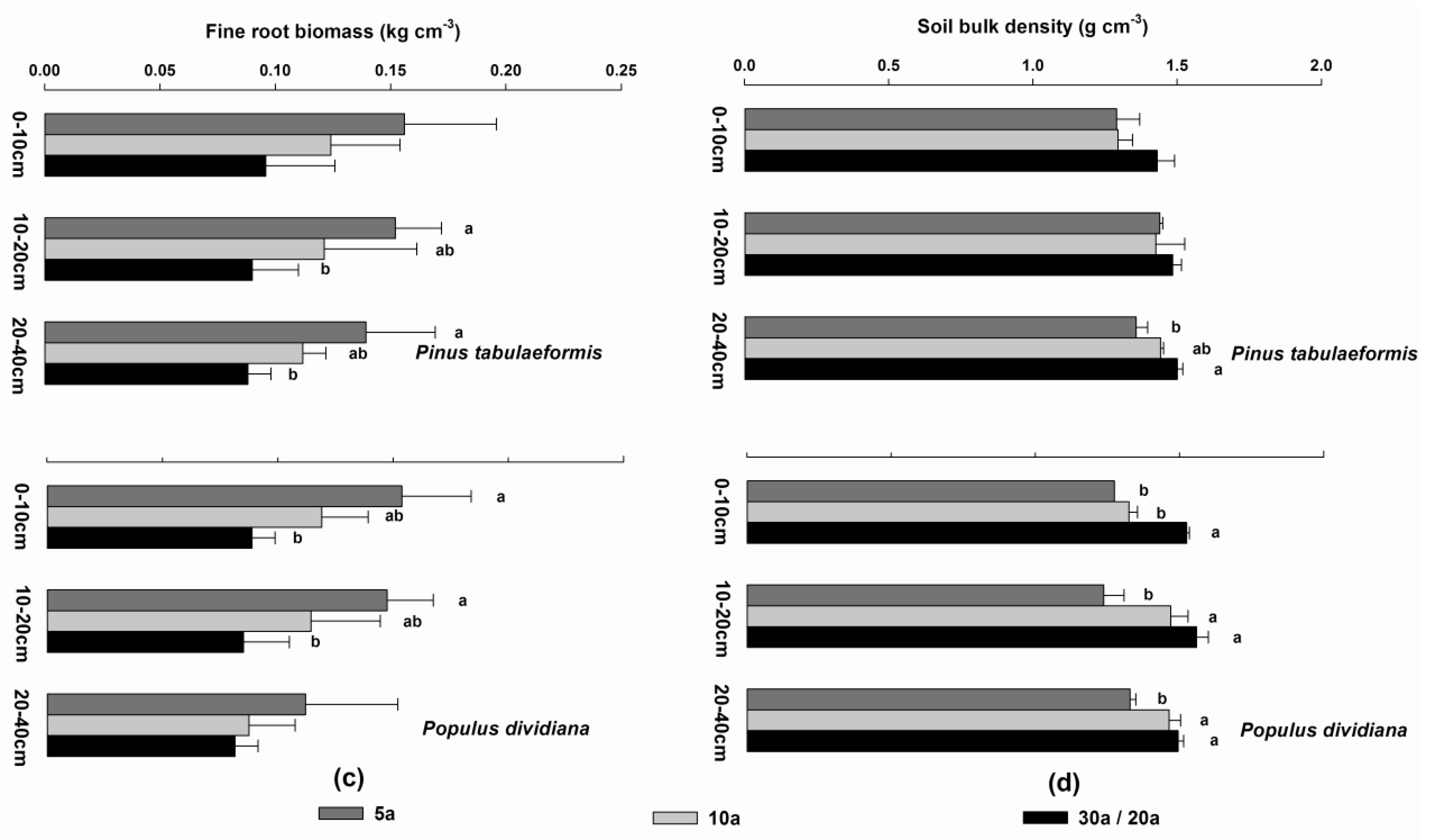
3.4. Soil Organic Carbon, Soil Total Nitrogen, Fine Root Biomass, and Soil Bulk Density
3.5. Relationships between Soil Respiration and Soil Temperature and Soil Water Content
3.6. Factors Related to Soil Respiration
4. Discussion
4.1. Stand Age and Soil Respiration
4.2. Forest Type and Soil Respiration
4.3. Temperature Sensitivity to Soil Respiration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Grace, J.; Rayment, M. Respiration in the balance. Nature 1999, 404, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Post, W.M.; Norby, R.J.; Classen, A.T. Modeling soil respiration and variations in source components using a multifactor global climate change experiment. Clim. Chang. 2011, 107, 459–480. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y.Q. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Ma, Y.C.; Piao, S.L.; Sun, Z.Z.; Lin, X.; Wang, T. Stand ages regulate response of soil respiration to temperature in a Larix principis-rupprechtii plantation. Agric. For. Meteorol. 2014, 184, 179–187. [Google Scholar] [CrossRef]
- Tang, J.W.; Bolstad, P.V.; Martin, J.G. Soil carbon fluxes and stocks in a Great Lakes forest chronosequence. Glob. Chang. Biol. 2009, 15, 145–155. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Luan, J.W.; Liu, S.R.; Wang, J.X.; Zhu, X.L.; Shi, Z.M. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Litton, C.M.; Ryan, M.G.; Knight, D.S.; Stahl, P.D. Soil-surface carbon dioxide efflux and microbial biomass in relation to tree density 13 years after a stand replacing fire in a lodgepole pine ecosystem. Glob. Chang. Biol. 2003, 9, 680–696. [Google Scholar] [CrossRef]
- Wiseman, P.E.; Seiler, J.R. Soil CO2 efflux across four age classes of plantation loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For. Ecol. Manag. 2004, 192, 297–311. [Google Scholar] [CrossRef]
- Xiao, W.F.; Ge, X.G.; Zeng, L.X.; Huang, Z.L.; Lei, J.P.; Lei, J.P.; Zhou, B.Z.; Li, M.H. Rates of litter decomposition and respiration in relation to soil temperature and water in different aged Pinus massoniana forests in the Tree Gorges Reservoir area, China. PLoS ONE 2014, 9, e101890. [Google Scholar]
- Wang, C.K.; Bond-Lamberty, B.; Gower, S.T. Soil surface CO2 flux in a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 107, 1–8. [Google Scholar]
- Yan, M.; Zhang, X.; Zhou, G.; Gong, J.; You, X. Temporal and spatial variation in soil respiration of poplar plantations at different developmental stages in Xinjiang, China. J. Arid Environ. 2011, 75, 51–57. [Google Scholar] [CrossRef]
- Gong, J.R.; Ge, Z.W.; An, R.; Duan, Q.W.; You, X.; Huang, Y.M. Soil respiration in poplar plantations in northern China at different forest ages. Plant Soil 2012, 360, 109–122. [Google Scholar] [CrossRef]
- Tedeschi, V.; Rey, A.; Manca, G.; Valentini, R.; Jarvis, P.G.; Borghetti, M. Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing. Glob. Chang. Biol. 2006, 12, 110–121. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Prescott, C.E. The influence of the forest canopy on nutrient cycling. Tree Physiol. 2002, 22, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, S.; Schulz, C.; Grimmeisen, W.; Borken, W. Carbon fluxes in coniferous and deciduous forest soils. Plant Soil 2012, 357, 355–368. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X.; Shi, P.; Zhang, Y. Comparing soil organic carbon dynamics in plantation and secondary forest in wet tropics in Puerto Rico. Glob. Chang. Biol. 2005, 11, 239–248. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Luan, J.W.; Liu, S.R.; Zhu, X.L.; Wang, J.X.; Liu, K. Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biol. Biochem. 2012, 44, 143–150. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, W.J.; Chen, W.L.; Yang, Y.H.; Zeng, H. Effect of forest age on soil autotrophic and heterotrophic research differ between evergreen and deciduous forests. PLoS ONE 2013, 8, e80937. [Google Scholar]
- Zhang, J.B.; Shangguan, T.L.; Meng, Z.Q. Changes in soil carbon flux and carbon stock over a rotation of poplar plantations in northwest China. Ecol. Res. 2011, 26, 153–161. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.C.; Wu, Y.H.; Shi, P.L.; She, J.; Zhou, P. Respiration in different primary succession stages on glacier forehead in Gongga mountain, China. PLoS ONE 2012, 7, e42354. [Google Scholar]
- Chen, Q.S.; Wang, X.B.; Han, X.G.; Wan, S.Q.; Li, L.H. Temporal and spatial variability and controls of soil respiration in a temperate steppe in northern China. Glob. Biogeochem. Cycles 2010, 24, 1–11. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob. Chang. Biol. 2001, 7, 667–677. [Google Scholar] [CrossRef]
- Reay, D.; Sabine, C.; Smith, P.; Hymus, G. Climate Change 2007: Spring-time for sinks. Nature 2007, 446, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Zhang, W.J.; Cao, J.S.; Shen, H.T.; Zeng, X.H.; Yu, Z.Q.; Zhao, X. Carbon storages in plantation ecosystems in sand source areas of North Beijing, China. PLoS ONE 2013, 8, e82208. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs, G.J.; Masera, O.; Andrasko, K.; Benetiz-Ponce, P.; Boer, R.; Dutschke, M.; Elsiddig, E.; Ford-Robertson, J.; Frumhoff, P.; Karjalainen, T.; et al. Forestry; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Larionova, A.A.; Rozanova, L.N.; Samoilov, T.I. Dynamics of gas exchange in the profile of a grey forest soil. Sov. Soil Sci. 1989, 3, 104–110. [Google Scholar]
- Zeng, X.H.; Zhang, W.J.; Shen, H.T.; Cao, J.S.; Zhao, X. Soil respiration response in different vegetation types at Mount Taihang, China. Catena 2014, 116, 78–85. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.D.; Zhang, W.J.; Ai, Z.P. Contribution of aboveground litter to soil respiration in Populus davidiana Dode plantations at different stand ages. J. Mt. Sci. 2016, 13, 1000–1012. [Google Scholar] [CrossRef]
- Staff, S.S. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; United States Department of Agriculture (USDA), Natural Resources Conservation Service: Washington, DC, USA, 1999.
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Chemical and microbial properties. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Lioyd, J.; Taylor, J.A. On the temperature-dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- McCarthy, D.R.; Brown, K.J. Soil respiration responses to topography, canopy cover, and prescribed burning in an oak-hickory forest in southeastern Ohio. For. Ecol. Manag. 2006, 237, 94–102. [Google Scholar] [CrossRef]
- Scott, N.A.; Tate, K.R.; Ford-Robertson, J. Soil carbon storage in plantation forests and pastures: Land-use change implications. Tellus B 1999, 51, 326–335. [Google Scholar] [CrossRef]
- Tanaka, K.; Hashimoto, S. Plant canopy effects on soil thermal and hydrological properties and soil respiration. Ecol. Model. 2006, 196, 32–44. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Bolstad, P. Belowground carbon allocation and soils of forests estimated from litter fall and IRGA-based soil respiration measurements. Agric. For. Meteorol. 2002, 113, 39–51. [Google Scholar] [CrossRef]
- Dickman, D.I.; Pregitzer, K.S. The Structure and Dynamics of Woody Plant Root Systems; Elsevier: London, UK, 1992; pp. 95–123. [Google Scholar]
- Grayston, S.J.; Waughan, D.; Jones, D. Rhizosphere carbon flows in trees, in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 1996, 5, 29–56. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steaudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to climate systems. Science 2002, 298, 2173–2175. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.H.; Su, B.; Wan, S.Q.; Luo, Y.Q. Labile, recalcitrant and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Grant Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Borken, W.; Davidson, E.A.; Savage, K.; Gaudinski, J.; Trumbore, S.E. Drying and wetting effects on carbon dioxide release from organic horizons. Soil Sci. Soc. Am. J. 2003, 67, 1888–1896. [Google Scholar] [CrossRef]
- Hibbard, K.A.; Law, B.E.; Reichstein, M.; Sulzman, J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 2005, 73, 29–70. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S. Global patterns of carbon dioxide emissions from soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef]
- Wang, C.K.; Yang, J.Y.; Zhang, Q.Z. Soil respiration in six temperate forests in China. Glob. Chang. Biol. 2006, 12, 2103–2144. [Google Scholar] [CrossRef]
- Li, H.J.; Yan, J.X.; Yue, X.F.; Wang, M.B. Significance of soil temperature and moisture for soil respiration in a Chinese mountain area. Agric. For. Meteorol. 2008, 148, 490–503. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Carrara, A.; Meiresonne, L.; Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol. 2003, 23, 1263–1270. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Kemp, P.R.; Ogle, K.; Fernández, R.J. Modifying the ‘pulse-reserve’ paradigm for deserts of North America: Precipitation pulses, soil water, and plant responses. Oecologia 2004, 141, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Funakawa, A.; Kilasara, M.; Kosaki, T. Effects of land management on CO2 flux and soil C stock in two Tanzanian croplands with contrasting soil texture. Soil Biol. Biochem. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Chen, H.; Tian, H.Q. Does a general temperature-dependent Q10 model of soil respiration exist at biome and global scale? J. Integr. Plant Biol. 2005, 47, 1288–1302. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Wang, T.; Sun, Z. Temperature sensitivity of soil respiration in different ecosystems in China. Soil Biol. Biochem. 2009, 41, 1008–1014. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperature mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Janssens, I.A.; Pilegaard, K. Large seasonal changes in Q10 of soil respiration in a beech forest. Glob. Chang. Biol. 2003, 9, 911–918. [Google Scholar] [CrossRef]
- Pang, X.Y.; Bao, W.K.; Zhu, B.; Cheng, W.X. Responses of soil respiration and its temperature sensitivity to thinning in a pine plantation. Agric. For. Meteorol. 2013, 171–172, 57–64. [Google Scholar] [CrossRef]
- Yuste, J.C.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob. Chang. Biol. 2004, 10, 161–169. [Google Scholar] [CrossRef]
- Xu, Z.F.; Tang, S.S.; Xiong, L.; Yang, W.Q.; Yin, H.J.; Tu, L.H.; Wu, F.Z.; Chen, L.H.; Tan, B. Temperature sensitivity of soil respiration in China’s forest ecosystems: Patterns and controls. Appl. Soil Ecol. 2015, 93, 105–110. [Google Scholar] [CrossRef]

| Forest Type | SA (years) | CD (%) | H (m) | DBH (cm) | Understory Vegetation Composition |
|---|---|---|---|---|---|
| Pinus tabulaeformis | 5 | 18 (3) | 1.1 (0.16) | 1.9 (0.15) | Carex rigescens (Franch.), Artemisia sacrorum Ledeb., Elymus dahuricus Turcz. ex Griseb., Potentilla ancistrifolia Bunge, Sanguisorba officinalis L. |
| 10 | 65 (7) | 2.8 (0.13) | 5.5 (0.34) | ||
| 30 | 77 (9) | 13.8 (0.77) | 10.2 (0.77) | ||
| Populus davidiana | 5 | 37 (5) | 8.6 (0.24) | 7.6 (0.34) | Artemisia verlotorum Lamotte, Potentilla discolor Bunge, Dendranthema indicum L. Des Moul., Setaria viridis (L.) P. Beauv. |
| 10 | 70 (9) | 20 (1.03) | 12 (0.91) | ||
| 20 | 83 (11) | 24.8 (2.08) | 23.1 (1.15) |
| Forest Type | 5 Years | 10 Years | 30 Years/20 Years | |
|---|---|---|---|---|
| SR (μmol·m−2·s−1) | Pinus tabulaeformis | 3.37 (0.26) a,A | 3.17 (0.21) b,A | 2.99 (0.20) c,A |
| Populus davidiana | 2.92 (0.19) a,A | 2.85 (0.35) a,b,A | 2.57 (0.11) b,B | |
| ST (°C) | Pinus tabulaeformis | 17.86 (1.13) a,A | 16.70 (1.01) b,A | 15.99 (0.95) b,A |
| Populus davidiana | 16.73 (1.25) a,B | 16.08 (1.28) a,b,A | 15.65 (1.30) b,B | |
| SWC (%) | Pinus tabulaeformis | 9.27 (0.99) b,B | 10.27 (0.43) a,b,B | 10.96 (0.35) a,B |
| Populus davidiana | 11.28 (0.22) b,A | 11.81 (0.04) a,b,A | 12.24 (0.26) a,A |
| Pinus tabulaeformis | Populus davidiana | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | p | Q10 | a | b | R2 | p | Q10 | ||
| 5 years | 1.13 | 0.06 | 0.48 | 0.00 | 1.85 | 5 years | 0.77 | 0.05 | 0.70 | 0.00 | 2.2 |
| 10 years | 1.11 | 0.06 | 0.32 | 0.01 | 1.85 | 10 years | 0.58 | 0.09 | 0.79 | 0.00 | 2.51 |
| 30 years | 0.96 | 0.07 | 0.28 | 0.02 | 1.99 | 20 years | 0.50 | 0.10 | 0.91 | 0.00 | 2.64 |
| All stands | 1.06 | 0.06 | 0.37 | <0.01 | 1.90 | All stands | 0.62 | 0.09 | 0.79 | <0.01 | 2.42 |
| SWC < 12.8% | 0.58 | 0.09 | 0.53 | <0.01 | 2.48 | ||||||
| SWC > 12.8% | 0.90 | 0.07 | 0.72 | <0.01 | 2.05 | ||||||
| ST | SWC | SOC | STD | FRB | SBD | pH | |
|---|---|---|---|---|---|---|---|
| SR | 0.881 * | −0.978 ** | −0.779 * | −0.482 | 0.740 * | −0.688 | −0.237 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, F.; Zhang, W.; Ai, Z.; Shen, H.; Liu, X.; Cao, J.; Manevski, K. Soil Respiration at Different Stand Ages (5, 10, and 20/30 Years) in Coniferous (Pinus tabulaeformis Carrière) and Deciduous (Populus davidiana Dode) Plantations in a Sandstorm Source Area. Forests 2016, 7, 153. https://doi.org/10.3390/f7080153
Zhao X, Li F, Zhang W, Ai Z, Shen H, Liu X, Cao J, Manevski K. Soil Respiration at Different Stand Ages (5, 10, and 20/30 Years) in Coniferous (Pinus tabulaeformis Carrière) and Deciduous (Populus davidiana Dode) Plantations in a Sandstorm Source Area. Forests. 2016; 7(8):153. https://doi.org/10.3390/f7080153
Chicago/Turabian StyleZhao, Xin, Fadong Li, Wanjun Zhang, Zhipin Ai, Huitao Shen, Xiuping Liu, Jiansheng Cao, and Kiril Manevski. 2016. "Soil Respiration at Different Stand Ages (5, 10, and 20/30 Years) in Coniferous (Pinus tabulaeformis Carrière) and Deciduous (Populus davidiana Dode) Plantations in a Sandstorm Source Area" Forests 7, no. 8: 153. https://doi.org/10.3390/f7080153







